Journal of Biomaterials and Nanobiotechnology
Vol.4 No.3A(2013), Article ID:33625,11 pages DOI:10.4236/jbnb.2013.43A003
Phenolic Compounds Hybrid Detectors
![]()
Department of Medicinal Chemistry and Microbiology, Faculty of Chemistry, Wrocław University of Technology, Wrocław, Poland.
Email: *jadwiga.soloducho@pwr.wroc.pl
Copyright © 2013 Jadwiga Sołoducho, Joanna Cabaj. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 29th, 2013; revised May 30th, 2013; accepted June 7th, 2013
Keywords: Biosensors; Phenolic Compound; Laccase; Tyrosinase; Horseradish Peroxidase; Thin Film; Immobilization; Conducting Polymers
ABSTRACT
Phenolic compounds are among the major classes of pollutants produced by industrial and agricultural activities. The amperometric biosensors have been mainly applied to the determination of phenolic compounds because of the advantages such as good selectivity, low cost, and easy automation. Amperometry is a method to measure the electric current that flows as a result of reactions generated at the electrode. Amperometric phenol biosensors are most often based on tyrosinase, laccase or horseradish peroxidase immobilized on the electrode surface. The immobilization of enzymes into ordered thin materials has attracted considerable attention over the past few years. The present researches have demonstrated that biomolecules immobilized in different matrixes retain their functional characteristics to a large extent. These new materials are of great interest for applications as biosensors and biocatalysts. Lately, also conducting polymers have attracted much interest in the development of biological sensors. The electrically conducting polymers are known as possessing many interesting features, which allow them to act as excellent materials for immobilization of biomolecules.
1. Introduction
Phenolic compounds are one of the popular pollutants of industrial wastes and, moreover, the compounds have high toxicity to the human organism when present above certain concentration limits this require rapid. The trend towards simplification of the analytical methods used in modern laboratories or in quality control of some industrial processes has led to setting up some electrometric procedures for determining phenol, based on biosensors [1-3]. Due to health and ecological risks caused by longand short-term exposure to these phenolic compounds, there is a considerable interest in their measurements in environmental matrices [4]. Various methods are available for the determination of these compounds, including chromatographic and spectrophotometric analyses, but these methods present some disadvantages, such as laborious sample pre-treatment, expensive, manpower and doubts about the sample integrity, which make them unsuitable for on-line monitoring [4,5].
In the example, using of microbial-based sensors to detect the concentrations of substances is based on the presence of specific enzyme systems which transform certain chemical compounds. The transformation processes can be accompanied by the appearance of electrochemically active products or utilization of reaction co-substrates, which enables the use of standard electrochemical techniques [6].
Biosensors can make ideal sensing systems to monitor the effects of pollution on the environment, due to their biological base, ability to operate in complex matrices, short response time and small size (Figure 1). The determination of phenol and its derivative compounds is of the environmental greatness, since these species are released into the environment by a large number of industries, e.g. the manufacture of plastics, dyes, drugs, antioxidants and waste waters from pulp and paper production. This group of biosensor is of great interest because of their application in food and pharmaceutical industry [7]. Furthermore, as polyphenols are electroanalytically active compounds that can be easily oxidized at inert electrodes, electrochemical sensors have also been used as tools for estimating the total phenolic content.

Figure 1. Simplified diagram of the biosensor effect.
Amongst enzymes, laccases and tyrosinases [8] or horse-radish peroxidase [9] are groups of oxidases that catalyze the transformation of a large number of phenolic compounds.
Phenolooxidases have wide substrate specificity and a great potential for the determination of phenolic compounds. Moreover, fungal laccases catalyze demethylation reactions an important and initial step of the biodegradation process of the lignin polymer chain, and subsequently decompose the lignin macromolecule by splitting aromatic rings and C-C bonds in the phenolic sub-structures [9].
Tyrosinaseand laccase-based amperometric biosensors have proved to be very useful for the determination of phenols and substituted phenols at low levels [10-12]. However, the use of this kind of analytical device has some limitations when employed for monioring, continuously, target contaminants in various environmental matrices. One of these limitations is the dependence on sample conditions, such as pH and ionic strength. In relation to the pH influence, usually the phenoloxidases-based biosensors show interesting sensitivity for pH between 3 and 7, with a very strong decrease at higher pH values [13,14].
The model of molecular assemblies often used in these types of biosensors design is prepared by LangmuirBlodgett (LB) and Langmuir-Schaefer (LS) techniques in which we have to the moment successful experience [8], Layer-by-Layer (LbL) or by employing different SelfAssembly Monolayers (SAMs) or electrolytic deposition. Construction of novel phenol detecting biosensor is a big challenge for modern technologies and the key element is modification of electrode by proteins using i.e., thin film preparation methods.
2. Need for Alternative Methods-Biosensors
The most broadly used methods for the determination of various phenols are high-performance liquid chromatography, liquid chromatography coupled with electrochemical detection, liquid chromatography coupled with mass spectrometry, capillary electrophoresis, gas chromatography, and gas chromatography coupled with mass spectrometry [15-18]. These methods offer proper selectivity and detection limits, but, they are not suitable for rapid processing of multiple samples and real-time detection. They involve highly trained operators, timeconsuming detection processes, and complex pre-treatment steps. The instruments are sophisticated and expensive. Further, the methods are unsuitable for field studies and in-situ monitoring of samples [19-21].
2.1. Biosensors for Phenolic Compounds
Biosensors based on the coupling of a biological entity with a suitable transducer offer an effective route for detection of phenolic compounds. For phenolic compounds determinationm biosensors modified with tyrosinase, peroxidase, laccase and polyphenol oxidase have been reported. Electrodes modified with these enzymes have the advantage that the detection of phenolic compounds can be carried out between −0.2 and 0.05 V versus SCE and the interface is minimized [7].
Electrochemical biosensors are rather cheap, simple to fabricate, and reusable. They have high stability and sensitivity. This kind of sensors can potentially be used for other species with the necessary modifications. Many phenolic compounds are successfully detected using electrochemical sensors as most sensors are oxidized at readily accessible potentials. Many phenolic compounds are successfully detected using electrochemical sensors as most sensors are oxidized at readily accessible potentials [21].
In example, chemically modified carbon electrodes have been designed by Yin et al. [19] for the detection of bisphenol A. Cobalt phthalocyanine modifier has been applied in electrodes to help decrease the redox potential. Increased sensitivity and selectivity have been achieved for bisphenol A in an aqueous solution. The detection limit was found as 1.0 × 10−8 M [19].
Electrochemical biosensor devices based on three enzymes (tyrosinase, laccase, horseradish peroxidase) use a similar approach to detection: the liberated quinones or phenoxy radicals, enzymaticaly oxidized, as mediators in the oxido-reduction enzyme cycle, are rereduced at the surface of the electrode, and a dramatic amplification of the biosensor response can be achieved by means of this partial substrate recycling (Figure 2).
2.1.1. Laccase and Tyrosinase Electrodes for Phenolic Compounds Detection
Laccaseand tyrosinase-based electrodes have been shown to be useful for the selective determination of phenols in environmental matrices [22-24]. The use of laccase is in great importance because it is more sensitive for chlorinated organic compounds, which is very significant in environmental respects. Due to the fact, laccase can react with phenolic compounds that are not reactive with tyrosinase.
