Journal of Biomaterials and Nanobiotechnology
Vol.3 No.2A(2012), Article ID:18985,7 pages DOI:10.4236/jbnb.2012.322032
Zero-Order Release Profiles from a Multistimuli Responsive Electro-Conductive Hydrogel
![]()
Instituto de Química, Universidade de São Paulo, São Paulo (SP), Brazil.
Email: *storresi@iq.usp.br
Received November 4th, 2011; revised March 14th, 2012; accepted April 20th, 2012
Keywords: Hydrogels; Conducting Polymers; Controlled Release
ABSTRACT
Electroconductive hydrogels has been extensively studied in drug delivery systems because they combine the properties of hydogels with the conducting polymers in only one material. In this work, pyrrole was polymerized electrochemically into poly(acrylic acid) hydrogel. This material kept the swelling properties that are characteristic of hydrogels and the electroactivity of the conducting polymers. The hydrogel (with and without the conducting polymer) swelling degree depends on both the ionic strength and pH. As this material is responsive to changes in the electrical potential, pH and ionic strength, the safranin release presented different delivery profiles, in accordance to variations and combinations of these stimuli. The most interesting result was the achievement of the linear safranin release indicating a zero-order kinetics.
1. Introduction
Conductive electroactive polymers (CEPs) [1,2] and hydrogels [3-5] are two of the most promising class of materials for biomedical applications The former ones, because its electrically modulated properties, could be engineered for devices like biosensors [6-9], drug delivery systems [10-12] and substrates for neural prostheses [13- 16]. The second ones, due its transport properties, high hydration levels and biocompatibility are extensively found in controlled delivery devices [17], biosensors, contact lenses, catheters, wound dresses and tourniquets [5]. In 1995, Guiseppi-Elie [18] described the synthesis of electroconductive hydrogels (ECHs), materials recognized as composites or blends made by the union of conducting polymers with hydrogels, resulting in a unique polymeric material that combines the special properties of their constituents: high swell ability in water, in vitro and in vivo biocompatibility and the high diffusivity of small molecules from hydrogels and high electrical conductivity, ON-OFF electrical and optical switching, electrochemical triggered redox properties and volume changes. Later, Wallace et al. reported the synthesis and application of a polyacrylamide/polypyrrole composite for controlled release applications [19]. Since the early works of Guiseppi-Eli and Wallace, ECH have been the subject of growing attention and today there are several works that shows the synthesis and application of ECHs in implantable devices (biosensors, controlled drug delivery, tissue engineering) and most of them involve conducting polymers such as polyaniline-PANI, polypyrrole-PPy and poly(3,4-ethylenedioxythiophene)—PEDOT and hydrogels networks (poly(vinyl alcohol)—PVA, polyacrylamide—PAAm, poly(hydroxyl ethyl methacrylate)—PHEMA, poly(acrylic acid)-PAA, poly(lactic-co-glycolic acid) —PLGA, etc) [20,21]. A number of routes have been proposed for synthesizing these ECHs, and they can be summarized into three categories, based on their fabrication route [20]: 1) Templated conducting polymer—hydrogel; 2) Conducting polymer deposited within a hydrogel matrix; 3) Conducting polymer—hydrogels formed from mixed precursors—either simultaneously or in a two-step process. The most common synthetic method is the polymerization (chemical or electrochemical) of the conducting polymer in a preformed matrix (category 2). As an example, our group has demonstrated the synthesis of a polyaniline-polyacrylamide composite by electropolymerization of conducting polymer inside an insulating hydrogel matrix of different pore sizes. This resulting new material was successfully applied in electrochemical controlled release devices [22-24]. A number of examples of synthesis and application of ECHs can be found in a recent review on this topic [20,21]. Ocusert™ system introduced by Alza Corporation in 1975 can be cited considering its technical and historical importance; it was the first topic formulation of controlled release for ocular use. The system, that is commercialized even today, is a reservoir type and releases pilocarpine trough an ethylene vinyl acetate copolymer membrane with a zero-order kinetics that provides a constant release of the drug over the time [25]. This special regime of release is often desired in order to obtain the needed therapeutic action and to avoid side effects. The problem is that most of controlled delivery devices based on ECH display other kind of release kinetics, typically a first order one, which limits its application. Usually, the structural characteristics of ECH, determined by the synthetic conditions, govern the release pattern of a determinate solute; and, environmental parameters such as temperature, pH and ionic strength only modify the release rate but not the mechanism that is inherent to the ECH. Our work presents the synthesis and characterization of an electroconductive hydrogel based on the electropolymerization of pyrrole in a poly(acrylic acid) hydrogel (named as polypyrrolepoly(acrylic acid) composite). This type of ECH posses a multistimuli responsive behavior: the anionic hydrogel matrix ensures a great swelling change driven by changes in pH and ionic strength of the release media while the conducting polymer provides the electrochemical switch capability based on changes in the polypyrrole charge and volume depending on its oxidation state. An unexpected synergetic effect between the hydrogel and the conducting polymer that causes an increase of the degree of swelling of the entire system when the pH is raised up to 13, is observed. More remarkable, the existence of a combination of pH and electrical potential applied to the polypyrrole-poly(acrylic acid) composite allows the release of safranin in a linear (constant) fashion. For the best of our knowledge, this work describes for the first time, a multistimuli responsive ECH that can display different release patterns (based on Fick’s Law), i.e., zeroorder or other mechanisms only as a function of external parameters.
2. Experimental
2.1. Poly(Acrylic Acid) Hydrogel Synthesis
Hydrogel was obtained by free radical polymerization of acrylic acid. The monomer and the crosslinking agent N, N’-methylene bis-acrylamide were dissolved and the solution was degassed for 10 minutes at room temperature. Then, potassium peroxydissulfate and N,N,N’,N’-tetramethylethylenediamine were added and they acted as oxidizer and catalyst, respectively. The moisture was placed in a cylindric plastic tube to obtain a cylindric hydrogel. After being removed from the tube, the hydrogels were immersed in water that was replaced periodically to remove unreacted substances.
2.2. Pyrrole Polymerization on Poly(Acrylic Acid) Hydrogel
The conducting polymer was electrochemically polymerized into poly(acrylic acid) hydrogel matrices. A constant potential of +0.6 V was applied until de charge achieves a value of 10.5 C. A platinum wire was inserted on hydrogel and was used as the working electrode. The reference and counter electrodes used were, respectively, Ag/AgCl (KCl(sat)) and a platinum sheet. The polymerization solution was pyrrole (0.4 mol·L–1) dissolved in sodium nitrate solution 1.0 mol·L–1. The composite obtained (polypyrrole-poly(acrylic acid) composite) is nemed as PPy-AA in the whole manuscript. Before the monomer polymerization, the swollen AA was sliced in small pieces and immersed in 1.0 mol·L–1 sodium nitrate solution for 24 hours. Pyrrole monomer was distilled using fractional distillation method prior to use. All the experiments were carried out with a potentiostat/galvanostat Autolab PSTAT 30.
2.3. Swelling Degree (Q)
Pieces of known mass of dried AA and PPy-AA hydrogels were placed in water or in sodium nitrate solutions of different concentration at room temperature. Periodically, hydrogels were carefully wiped with a filter paper and weighted. The swelling degree was calculated applying the Equation (1):
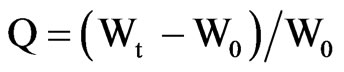 (1)
(1)
where Q is the swelling degree and W0 and Wt are hydrogel mass at the dried state and at different times t.
2.4. Cyclic Voltametry
The electroactivity of PPy-AA and AA were determined by cyclic voltammetry. In this case, the working electrodes were the hydrogel with and without polypyrrole and the counter and reference electrode were the same used on conducting polymer polymerization. Prior this characterization, the AA and PPy-AA were put in sodium nitrate solution 1.0 mol·L–1 for 24 hours, and the cyclic voltammetry experiemts were performed in this solution.
2.5. Safranin Loading and Delivery
AA and PPy-AA hydrogels were immersed in a safranin solution (0.01 mol·L–1) for 5 hours and after this time, they were used as working in the potentiostatic controlled release experiments. The counter and reference were the same used at pyrrole polymerization in poly(acrylic acid) hydrogel. The hydrogels/composites were washed with distillated water in order to remove the excess of safranin and then placed on sodium nitrate solution on pH 6.4 or 3.8 and the potential was applied. The solution was sampled at regular times and the amount of released safranin was determined by UVVIS spectrophotometry by using a Hewlett Packard 8453 diode array spectrophotometer at a fixed wavelength of 519 nm.
3. Results and Discussion
The chronoamperogram for the pyrrole polymerization is ilustred it Figure 1. It can be observed that the current increases with time indicating that an electroactive material is being formed. This is because the pyrrole polymerization stars at the platinum wire and after be covered totally, the polymerization branches on the hydrogel structure until that the conducting polymer fills all the material. This process can be observed on photos inserted in the graphic that was obtained during the polymerization. The hydrogel’s ability to absorb water and biological fluids is related to the flexibility and the hydrophilic nature of the polymeric network. When the hydrogel is immersed in aqueous medium, water and others molecules diffuse through the pores due to their interaction with the functional groups of the polymer.