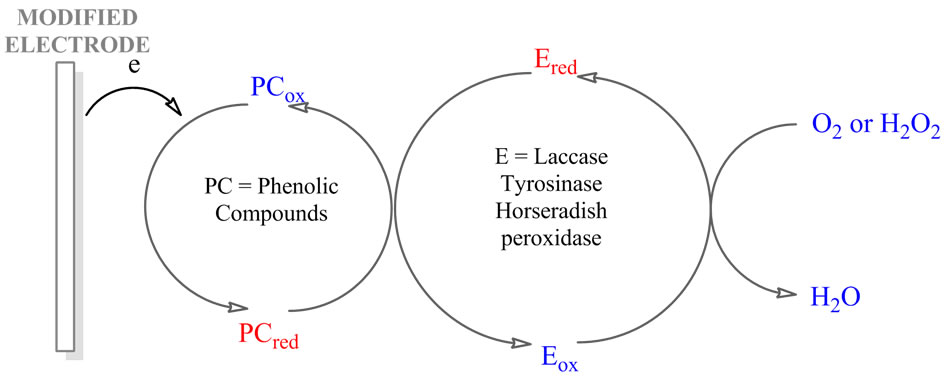
Figure 2. Mechanism of the reactions on the laccase/ tyrosinase/horseradish peroxidase biosensor. PC: Phenolic compound; E: Enzyme; red and ox are the reduced and oxidized forms.
Laccase and tyrosinase are both copper-containing oxidases catalyzing the reduction of molecular oxygen by different electron donors, e.g. phenolic compounds. One of the most important points in using laccase is the sensitivity for phenolic compounds that are toxic. In catalyzed reactions the oxygen is reduced directly to water without the intermediate formation of hydrogen peroxide (Figure 3) [22]. Mechanism of laccase catalysys sometime requires also the mediator (Figure 3). The substrate for laccase is also molecular oxygen, hence the enzyme plays a role of terminal electron acceptor in a four electron process in which water is the final product.
The two enzymes (laccase, tyrosinase) display different substrate specificities and mechanisms, hence the coimmobilization of laccase and tyrosinase on the transducer element of an electrochemical sensor allows more phenolic compounds to be detected [22].
For the design of biosensor different methods of enzyme immobilization have been employed. They include the modification of solid graphite [25], incorporation of enzyme into carbon paste, immobilization on surface of different membranes [26] Langmuir-Blodgett hybrid films [27,28]. The most sensitive biosensors are based on tyrosinases [29], however, in order to low stability of this class of enzymes, these devices usually present short lifetimes [30]. Alternatively to tyrosinases—laccases are often used.
However, an exhaustive overview in the basic aspects of immoblilization of laccase and tyrosinase has been reported. Whereas, to retain enzyme’s specific biological function, their immobilization on solid matrix is a key factor in preparing biosensors. So far several immobilization strategies have been commonly used to immobilize small molecules onto appropriately functionalized glass slides, including covalent immobilization with Staudingeer ligation [31]. The immobilization methods for laccase or tyrosinase such as physical adsorption [27]covalent attachment [30], incorporation within carbon paste [8], immobilization in polymer films [32], entrapment in some sol-gel matrices [8] have been also reported in the literature. Vianello et al. presented a highsensitivity flow biosensor based on a monomolecular layer of laccase immobilized on a gold support. This biosensor detects phenols in the low micromolar range, i.e. below European Community limits [30].
In particular, several biosensors based on tyrosinase were developed for determination of phenols (Figure 4) [33]. The tyrosinase was immobilized on an electrode’s surface as a thin film or in a membrane on a Clark oxygen electrode [34], chemically bonded to a solid graphite electrode [25] or controlled-pore glass [35] and using electropolymerization of an amphilic pyrrole derivative-enzyme mixture [36]. Tyrosinase was also adsorbed on the surface of phospholipids LangmuirSchaefer film [27].
A general problem for many tyrosinase biosensors is the lack of the necessary operational and storage stability needed for commercial exploitation, and is currently a major obstacle to solve in the biosensor area. The instability of tyrosinase biosensors in pure standard solutions is mainly due to that: quinones suffer from high instability in water, and formation of intermediate radicals in both the enzymatic and electrochemical reactions. Radicals can react and polymerise to polyaromatics which can inactivate the biocatalyst and foul the electrode [37,38].
For example, Nistor et al. has presented the possibility of using Nafion modified tyrosinase electrodes for obtaining biosensors with improved stability for the screening of phenolic compounds in waste waters. The immobilisation of tyrosinase in cationic exchange membranes
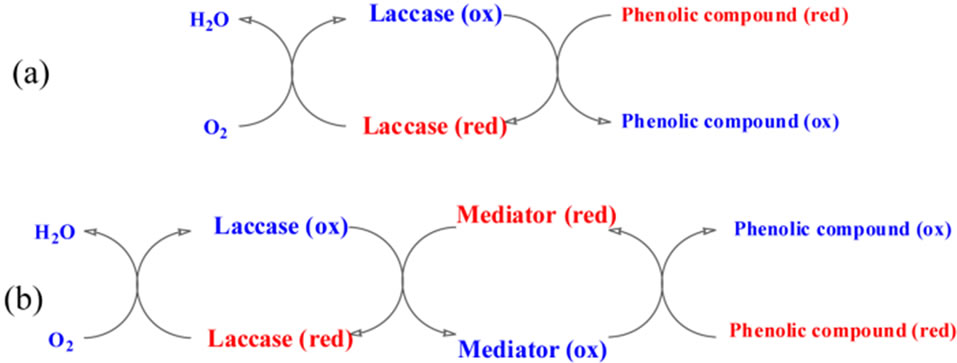
Figure 3. Mechanism of the reactions on the laccase (a) without mediator, (b) with mediator.
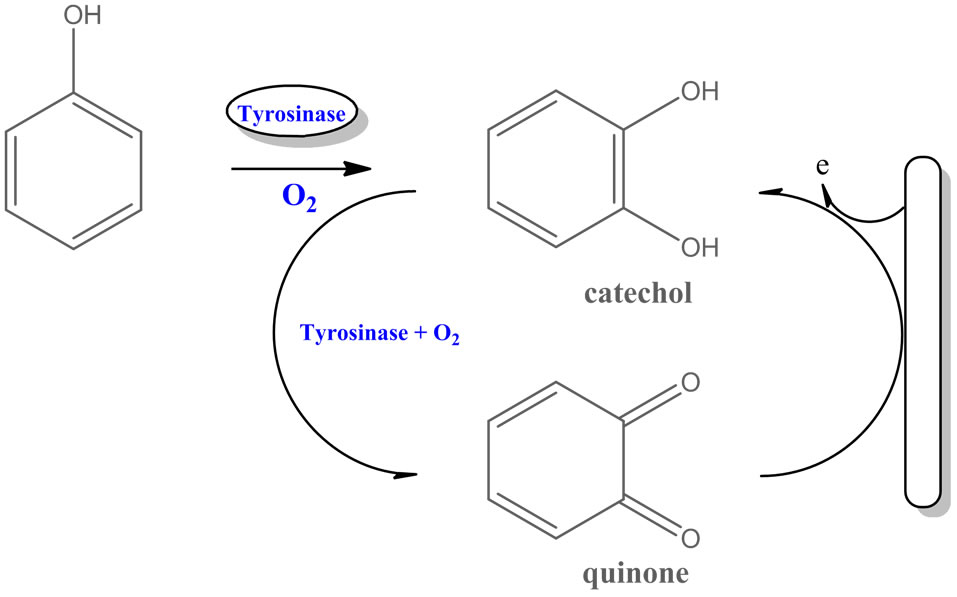
Figure 4. Mechanism of reactions catalyzed by tyrosinase.
has some advantages (exclusion of anionic interferences and altered selectivity of the sensors due to preconcentration of certain monophenols into the membranes) [23].