The elasticity of the hydrogel is related to the degree of crosslinking and, in this specific case, to the presence of the conducting polymer. Figure 2 shows the swelling degree measurements performed for AA and PPy-AA in
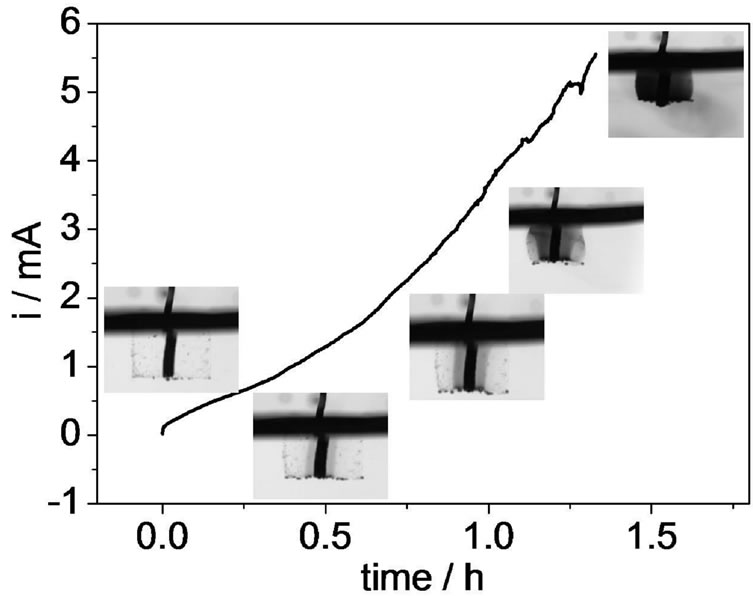
Figure 1. i/t profile recorded during pyrrole polymerization inside the polyacrylic acid matrix. Electrolytic solution: 0.4 mol·L–1 pyrrole +0.1 mol·L–1 NaNO3. Eapp: +0.6 V. Inserts: photos obtained during the conducting polymer polymerization.
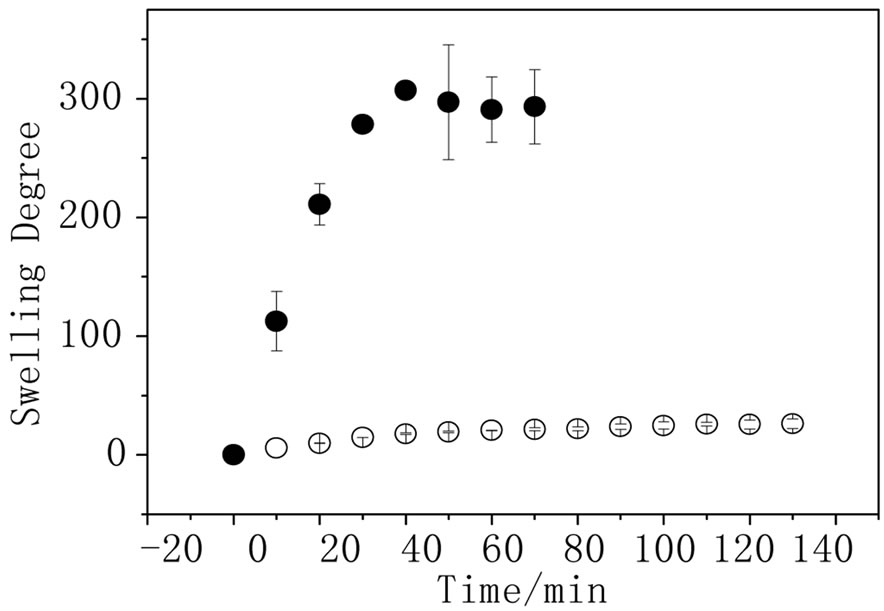
Figure 2. Swelling degree as function of time for ● AA and ○ PPy-AA in water (n = 3).
water. It can be observed that the presence of the polypyrrole decreases the water absorption from 300 to 25; this result is expected due to the fact that the conducting polymer was polymerized inside the hydrogel pores and the entanglement of the polypyrrole chains in AA would reduce the material elasticity and the swelling degree. Figure 3(a) shows the hydrogel swelling degree dependence on time. All the measurements were done at the same pH (6.4) but in sodium nitrate solutions of different concentrations. The hydrogel swelling decreases with the increase of sodium nitrate concentration, as expected in agreement with the thermodynamics basis of the equilibrium swelling theory that predicts that osmotic swelling pressure decreases as the ionic strength in the solution outside the hydrogel increases. As the osmotic pressure decreases, the Gibbs’s free energy of swelling also decreases and, consequently, the degree of swelling goes in the same direction [3]. The same outline was observed for the hydrogel filled with polypyrrole (Figure 3 (b)). For this experiment, the concentration of the sodium nitrate solution was less than those used for the hydrogel without conducting polymer because for concentrations above 0.25 mol·L–1 , the swelling degree was unaltered; so that, no differences of the swelling degree could be analyzed. For the achievement of a dual response system, the PPy-AA must keep the capability of changing the swelling degree due to changes in the pH. In order to verify this behavior, swelling experiments were carried out in pHs 3.8, 6.4 and 13.0 but keeping the concentration of sodium nitrate solution constant at 0.25 mol·L–1.