2.1.2. Horseradish Peroxidase Electrodes for Phenolic Compounds Detection
Horseradish peroxidase (HRP) catalyzed reactions with phenols are much faster than polyphenol oxidases (PPO) ones, moreover, HRP-based electrodes show much higher sensitivity in comparison to PPO-based sensors. Due to the fact, the application of HRP on working electrodes may be advised for faster and effective phenol measurements.
Horseradish peroxidase is a popular enzyme for phenol detection due to its high sensitivity towards a great number of phenolic compounds.
Many different methods such as covalent immobilization [39], sol-gel derived matrix [40], recently LbL assembly was employed for modification of electrodes [41]. And still the combination of oxidoreductases and amperometric electrodes is the most commonly studied biosensor concept (Figure 5).
Amperometric biosensors for the detection of phenolic compounds have been introduced as a mono-enzyme system using tyrosinase, laccase or HRP. Tyrosinase biosensors are restricted to the monitoring of phenolic compounds having at least one o-position free.
Whereas, laccase biosensors give response to phenolic compounds with free pand m-position with a complicated catalytic cycle. HRP is less selective to phenolics and capable of giving response to a wide number of phenol derivatives, and is highly stable as well as efficient for different biosensor designs. HRP is oxidized by hydrogen peroxide and re-reduced by phenols. Phenoxy radicals, formed during the enzymatic oxidation of phenolic compounds in the presence of hydrogen peroxide, were reduced electrochemically on the electrode surface; the reduction current is proportional to concentration of phenolic compound [42,43].
The direct adsorption of HRP molecules on electrode surfaces causes denaturation and loss of activity including the slow electron transfer due to the active sites
Figure 5. Amperometric biosensor concept.
of enzyme have the long distance between the active sites and electrode surface [44]. Therefore, nanomaterials like gold nanoparticles have been applied as a promoter to enhance the electron transfer as was proposed by Kumpangpet et al. [45].
Moreover, Imabayaschi et al. reported the HRP biosensor constructed by enzyme covalently immobilized on the mercaptonic acid self-assembled monolayer on the gold electrode [39]. The most simple electrode for the detection of peroxide consists of a layer of peroxidase molecules adsorbed onto the electrode surface. If the electrode is placed into a sample and poised at a potential more negative than 0.6V vs. SCE then a proportionality between the registered reduction current and the peroxide concentration is observed. This phenomenon was observed for horseradish peroxidase adsorbed on carbon black, graphite, carbon fibers, gold, and platinum electrodes [46].
The response of the peroxidase biosensors to phenolic compounds is based on the double displacement in which two substrates, H2O2 and the electron-donating phenolic compounds are involved (Figure 6). At the electrode surface, peroxidase molecules are oxidized by H2O2 followed of its reduction by phenolic compounds. In the last reaction, the phenolic compounds are mainly converted into quinones or free radical products, which are electroactives and can be electrochemically reduced on the electrode surface. The reduction current is proportional to the phenolic compounds concentration in the solution, as long as the H2O2 concentration is not limiting [46].
The monitoring of the enzyme reaction is accomplished by the electrode reduction of the phenoxy radicals formed, the current being proportional to the concentration of phenolic compounds as long as the H2O2 concentration is not limiting. Therefore, an excess of H2O2 should be added to the working solution in order for the biosensor to be able to respond to the phenolic compounds [47]. However, it is well known that the presence of a high concentration of H2O2 causes inhibition of the activity of peroxidase [48].

Figure 6. Scheme of the reactions at the electrode modified with horseradish peroxidase.
Serra et al. reported the sensing system for phenolic compounds where horseradish peroxidase is mixed with glucose oxidase (GOx). In this biosensor, GOx was responsible for generating in situ H2O2 needed for the enzyme reaction with the phenolic compounds [48]. For the sensor design, matrices of graphite and Teflon were selected. The enzymatic electrodes were constructed by simple physical inclusion of the enzymes (HRP, GOx) into the bulk of graphite-Teflon pellet with no covalent attachments.
Serra et al. described also the three enzyme system with HRP, GOx and tyrosinase to monitor possibly large number of phenolic compounds [47].
3. Modification of Electrodes by Conducting Polymers
A new class of polymers known as intrinsically conducting polymers or electroactive conjugated polymers has been extremely famous. This kind of materials exhibit interesting electrical and optical properties previously found mostly in inorganic units. Conducting polymers vary from all the popular inorganic semiconductors (i.e., silicon). These materials exhibit intrinsic electronic conductivity ranging from about 10−14 to 102 S∙cm−1 due to extension of the doped state [49]. In the neutral (undoped) state these materials are only semiconducting and electronic conductivity only appears when the material is doped with small sized ions (e.g. when electrons or holes are injected into the super orbital).
Many applications of conducting polymers including analytical chemistry and biosensing devices have been reviewed by various researchers [50-52]. They have broadened the possibility of modification of surface of conventional electrodes providing new and interesting features. Semiconducting organic materials were applied in electrocatalysis, membrane separations and chromatography. They also create new technological possibilities in design of chemical and biochemical sensors [50,53].
Importance of Conducting Polymers to Sensor Devices
Conducting polymers have attracted much interest as a suitable matrix of enzymes, they enhance speed, sensitivity and versatility of biosensors in diagnostics to measure different analytes. Conducting polymers are thus finding ever increasing use in diagnostic medical reagents [54]. Conducting polymers have attracted much attention as a suitable matrix for the entrapment of enzymes [55,56]. The techniques of incorporating enzymes into electro-depositable conducting polymeric films permit the localization of biologically active molecules on electrodes of any size or geometry and is particularly appropriate for the fabrication of multi-analyte microamperometric biosensors [57].
Semiconducting polymers have proper flexibility in the available chemical structure, which can be modified as need. By chemical modeling and synthesis, it is possible to modulate the required electronic and mechanical properties of material. Morover, the polymer itself may be modified to bind protein molecules [58,59]. The valid advantage offered by conducting polymers is that, the electrochemical synthesis enables the direct deposition of the polymer on the electrode surface (i.e., simultaneously trapping the protein molecules) [60]. It is potential also to control the distribution of the immobilized biocatalysts, the film thickness and modulate the enzyme activity by changing the electrical state of the polymer.
Synthetic and biological receptors may be applied to manipulate the sensitivity of a conducting polymer for different types of analyte [61,62]. Certain conducting polymers that have been modified with various receptors are listed in Table 1. To immobilize the receptor, it is bonded to the polymer matrix through covalent or noncovalent interactions. Physical adsorption [27], the Langmuir-Blodgett technique [8], layer-by layer deposition technique [63], and mechanical embedding method [64] are used to bind the receptor to the matrix through different ionic interactions. Gerard et al. [65] have discussed the advantages and limitations of these techniques.
Conducting polymers have also the ability to efficient transfer of charge produced by the biochemical reaction to electronic circuit [73]. Moreover conducting polymers may cover defined areas of electrodes. This exceptional feature concern the possibility to encapsulate enzymes during electrochemical process according to amperometric biosensors [74].
Among the conducting polymers/materials, polypyrroles play a leading role due to their versatile applicability and the wide variety of molecular (redox) species covalently linked to a pyrrole group [75]. Nakabayashi et al. reported an amperometric biosensor for detection of H2O2 based on electron transfer between HRP and ferrocene as a mediator [76]. Likewise, Thanachasai et al. developed novel H2O2 biosensor based on peroxide carrying poly(pyrrole-co-[4-(3-pyrrolyl) butanesulfonate]) [77]. Yasuzawa et al. showed the feature of glucose sensors based on the immobilized glucose oxidase in polypyrrole [78]. The biosensing device was prepared by electro polymerization of 3-(1-pyrrolyl) propionic acid in the presence of the biocatalyst following the treatment with water soluble carbodiimide to provide covalent linkage between glucose oxidase and polypyrrole.
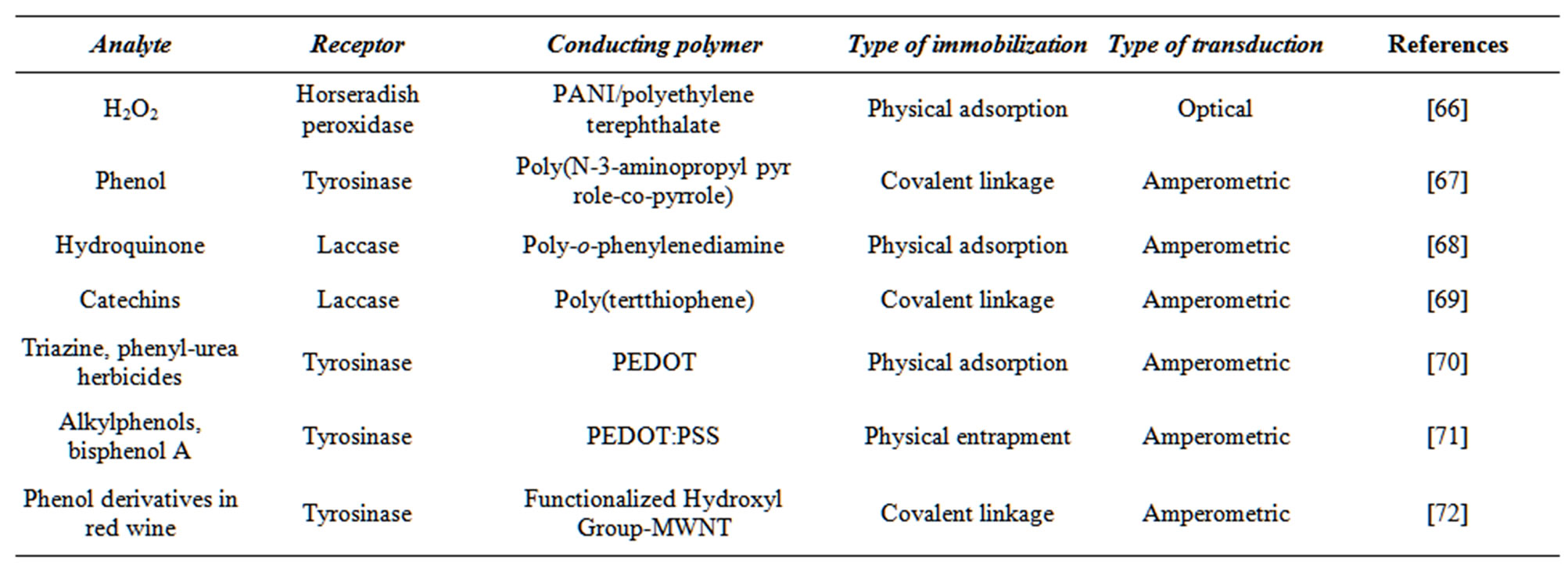
Table 1. Examples of conducting polymer-based biosensors for phenol compounds detection.
A lot of enzymes have been immobilized by physical adsorbtion on a number of conducting polymers by [66,68,70]. This is the simplest method of enzyme immobilization. The binding forces involved are hydrogen bonds, multiple salt linkages, Van der Waal’s forces etc. [79].
4. Protein-Monolayer Engineering
Protein monolayer electrochemistry is an effective technique used to study interactions between redox proteins and synthetic adsorptive platforms. The effectiveness of this strategy, however, is dependent on the ability to engineer an adsorption interface with a high degree of molecular level control.
A relevant path in this context is to use structural motifs of existing proteins as stable scaffolds, which, by appropriate mutations, deletions, insertions, or fusions create protein structures with desired functionality [80].
The SAMs structures can be ideally composed of tightly packed and well ordered chains, although several factors may lead to the formation of defects and irregularities [81]. Due to that, the nanometric size of proteins, as well as their diversity, makes that complex interesting to explore the utilization of such structures in molecular electronic devices [82].
Monolayers engineering aims to create complex molecular assemblies with a specific layered structure. The techniques applied are based mainly on the original Langmuir-Blodgett (LB) [8] or Langmuir-Schaefer (LS) [8] methods, very often combined with self-assembly [81] and adsorption processes. Generally, monolayers of amphiphilic organic molecules are formed at the liquid-air interface in the LB through by first spreading and then compressing the organic surface layer to a defined surface pressure (Figure 7).
However, protein monolayers are usually prepared by adopting the horizontal-lift LS technique for transfer onto the solid substrate. For enzymes, an adsorption through has proved to be even more effective in preserving the native protein function in the engineered monolayers [8].
As proteins are not ideal amphiphilic molecules, the techniques need to be adapted, either by chemical methods (e.g., derivatization methods [83] or varying the subphase composition [84]) or by applying some mimetic systems of biological membranes, due to preserve their native structure and function in monolayer.
The quality of protein-monolayer formation at the air-water interface is related to the degree of preservation of the native properties of all proteins. The magnitude of the electrostatic forces maintaining the protein structure is comparable with that of the surface tension. Proteins tend to form stable monolayers at the air-water interface because of their mixture of hydrophilic and lipophilic groups. Often spreading species such as proteins at the air-water interface can affect the conformation of the molecule such as causing unfolding. For example insulin or ovalbumin unfold completely whereas myoglobin and cytochrome C are only partially unfolded [85]. This again is thought to be a function of the ratio of polar to non-polar amino acids residues. Highly polar proteins such as xanthine oxidase do not form stable monolayers [84]. In all these circumstances the convenient matrix may be required. For instance, according to Girart-Ergot et al. [86] enzyme bioactivity in mixed lipid LB films is preserved due to the lipid molecular assembly protects the enzyme, positioning the polypeptide moiety in such a way as to allow the recognition and signal events.
The possibility of preparing multilayer films opens the perspectives of characterizing these mimetic systems using a wider range of techniques in opposition to LB method, which usually restricts the phospholipids films
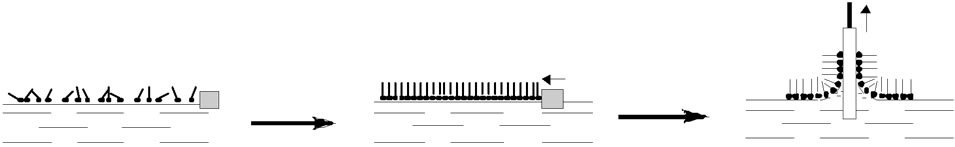
Figure 7. Formation of Langmuir/Langmuir-Blodgett layers.
to one or two layers [87]. However, despite this disadvantage, the LB technique is still a distinctive way to produce phospholipids structured as monoor bilayers like they are found in the cell membrane models.
Protein Monolayers Electronic Properties
The electronic properties of the protein monolayers are currently characterized by using the CP-AFM technique. This method allowed for continuous monitoring the effect of externally applied force on the current flowing through the junction [88-90]. To study these effects I-V curves were obtained under varying forces. Current was not observed when forces were lower than 23 nN.
First insight into the effect of applied force on the electronic behavior of protein layer was obtained by monitoring the changes in the low voltage conductance of the proteins, which was estimated from the slope of the I-V curve in the range of [V] ≤ 0.2 V for each of the I-V curve. An increase in the conductance with applied force was detected for azurin by Davis et al. [88], although with much stronger exponential dependence. The observed dependence can be explained by an increase in the contact area (i.e., increase in the number of conduction channels), and elastic deformation, i.e., a decrease in the effective length of the conduction channel [88].