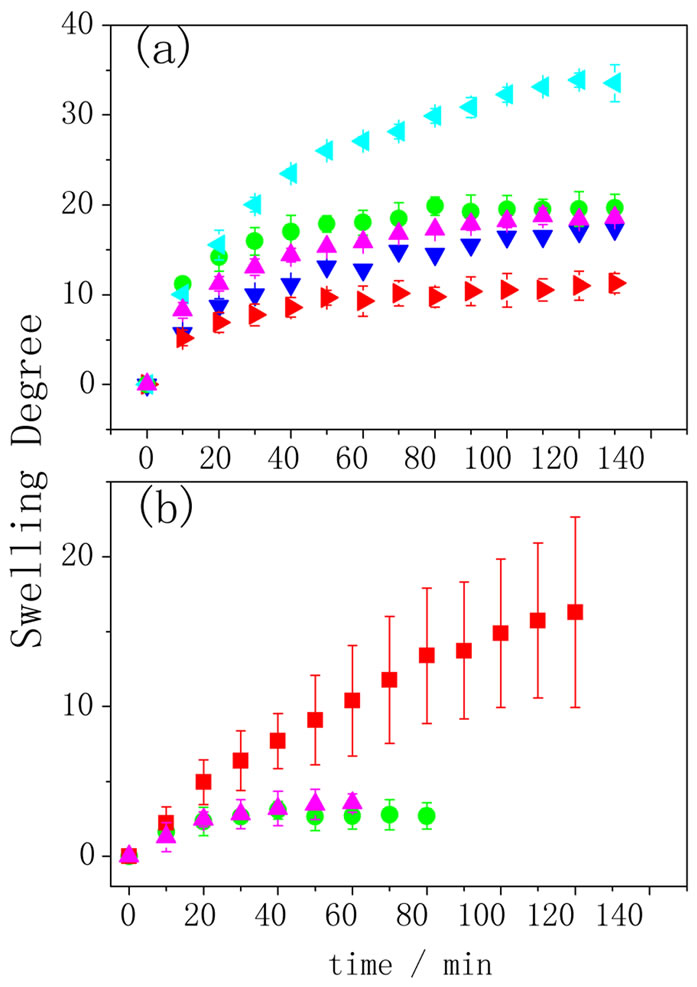
Figure 3. Swelling degree as function of time for (a) AA in NaNO3 ◄ 0.25, ● 0.5, ▲ 1.0, ▼2.0, ►4.0 mol·L–1; (b) PPyAA in NaNO3 ■ 0.125, ▲ 0.25, ● 0.5 mol·L–1 (n = 3).
Figure 4 depicts the swelling behavior of both the hydrogel and the composite in different pHs. As it can be observed, when AA is immersed in pH higher than 4.2, the pKa of poly(acrylic acid), carboxylate groups are formed and to neutralize the charge, water and protons penetrate the hydrogel. So, the swelling degree was higher for pHs 13 and 6.4 than for 3.8. The swelling behavior of PPy-AA (Figure 4(b)) was also higher for pH 13 than for 3.8 and 6.4, these two last ones being almost equal and lower than the values obtained for the AA hydrogel. Here, a striking feature must be pointed out related to the fact the swelling of the PPy-AA composite at pH 13 is practically twice compared to that observed for the AA hydrogel in the same pH. The pKa of PPy was measured to be 8.6 [26], above this value an electrostatic repulsion between the polyacrylate and polypyrrole chains is expected due to the unpaired electrons on the nitrogens, since the polypyrrole is deprotonated. That is to say, that the interaction between the hydrogel and the conducting polypyrrole chains will be weaker. Moreover, conformational changes of the conducting polymer are expected leading to more linear chains due the presence of the polypyrrole’s unbounded amine electrons and these two factors together, result in a more open pore structure and , consequently, to a higher swelling degree as depicted in the scheme shown in Figure 5. Only at pH below 8.6, an effective interaction among the two components of the polymeric system is present, and because of this interaction, the conducting polymer chains are completely spread into the hydrogel matrix, leading to a state of mini-
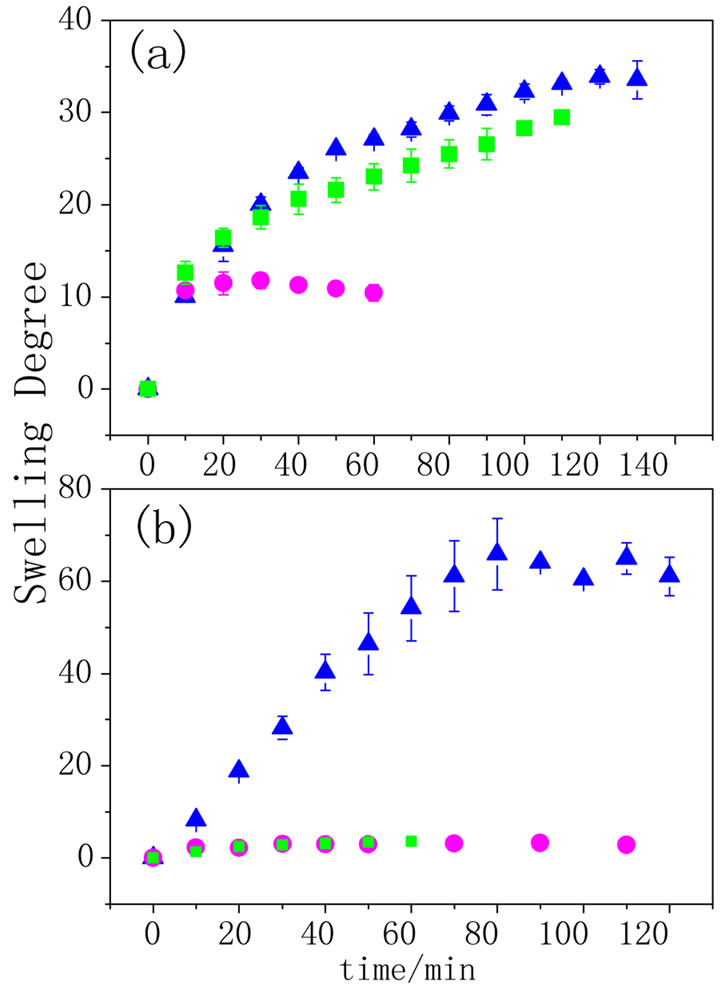
Figure 4. Swelling degree as function of time for (a) AA and (b) PPy-AA in 0.25 mol·L–1 solution at different pHs: ● 3.8, ■ 6.4 e ▲ 13 (n = 3).