5. Conclusions
For nearly 50 years we have witnessed tremendous progress in the development of electrochemical biosensors. Elegant research on new sensing concepts, coupled with numerous technological innovations, has thus opened the door to widespread applications of electrochemical biosensors.
Phenolic compounds generated in different industrial activities and discharged as waste in waters are important environmental pollutants because of their toxicity. Although well-established spectrophotometric and chromatographic methods are currently used for the determination of phenols in waters, these methods are longtime ones, and unsuitable for in situ monitoring. Sensors using biological recognition elements constitute an evident alternative to overcome these hindrances.
Major fundamental and technological advances have been made for enhancing the capabilities and improving the reliability of chemical measuring devices.
As this field enters its fifth decade of intense research, we could expect significant efforts that couple the fundamental sciences with technological advances.
6. Acknowledgements
Financial support from the Wrocław University of Technology and Polish Ministry of Science and Higher Education Grant No. 2012/05/B/ST5/00749 authors are gratefully acknowledged.
REFERENCES
- E. A. Cummings, S. Linquette-Mailley, P. Mailley, S. Cosnier, B. R. Eggins and E. T. McAdams, “A Comparison of Amperometric Screen-Printed, Carbon Electrodes and Their Application to the Analysis of Phenolic Compounds Present in Beers,” Talanta, Vol. 55, No. 3, 2001, pp. 1015-1027. doi:10.1016/S0039-9140(01)00532-X
- H. M. Tan, S. P. Cheong and T. C. Tan, “An Amperometric Benzene Sensor Using Whole Cell Pseudomonas putida ML2,” Biosensors & Bioelectronics, Vol. 9, No. 1, 1994, pp. 1-8. doi:10.1016/0956-5663(94)80008-1
- S. C. Chang and C. J. Mc Neil, “Disposable TyrosinasePeroxidase Bi-Enzyme Sensor for Amperometric Detection of Phenols,” Biosensors & Bioelectronics, Vol. 17, No. 11, 2002, pp. 1015-1023. doi:10.1016/S0956-5663(02)00094-5
- J. Wang and Q. Chen, “Remote Electrochemical Biosensor for Field Monitoring of Phenolic Compounds,” Analytica Chimica Acta, Vol. 312, No. 1, 1995, pp. 39-44. doi:10.1016/0003-2670(95)00207-G
- J. Wang, J. Lu, S. Y. Ly, B. Tian, W. K. Adeniyi and R. A. Armendariz, “Lab-on-a-Cable for Electrochemical Monitoring of Phenolic Contaminants,” Analytical Chemistry, Vol. 72, No. 11, 2000, pp. 2659-2663. doi:10.1021/ac991054y
- T. Kuwahara, K. Oshima, M. Shimomura and S. Miyauchi, “Glucose Sensing with Glucose Oxidase Immobilized Covalently on the Films of Thiophene Copolymers,” Synthetic Metals, Vol. 152, No. 1-3, 2005, pp. 29-32. doi:10.1016/j.synthmet.2005.07.096
- J. Cabaj and J. Sołoducho, “Hybrid Film Biosensor for Phenolic Compounds Detection in Environmental Biosensors,” V. Somerset, Ed., InTech, Rijeka, 2011.
- J. Cabaj, J. Sołoducho and A. Nowakowska-Oleksy, “Langmuir-Blodgett Film Based Biosensor for Estimation of Phenol Derivatives,” Sensors and Actuators B, Vol. 143, No. 2, 2010, pp. 508-515. doi:10.1016/j.snb.2009.09.047
- K. F. Fernandes, C. S. Lima, F. M. Lopes and C. H. Collins, “Hydrogen Peroxide Detection System Consisting of Chemically Immobilised Peroxidase and Spectrometer,” Process Biochemistry, Vol. 40, No. 11, 2005, pp. 3441-3445. doi:10.1016/j.procbio.2005.04.003
- S. S. Rosatto, G. Oliveira-Neto and L. T. Kubota, “Effect of DNA on the Peroxidase Based Biosensor for Phenol Determination in Waste Waters,” Analytica Chimica Acta, Vol. 13, No. 6, 2001, pp. 445-450.
- G. Marko-Varga, J. Emneus, L. Gorton and T. Ruzgas, “Development of Enzyme Based Amperometric Sensors for the Determination of Phenolic Compounds,” Trends in Analytical Chemistry, Vol. 14, No. 1, 1995, pp. 319- 328. doi:10.1016/0165-9936(95)97059-A
- A. L. Ghindilis, V. P. Gavrilova and A. I. Yaropolov, “Laccase-Based Biosensor for Determination of Polyphenols: Determination of Catechols in Tea,” Biosensors & Bioelectronics, Vol. 7, No. 2, 1992, pp. 127-131. doi:10.1016/0956-5663(92)90017-H
- R. S. Freire, N. Duran and L. T. Kubota, “Effects of Fungal Laccase Immobilization Procedures for the Development of a Biosensor for Phenol Compounds,” Talanta, Vol. 54, No. 4, 2001, pp. 681-686. doi:10.1016/S0039-9140(01)00318-6
- J. Wang, R. S. Freire, N. Durán, S. Thongngamdee and L. T. Kubota, “Mixed Enzyme (Laccase/Tyrosinase)-Based Remote Electrochemical Biosensor for Monitoring Phenolic Compounds,” Analyst, Vol. 127, No. 2, 2002, pp. 258-261. doi:10.1039/b110011d
- Y. Wen, B. Zhou, Y. Xu, S. Jin and Y. Feng, “Analysis of Estrogens in Environmental Waters Using Polymer Monolith In-Polyether Ether Ketone Tube Solid-Phase Microextraction Combined with High-Performance Liquid Chromatography,” Journal of Chromatography A, Vol. 1133, No. 1-2, 2006, pp. 21-28. doi:10.1016/j.chroma.2006.08.049
- G. Gatidou, N. Thomaidis, A. Stasinakis and T. Lekkas, “Simultaneous Determination of the Endocrine Disrupting Compounds Nonylphenol, Nonylphenol Ethoxylates, Triclosan and Bisphenol A in Wastewater and Sewage Sludge by Gas Chromatography-Mass Spectrometry,” Journal of Chromatography A, Vol. 1138, No. 1-2, 2007, pp. 32-41. doi:10.1016/j.chroma.2006.10.037
- A. M. Comerton, R. C. Andrews and D. M. Bagley, “Practical Overview of Analytical Methods for Endocrine-Disrupting Compounds, Pharmaceuticals and Personal Care Products in Water and Wastewater,” Philosophical Transactions of Royal Society A, Vol. 367, No. 1904, 2009, pp. 3923-3939.
- M. A. Mottaleb, S. Usenko, J. G. O’Donnell, A. J. Ramirez, B. W. Brooks and C. K. Chambliss, “Gas Chromatography-Mass Spectrometry Screening Methods for Select UV Filters, Synthetic Musks, Alkylphenols, an Antimicrobial Agent, and an Insect Repellent in Fish,” Journal of Chromatography A, Vol. 1216, No. 5, 2009, pp. 815-823. doi:10.1016/j.chroma.2008.11.072
- H. S. Yin, Y. Zhou and S.-Y. Ai, “Preparation and Characteristic of Cobalt Phthalocyanine Modified Carbon Paste Electrode for Bisphenol A Detection,” Journal of Electroanalytical Chemistry, Vol. 626, No. 1-2, 2009, pp. 80-88. doi:10.1016/j.jelechem.2008.11.004
- C. R. Suri, R. Boro, Y. Nangia, S. Gandhi, P. Sharma, N. Wangoo, K. Rajesh and G. S. Shekhawat, “Immunoanalytical Techniques for Analyzing Pesticides in the Environment,” Trends in Analytical Chemistry, Vol. 28, No. 1, 2009, pp. 29-39. doi:10.1016/j.trac.2008.09.017
- A. F. Le Blanc, C .Albrecht, T. Bonn, P. Fechner, G. Proll, F. Proll, M. Carlquist and G. Gauglitz, “A Novel Analytical Tool for Quantification of Estrogenicity in River Water Based on Fluorescence Labelled Estrogen Receptor,” Analytical and Bioanalytical Chemistry, Vol. 395, No. 6, 2009, pp. 1769-1776. doi:10.1007/s00216-009-3038-8
- A. I. Yaropolov, A. N. Kharybin, J. Emnéus, G. MarkoVarga and L. Gorton, “Flow Injection Analysis of Phenols at a Graphite Electrode Modified with Co-Immobilized Laccase and Tyrosinase,” Analytica Chimica Acta, Vol. 308, 1995, pp. 137-144. doi:10.1016/0003-2670(94)00404-A
- C. Nistor, J. Emnéus, L. Gorton and A. Ciucu, “Improved Stability and Altered Selectivity of Tyrosinase Based Graphite Electrodes for Detection of Phenolic Compounds,” Analytica Chimica Acta, Vol. 387, No. 3, 1999, pp. 309-326. doi:10.1016/S0003-2670(99)00071-9
- R. Tungel, T. Rinken, A. Rinken and T. Tenno, “Immobilisation and Kinetic Study of Tyrosinase for Biosensor Construction,” Analytical Letters, Vol. 32, No. 2, 1999, pp. 235-249. doi:10.1080/00032719908542818
- D. G. Zhu, M. C. Petty, H. Ancelin and J. Yarwood, “On the Formation of Langmuir-Blodgett Films Containing Enzymes,” Thin Solid Films, Vol. 176, No. 1, 1989, pp. 151-156. doi:10.1016/0040-6090(89)90372-6
- J. Anzai, S. Lee and T. Osa, “Enzyme-Immobilized Langmuir-Blodgett Membranes for Biosensor Application. Use of Highly Branched Polyethyleneimine as a Spacer for Immobilizing α-Chymotrypsin and Urease,” Die Makromolekulare Chemie Rapid Communications, Vol. 10, No. 4, 1989, pp. 167-170. doi:10.1002/marc.1989.030100404
- I. A. Nagovitsyn and G. K. Chudinova, “An Immunosensor Based on Langmuir-Blodgett Films and Infrared Fluorescence Detection,” Biochemistry, Biophysics and Molecular Biology, Vol. 382, No. 2, 2002, pp. 267-269.
- D. I. Cherny, A. Fourcade, F. Svinarchuk, P. E. Nielsen, C. Malvy and E. Delfin, “Analysis of Various SequenceSpecific Triplexes by Electron and Atomic Force Microscopies,” Biophysical Journal, Vol. 74, No. 2, 1998, pp. 1015-1023. doi:10.1016/S0006-3495(98)74026-3
- F. Antolini, S. Paddeu and C. Nicolini, “Heat Stable Langmuir-Blodgett Film of Glutathione-S-Transferase,” Langmuir, Vol. 11, No. 7, 1995, pp. 2719-2725. doi:10.1021/la00007a062
- S. Paddeu, A. Fanigliulo, M. Lanzi, T. Dubrovsky and C. Nicolini, “LB-Based PAB Immunosystem: Activity of an Immobilized Urease Monolayer,” Sensors & Actuators, B, Vol. 25, No. 1-3, 1995, pp. 876-882. doi:10.1016/0925-4005(95)85193-3
- L. Caseli, A. C. Perinotto, T. Viitala, V. Zucolotto and O. N. Oliveira, “Immobilization of Alcohol Dehydrogenase in Phospholipid Langmuir-Blodgett Films to Detect Ethanol,” Langmuir, Vol. 25, No. 5, 2009, pp. 3057-3061. doi:10.1021/la8037445
- J. Cabaj, K. Idzik, J. Sołoducho, A. Chyla, J. Bryjak and J. Doskocz, “Well Ordered Thin Films as Practical Components of Biosensors,” Thin Solid Films, Vol. 516, No. 6, 2008, pp. 1171-1174. doi:10.1016/j.tsf.2007.06.082
- T. Mai Anh, S. V. Dzyadevych, A. P. Soldatkin, N. Duc Chien, N. Jaffrezic-Renault and J.-M. Chovelon, “Development of Tyrosinase Biosensor Based on pH-Sensitive Field-Effect Transistors for Phenols Determination in Water Solutions,” Talanta, Vol. 56, No. 4, 2002, pp. 627- 634. doi:10.1016/S0039-9140(01)00611-7
- L. C. Clark Jr. and C. Lyons, “Electrode Systems for Continuous Monitoring in Cardiovascular Surgery,” Annals of the New York Academy of Sciences, Vol. 102, 1962, pp. 29-45. doi:10.1111/j.1749-6632.1962.tb13623.x
- A. M. Girelli, E. Mattei, A. Messina and D. Papaleo, “Immobilization of Mushroom Tyrosinase on Controlled Pore Glass: Effect of Chemical Modification,” Sensors and Actuators B, Vol. 125, No. 1, 2007, pp. 48-54. doi:10.1016/j.snb.2007.01.035
- J. -L. Besombes, S. Cosnier and P. Labbe, “Improvement of Poly(Amphiphilic Pyrrole) Enzyme Electrodes via the Incorporation of Synthetic Laponite-Clay-Nanoparticles,” Talanta, Vol. 44, No. 12, 1997, pp. 2209-2215. doi:10.1016/S0039-9140(97)00039-8
- J. N. Rodrı́guez-López, J. Tudela, R. Varón, F. Garcı́aCarmona and F. Garcı́a-Cánovas, “Analysis of a Kinetic Model for Melanin Biosynthesis Pathway,” The Journal of Biological Chemistry, Vol. 267, No. 6, 1992, pp. 3801- 3810.
- H. Kotte, B. Gruendig and K.-D. Vorlop, “Methylphenazonium-Modified Enzyme Sensor Based on Polymer Thick Films for Subnanomolar Detection of Phenols,” Analytical Chemistry, Vol. 67, No. 1, 1995, pp. 65-70. doi:10.1021/ac00097a011
- S. Imabayashi, Y. T. Kong and M. Watanabe, “Amperometric Biosensor for Polyphenol Based on Horseradish Peroxidase Immobilized on Gold Electrodes,” Electroanalysis, Vol. 13, No. 5, 2001, pp. 408-412.
- J. Anzai, J. Hashimoto, T. Osa and T. Matsuo, “Penicillin Sensors Based on an Ion-Sensitive Field Effect Transistor Coated with Stearic Acid Langmuir-Blodgett Membrane,” Analytical Sciences, Vol. 4, No. 3, 1988, pp. 247-250. doi:10.2116/analsci.4.247
- M. Sriyudthsak, H. Yamagishi and T. Moriizumi, “Enzyme-immobilized Langmuir-Blodgett Film for a Biosensor,” Thin Solid Films, Vol. 160, No. 1-2,1988, pp. 463- 470. doi:10.1016/0040-6090(88)90092-2
- S. Korkut Ozoner, F. Yilmaz, A. Celik, B. Keskinler and E. Erhan, “A Novel Poly(Glycidly Methacrylate-co-3- Thienylmethyl Methacrylate)-Polypyrrole-Carbon Nanotube-Horseradish Peroxidase Composite Film Electrode for the Detection of Phenolic Compounds,” Current Applied Physics, 2011, in press.