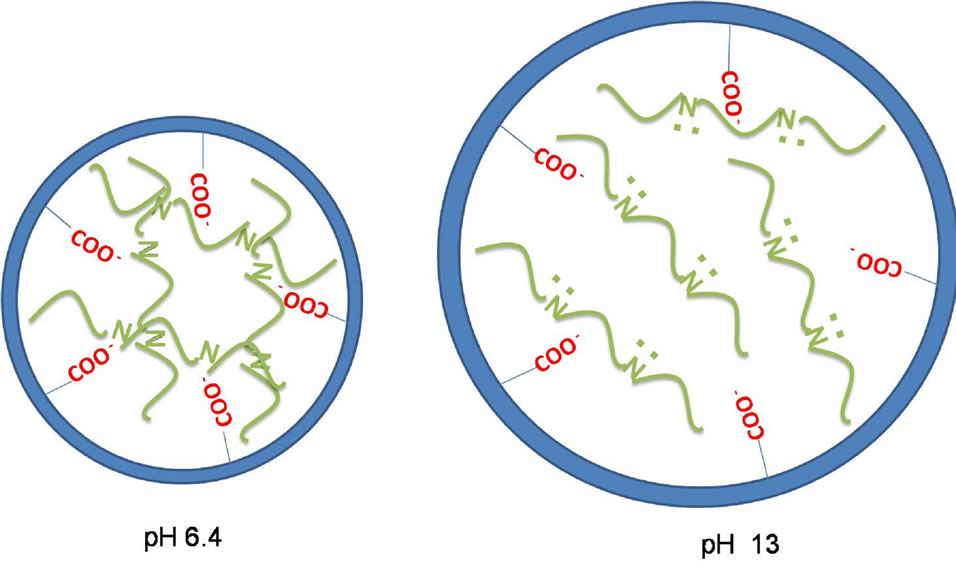
Figure 5. Scheme of PPy-AA structure at pH 6.4 (left) and pH 13 (right). The blue circle is the representation of one AA pore and their terminal carboxilate groups (in red). In green, polypyrrole chains are depictede with (pH 13) and without (pH 6.4) the polypyrrole’s unpaired nitrogen electrons.
mum swelling. It must be noted that in most cases, the error bars were less than the height of the symbol used to represent each curve indicating that this behavior is repetitive in at less all the experiments performed (n = 3). Figure 6 shows the i/E potentiodynamic profiles of the hydrogels with and without polypyrrole. It can be observed the characteristic voltammetric profile of polypyrrole which is maintained in the PPy-AA material [27], corroborating that the conducting polymer was formed inside the AA hydrogel. The oxidation and reduction peaks at 0.2 and 0.4 V, respectively, are associated with the oxidation/reduction processes that are accompanied with the expulsion/injection of anions, cations and water from the PPy-AA. This experiment is of relevant importance because is a clear indication of the electroactivity of the composite and the possibility of tuning the swelling behavior by the application of an electric field. In order to investigate the release kinetics and the possibility of electrochemical control, safranine was chosen as model molecule due to its size, structure and with the sake of comparison with former works performed in our group involving other composites such as polyacrylamide/PPy and Polycrylamide/PANI [22-24] Release profiles can be analyzed by using a molecular model on the light of the Fick’s law. Hitger and Peppas [28] proposed a model that describes the solute delivery mechanism from polymeric materials that is represented by the Equation (2):
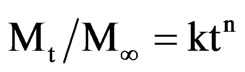 (2)
(2)
where: Mt is the amount of solute delivered at time t and M∞ is the amount at the equilibrium, so, Mt/M∞ is the molecular fraction released. The constant k is related to the geometry and the material structure, t is the time and n is the diffusional exponent that is characteristic of the release mechanism. For the system studied here (delivery from cylinders), the relation is available only for the first 20% of the molecule released and k = 4 (D/πa)21/2. Where D is the diffusion coefficient of the drug delivered and a is the cylinder radius.
The n value, for this system, implies in different meanings. If the value is 0.5, it indicates that the drug delivery process is diffusion controlled. If n = 1, it means that the process is independent on the time and it is controlled by the relaxation of the macromolecular network. When n value is between 0.5 and 1, the release process is anomalous, co-existing both the diffusion control and swelling by relaxation.
Figure 7 shows the safranine release profiles obtained for PPy-AA hydrogels at different potentials and pHs. Two potential values of +0.4 and –0.4 V were used. These values were chosen because the negative value is close to
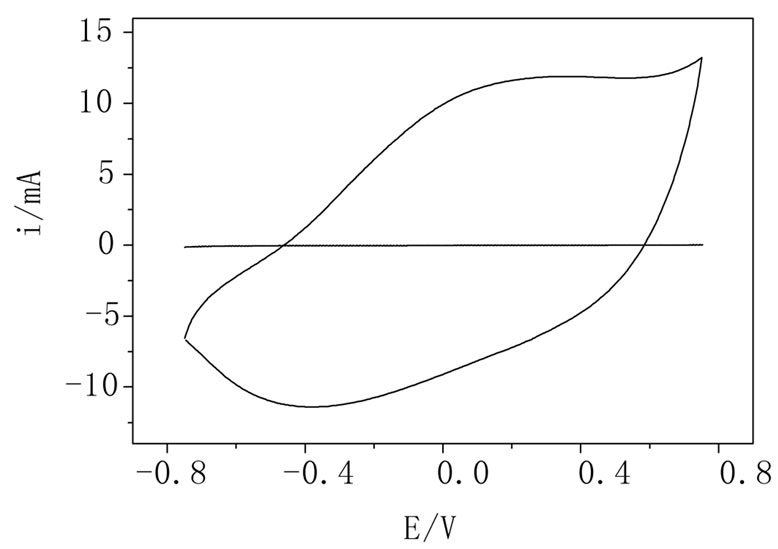
Figura 6. i/E potentyodynamic profiles of AA (dotted line) and PPy-AA (full line) hydrogels in NaNO3 1.0 mol·L–1 solution. Scan rate: 0.05 V·s–1.
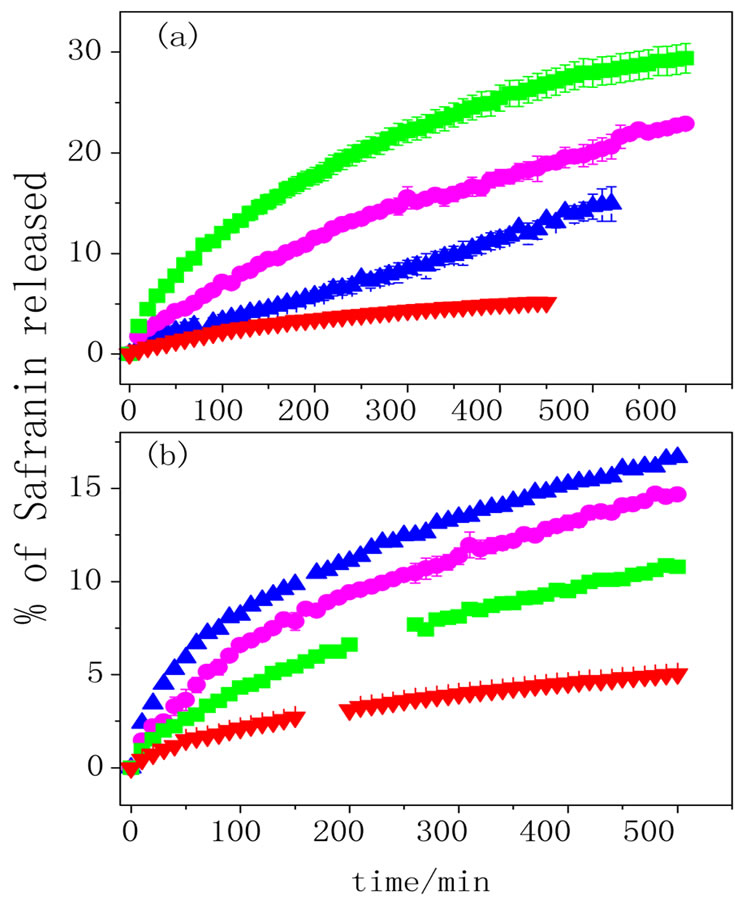
Figure 7. Safranine release profiles recorded in 1.0 mol·L–1 NaNO3 solution at (a) pH 6.4 and (b) pH 3.8 for PPy-AA hydrogel at ■ +0.4 V, ▲ –0.4 V and ● OPC. AA hydrogel is also shown for comparison ▼ (n = 3).
polypyrrole reduction potential and the positive one corresponds to an oxidized state. The release profiles of PPy-AA obtained at open circuit conditions and for AA hydrogel are also shown for comparison.