- S. Korkut, B. Keskinler and E. Erhan, “An Amperometric Biosensor Based on Multiwalled Carbon Nanotube-Poly (Pyrrole)-Horseradish Peroxidase Nanobiocomposite Film for Determination of Phenol Derivatives,” Talanta, Vol. 76, No. 5, 2008, pp. 1147-1152. doi:10.1016/j.talanta.2008.05.016
- H. Yin, S. Ai, W. Shi and L. Zhu, “A Novel Hydrogen Peroxide Biosensor Based on Horseradish Peroxidase Immobilized on Gold Nanoparticles-Silk Fibroin Modified Glassy Carbon Electrode and Direct Electrochemistry of Horseradish Peroxidase,” Sensors and Actuators B: Chemical, Vol. 137, No. 2, 2009, pp. 747-753.
- R. Kumpangpet, B. Jongsomjit, C. Thanachayanont and S. Prichanont, “Solid Oxide Fuel Cell Technology,” Engineering Journal, Vol. 16, No. 3, 2012, pp. 45-52. doi:10.4186/ej.2012.16.3.45
- X. Chen, C. Ruan, J. Kong and J. Deng, “Characterization of the Direct Electron Transfer and Bioelectrocatalysis of Horseradish Peroxidase in DNA Film at Pyrolytic Graphite Electrode,” Analytica Chimica Acta, Vol. 412, No. 1-2, 2000, pp. 89-98. doi:10.1016/S0003-2670(99)00877-6
- B. Serra, B. Benito, L. Agui, A. J. Reviejo and J. M. Pingarron, “Graphite-Teflon-Peroxidase Composite Electrochemical Biosensors. A Tool for the Wide Detection of Phenolic Compounds. Electroanalysis, Vol. 13, No. 8-9, 2001, pp. 693-700. doi:10.1002/1521-4109(200105)13:8/9<693::AID-ELAN693>3.0.CO;2-3
- W. Scheller, F. Schubert and J. Fedrowitz, “Frontiers in Biosensorics I. Fundamental Aspects,” Birkhauser, Basel, 1997.
- G. Bidan, “Electro Conducting Conjugated Polymers: New Sensitive Matrices to Build up Chemical or Electrochemical Sensors,” Sensors and Actuators B: Chemical, Vol. 6, No. 1-3, 1992, pp. 45-56. doi:10.1016/0925-4005(92)80029-W
- M. Trojanowicz, A. Lewenstam, T. Krawczynski vel Krawczyk, I. Lähdesmäki and W. Szczepek, “Flow Injection Amperometric Detection of Ammonia Using a Polypyrrole-Modified Electrode and Its Application in Urea and Creatinine Biosensors,” Electroanalysis, Vol. 8, No. 3, 1996, pp. 233-243. doi:10.1002/elan.1140080307
- A. Guiseppi-Elie, C. Lei and R. H. Baughman, “Direct Electron Transfer of Glucose Oxidase on Carbon Nanotubes,” Nanotechnology, Vol. 13, No. 5, 2002, pp. 559-564. doi:10.1088/0957-4484/13/5/303
- W. Schuhmann, “Conducting Polymers and Their Application in Amperometric Biosensors,” Microchimica Acta, Vol. 121, No. 1-4, 1995, pp. 1-29. doi:10.1007/BF01248237
- P. N. Barlett and J. M. Cooper, “A Review of the Immobilization of Enzymes in Electropolymerized Films,” Journal of Electroanalytical Chemistry, Vol. 362, No. 1-2, 1993, pp. 1-12.
- N. Gupta, S. Sharma, I. A. Mir and D. Kumar, “Advances in Sensors Based on Conducting Polymers,” Journal of Scientific & Industrial Research, Vol. 65, 2006, pp. 549- 557.
- S. B. Adeloju and G. G. Wallace, “Conducting Polymers and the Bioanalytical Sciences: New Tools for Biomolecular Communication. A Review,” Analyst, Vol. 121, No. 6, 1996, pp. 699-703. doi:10.1039/an9962100699
- W. J. Sung and Y. H. Bae, “A GL on Electropolymerized Conducting Polymer with Polyanion-Enzyme Conjugated Dopant.” Analytical Chemistry, Vol. 72, No. 9, 2000, pp. 2177-2181. doi:10.1021/ac9908041
- P. R. Unwin and A. J. Bard, “Scanning Electrochemical Microscopy. 9. Theory and Application for Feedback Mode to the Measurement of the Following Chemical Reaction Rates in Electrode Process,” The Journal of Physical Chemistry, Vol. 95, No. 20, 1991, pp. 7814-7824. doi:10.1021/j100173a049
- M. Situmorang, J. J. Gooding, D. B. Hibbert and D. Barnett, “Development of Potentiometric Biosensors Using Electrodeposited Polytyramine as the Enzyme Immobilization Matrix,” Electroanalysis, Vol. 13, No. 18, 2001, pp. 1469-1474. doi:10.1002/1521-4109(200112)13:18<1469::AID-ELAN1469>3.0.CO;2-U
- A. Mulchandani and C.-L. Wang, “Bienzyme Sensors Based on Poly(Anilinomethylferrocene)-Modified Electrodes,” Electroanalysis, Vol. 8, No. 5, 1996, pp. 414-419. doi:10.1002/elan.1140080503
- G. Vasapollo, R. Del Sole, L. Mergola, M. R. Lazzoi, A. Scardino, S. Scorrano and G. Mele, “Molecularly Imprinted Polymers: Present and Future Prospective,” International Journal of Molecular Sciences, Vol. 12, No. 9, 2011, pp. 5908-5945. doi:10.3390/ijms12095908
- B. Adhikari and S. Majumdar, “Polymers in Sensor Applications,” Progress in Polymer Science, Vol. 29, No. 7, 2004, pp. 699-766. doi:10.1016/j.progpolymsci.2004.03.002
- T. Ahuja, A. Mir, I. Kumar and D. Rajesh, “Biomolecular Immobilization on Conducting Polymers for Biosensing Applications,” Biomaterials, Vol. 28, No. 5, 2007, pp. 791-895. doi:10.1016/j.biomaterials.2006.09.046
- M. K. Ram, M. Adami, S. Paddeu and C. Nicolini, “Nanoassembly of Glucose Oxidase on the in Situ Self-Assembled Electrochemical Characterizations,” Nanotechnology, Vol. 11, No. 2, 2000, pp. 112-119. doi:10.1088/0957-4484/11/2/312
- J. Kan, X. Pan and C. Chen, “Polyaniline-Uricase Biosensor Prepared with Template Process,” Biosensors and Bioelectronics, Vol. 19, No. 12, 2004, pp. 1635-1640. doi:10.1016/j.bios.2003.12.032
- M. Gerard, A. Chaubey and B. D. Malhotra, “Application of Conducting Polymers to Biosensors,” Biosensors and Bioelectronics, Vol. 17, No. 5, 2000, pp. 345-359. doi:10.1016/S0956-5663(01)00312-8
- K. F. Fernandes, C. S. Lima, F. M. Lopes and C. H. Collins, “Hydrogen Peroxide Detection System Consisting of Chemically Immobilised Peroxidase and Spectrometer,” Process Biochemistry, Vol. 40, No. 11, 2005, pp. 3441-3445. doi:10.1016/j.procbio.2005.04.003
- Rajesh, W. Takashima and K. Kaneto, “Amperometric Phenol Biosensor Based on Covalent Immobilization of Tyrosinase onto an Electrochemically Prepared Novel Copolymer Poly (N-3-Aminopropyl Pyrrole-Co-Pyrrole) Film,” Sensors and Actuators B: Chemical, Vol. 102, No. 2, 2004, pp. 271-277. doi:10.1016/j.snb.2004.04.028
- B. Pałys, A. Bokun and J. Rogalski, “Poly-o-Fenylenediamine as Redox Mediator for Laccase,” Electrochimica Acta, Vol. 52, No. 24, 2007, pp. 7075-7082. doi:10.1016/j.electacta.2007.05.029
- A. Rahman, H.-B. Noh and Y.-B. Shim, “Direct Electrochemistry of Laccase Immobilized on Au Nanoparticles Encapsulated-Dendrimer Bonded Conducting Polymer: Application for a Catechin Sensor,” Analytical Chemistry, Vol. 80, No. 21, 2008, pp. 8020-8027. doi:10.1021/ac801033s
- C. Verdine, S. Fabiano and C. Tran-Minh, “Amperometric Tyrosinase Based Biosensor Using an Electrogenerated Polythiophene Film as an Entrapment Support,” Talanta, Vol. 59, No. 3, 2003, pp. 535-544. doi:10.1016/S0039-9140(02)00540-4
- E. Moczko, G. Istamboulie, C. Calas-Blanchard, R. Rouillon and T. Noguer, “Biosensor Employing ScreenPrinted PEDOT:PSS for Sensitive Detection of Phenolic Compounds in Water,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 50, No. 11, 2012, pp. 2286- 2292. doi:10.1002/pola.26009
- J.-H. Yang, J.-C. Lee and S.-H. Choi, “Tyrosinase-Immobilized Biosensor Based on the Functionalized Hydroxyl Group-MWNT and Detection of Phenolic Compounds in Red Wines,” Journal of Sensors, Vol. 2009, No. 2009, 2009, pp. 1-9. doi:10.1155/2009/916515
- P. De Taxis du Poet, S. Miyamoto, et al., “Direct Electron Transfer with Glucose Oxidase Immobilized in an Electropolymerized Poly-N-Methylpyrrole Film on a Gold Microelectrode,” Analytica Chimica Acta, Vol. 235, 1990, pp. 255-264. doi:10.1016/S0003-2670(00)82082-6
- Y. Iwakura, M. Asano and Y. Kavade, “Male Sterility of Transgenic Mice Carrying Exogenous Mouse InterferonBeta Gene under the Control of the Metallothionein Enhancer-Promoter,” The EMBO Journal, Vol. 7, No. 12, 1988, pp. 3757-3762.
- Y. Li, W. Zhang, J. Chang, J. Chen and G. Li, “‘Click’ on Conducting Polymer Coated Electrodes: A Versatile Platform for the Modification of Electrode Surface,” Macromolecular Chemistry Physics, Vol. 209, No. 3, 2008, pp. 322-329. doi:10.1002/macp.200700436
- Y. Nakabayashi and H. Yoshikawa, “Amperometric Biosensors for Sensing of Hydrogen Peroxide Based on Electron Transfer between Horseradish Peroxide and Ferrocene as a Mediator,” Analytical Sciences, Vol. 16, No. 6, 2000, pp. 609-613. doi:10.2116/analsci.16.609
- S. Thanachasai, S. Rokutanzono, S. Yoshida and T. Watanabe, “Novel Hydrogen Peroxide Sensors Based on Peroxidase-Carrying Poly{Pyrrole-Co-[4-(3-Pyrrolyl)Butanesulfonate]} Copolymer Films,” Analytical Sciences, Vol. 18, No. 7, 2002, pp. 773-777. doi:10.2116/analsci.18.773
- M. Yasuzava, T. Nieda, T. Hirano and A. Kunugi, “Properties of Glucose Sensors Based on the Immobilization of Glucose Oxidase in N-Substituted Polypyrrole Film,” Sensors and Actuators B: Chemical, Vol. 66, No. 1-3, 2000, pp. 77-79. doi:10.1016/S0925-4005(99)00453-0
- M. Gerard, A. Chaubey and B. D. Malhotra, “Application of Conducting Polymers to Biosensors,” Biosensors and Bioelectronics, Vol. 17, No. 5, 2002, pp. 345-359. doi:10.1016/S0956-5663(01)00312-8
- S. Terrettaz, W.-P. Ulrich, H. Vogel, Q. Hong, L. G. Dover and J. H. Lakey, “ Stable Self-Assembly of a Protein Engineering Scaffold on Gold Surfaces,” Protein Sciences, Vol. 11, No. 8, 2002, pp. 1917-1925. doi:10.1110/ps.0206102
- X. F. Ang, Z. Chen, C. C. Wong and J. Wei, “Effect of Chain Length on Low Temperature Gold-Gold Bonding by Self-Assembled Monolayers,” Applied Physics Letters, Vol. 92, 2008, Article ID: 13913. doi:10.1063/1.2906905
- M. W. Shinwari, M. J. Deen, E. B. Starikov and G. Cuniberti, “Electrical Conductance in Biological Molecules,” Advanced Functional Materials, Vol. 20, No. 12, 2010, pp. 1865-1883. doi:10.1002/adfm.200902066
- A. Riccio, M. Lanzi, C. Antolini, C. De Nitti, C. Tavani and C. Nicolini, “Ordered Monolayer of Cytochrome c via Chemical Derivatization of Its Outer Arginine,” Langmuir, Vol. 12, No. 6, 1996, pp. 1545-1549. doi:10.1021/la950420f
- V. Erokhin, P. Facci and C. Nicolini, “Two-Dimensional Order and Protein Thermal Stability: High Temperature Preservation of Structure and Function,” Biosensors and Bioelectronics, Vol. 10, No. 1-2, 1995, pp. 25-34. doi:10.1016/0956-5663(95)96792-W
- K. S. Birdi, “Self-Assembly Monolayer Structures of Lipids and Macromolecules at Interfaces,” Kluwer Academic Press, Dordrecht, 1999.
- A. P. Girart-Ergot, S. Godoy and L. J. Blum, “Enzyme Association with Lipidic Langmuir-Blodgett Films: Interests and Applications in Nanobioscience,” Advances in Colloid and Interface Science, Vol. 116, No. 1-3, 2005, pp. 205-225. doi:10.1016/j.cis.2005.04.006
- P. H. B. Aoki, P. Alessio, M. L. Rodriguez-Mendez, J. A. De Saja Saez and C. J. L. Constantino, “Taking Advantage of Electrostatic Interactions to Grow,” Langmuir, Vol. 25, No. 22, 2009, pp. 13062-13070. doi:10.1021/la901923v
- J. J. Davis, D. A. Morgan, C. L. Wrathmell, D. N. Axford, J. Zhao and N. Wang, “Molecular Bioelectronics,” Journal of Materials Chemistry, Vol. 15, No. 22, 2005, pp. 2160-2174. doi:10.1039/b417712f
- J. W. Zhao, J. J. Davis, M. S. P. Sansom and A. Hung, “Exploring the Electronic and Mechanical Properties of Protein Using Conducting Atomic Force Microscopy,” Journal of the American Chemical Society, Vol. 126, No. 17, 2004, pp. 5601-5609. doi:10.1021/ja039392a
- J. Zhao and J. J. Davis, “Force Dependent Metalloprotein Conductance by Conducting Atomic Force Microscopy,” Nanotechnology, Vol. 14, No. 9, 2003, pp. 1023-1028. doi:10.1088/0957-4484/14/9/317
NOTES
*Corresponding author.