From these data and applying Equation (2), the diffusion coefficients of safranine for the different release conditions were obtained and they are shown in Figure 8.
Considering the AA hydrogel, D increases when the pH decreases. This can be explained considering that at pH 6.4 the carboxylic groups from acrylic acid are negatively charged and as safranin is a cationic molecule, an electrostatic attraction occurs between then, decreasing the diffusion when compared with pH 3.8. In this pH, the acrylic acid is neutral (pH < pKa), so that, safranine can diffuse without any constriction. The same behavior is observed for the PPy-AA at open circuit potential but the D values obtained are lower than those observed for AA hydrogel, due to the presence of the conducting polymer that will provoke a loss of elasticity of the hydrogel network and a diminution of the free path for safranine release.
When an electric field is applied, the release process is substantially changed upon the potential. Figure 7(a) shows that at pH 6.4 both, the amount of safranine released and the release rate change in the sense +0.4V > OCP > –0.4 V. As the diffusion coefficient is related to the initial release rate, it increases when the potential varies from –0.4 to +0.4 V (Figure 8). When the pH is 3.8 (Figure 7(b)), the release behavior is striking different and exactly opposite from that observed at pH 6.4 (Figure 7(a)). In this case, the amount and the rate of released dye change in the sense –0.4 V > OCP > +0.4 V. So does the diffusion coefficient as it is shown in Figure 8.
The whole kinetic behaviour of the release process upon both stimuli, potential and pH, will mostly depend

Figure 8. Safranine diffusion coefficient (D) calculated from data shown in Figure 7. Black Bars (pH 6.4) and grey Bars (pH 3.8). D value obtained for PPy-AA hydrogel at –0.4 V and pH 6.4 was very low (8.7 10 - 10 cm·min–1) at it is not possible to displayed in the present scale.
on the intermolecular and/or electrostatic interactions between the hydrogel and the conducting polymer chains. In a general words, strong interactions suppose that the chains are very close leading to higher space inside the pores favoring safranine and water diffusion; consequently, increasing both D and the amount of safranine released. When interactions are weak, polypyrrole chains are not so close of hydrogel ones and they are expected to be spread inside the pores. This would lead to a constriction for safranine diffusion provoked by the diminution of the mean free path of the dye. More specifically, at pH 6.4, the hydrogel is in its anionic form and when a negative potential is applied, the polypyrrole is neutral. At this situation, the interaction between the two polymeric species (AA and PPy) is the weakest possible among all studied here; therefore, the conducting polymer chains tend to spread inside the hydrogel pores and this, in turn, will lead to a specific pore structure that will hinder safranine diffusion. On the other hand, when the PPy is oxidized (+0.4 V), intermolecular interactions between the two polymers are stronger and this fact leads to a closer contact of the polymer chains and opened pores that consequently, will increase the free safranin release.
When the pH is changed to 3.8, the situation is opposite; as AA is neutral (protonated) stronger interactions with PPy chains will take place when it is reduced and, as discussed above, this situation leads to easier safranin diffusion due the larger space inside the pores. When PPy chains are positively charged (oxidized at 0.4 V), electrostatic repulsion comes to between PPy and AA chains resulting in the spread of PPy inside the pores avoiding the dye release.
The values of the diffusional exponent (n) calculated for each curve was ca. 0.5 meaning that safranine release was diffusionally controlled, except for the delivery at pH 6.4 and –0.4 V. In this case the n value was 1, indicating that the diffusion was time independent, and the curve presents a zero order profile. This behaviour is also explained by the distribution of polypyrrole inside the hydrogel pores that is a consequence of the polymerpolymer interactions, as described above. This particular combination of stimuli (pH 6.4 and –0.4 V) provokes a situation where the rate limiting step is not the safranine diffusion but the “molecular movement” through the free space generated by PPy chains distribution. This kinetic phenomenon would be equivalent to a heterogeneous catalytic process (zero order) limited by the catalyst surface. Envisaging a practical application, this kind of release profile shows therapeutic advantages because it will allow a much better control of the released amount because it varies linearly with time.
4. Conclusion
In this work, polypyrrole was electropolymerized inside a poly(acrylic acid) hydrogel. The polymerization began at the platinum wire and it spread within the whole hydrogel. Both, swelling degree and conducting polymer electroactivity were maintained at the material obtained (PPy-AA). The swelling degree depends not only on the pH but also on the ionic strength, due to the anionic nature of the hydrogel. The molecular delivery was monitored at different potentials and the safranin diffusion behavior depends on both pH and potential. The proper combination of the different stimuli allows reaching special release regime of zero order with relevant potential applications in therapeutic fields.
5. Acknowledgements
The authors thank FAPESP (09/53199-3) and Instituto Nacional de Bionanalitica for financial support. S.H.T thanks CNPq 141950/2009-9 for the scholarship granted.
REFERENCES
- M. Asplund, T. Nyberg and O. Inganas, “Electroactive Polymers for Neutral Interfaces,” Polymer Chemistry, Vol. 1, No. 9, 2010, pp. 1374-1391. doi:10.1039/c0py00077a
- T. Skotheim and J. R. Reynolds, “Handbook of Conducting Polymers: Conjugated Polymers Processing and Applications 3rd Edition,” Taylor and Francis, New York, 2007.
- S. H. Gehrke and P. I. Lee, “Hydrogels for Drug Delivery Systems,” In: P. Tyle, Ed., Specialized Drug Delivery Systems, Marcel Dekker, New York, 1990, pp. 333-392.
- P. Gupta, K. Vermani and S. Garg, “Hydrogels: From Controlled Release to pH Responsive Drug Delivery,” Drug Discovery Today, Vol. 7, No. 10, 2002, pp. 569-579. doi:10.1016/S1359-6446(02)02255-9
- C. C. Lin and A. T. Metters, “Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling,” Advanced Drug Delivery Reviews, Vol. 58, No. 12-13, 2006, pp. 1379-1408. doi:10.1016/j.addr.2006.09.004
- S. A. Jaffari and A. P. F. Turner, “Recent Advances in Amperometric Glucose Biosensors for in Vivo Monitoring,” Physiological Measurement, Vol. 16, No. 1, 1995, pp. 1-15. doi:10.1088/0967-3334/16/1/001
- N. Wisniewski, F. Moussy and W. M. Reichert, “Characterization of Implantable Biosensors Membrane Biofuling,” Fresenius’ Journal of Analytical Chemistry, Vol. 366, 2000, pp. 611-621.
- O. M. Schuvailo, O. O. Soldatkin, A. Lefebvre, R. Cespuglio and A. P. Soldatkin, “Highly Selective Microbiosensors for in Vivo Measurement of Glucose, Lactate and Glutamate,” Analytical Chimica Acta, Vol. 573-574, 2006, pp. 110-116. doi:10.1016/j.aca.2006.03.034
- A. Ramanavicius, K. Habermuller, E. Csoregi, V. Laurinavicius and W. Schumann, “Polypyrrole Entrapped Quinohemoprotein Alcohol Dehydrogenase. Evidence for Direct Electron Transfer via Conducting Polymer Chains,” Analytical Chemistry, Vol. 71, No. 16, 1999, pp. 3581- 3586. doi:10.1021/ac981201c
- B. Zinger and L. L. Miller, “Timed Release of Chemicals from Polypyrrole Films,” Journal of the American Chemical Society, Vol. 106, No. 22, 1984, pp. 6861-6863. doi:10.1021/ja00334a076
- A. N. K. Lau and L. L. Miller, “Electrochemical Behavior of a Dopamine Polymer. Dopamine Release as a Primitive Analog of a Synapse,” Journal of the American Chemical Society, Vol. 105, No. 16, 1983, pp. 5271-5277. doi:10.1021/ja00354a016
- J. M. Pernaut and J. R. Reynolds, “Use of Conducting Electroactive Polymers for Drug Delivery and Sensing of Bioactive Molecules. A Redox Chemistry Approach,” The Journal of Physical Chemistry B, Vol. 104, No. 17, 2000, pp. 4080-4090. doi:10.1021/jp994274o
- X. Cui, V. A. Lee, Y. Raphael, J. A. Wiler, J. F. Hetke, D. J. Anderson and D. C. Martin, “Surface Modification of Neutral Recording Electrodes with Conducting Polymer/ Biomolecule Blends,” Journal of Biomedical Materials Research, Vol. 56, No. 2, 2001, pp. 261-272. doi:10.1002/1097-4636(200108)56:2<261::AID-JBM1094>3.0.CO;2-I
- P. M. George, A. W. Lyckman, D. A. LaVan, A. Hegde, Y. Leung, R. Avasare, C. Testa, P. M. Alexander, R. Langer and M. Sur, “Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics,” Biomaterials, Vol. 26, No. 17, 2005, pp. 3511-319.
- X. Cui, J. Wiler, M. Dzaman, R. A. Altschuler and D. C. Martin, “In Vivo Studies of Polypyrrole/Peptide Coated Neural Probes,” Biomaterials, Vol. 24, No. 5, 2003, pp. 777-787. doi:10.1016/S0142-9612(02)00415-5
- J. Y. Wong, R. Langer and D. E. Ingber, “Electrically Conducting Polymers Can Noninvasively Control the Shape and Growth of Mammalian Cells,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 91, No. 8, 1994, pp. 3201-3204. doi:10.1073/pnas.91.8.3201
- S. Murdan, “Electro-Responsive Drug Delivery from Hydrogels,” Journal of Controlled Release, Vol. 91, 1994, pp. 3201-3204.
- A. Guiseppi-Elie and N. F. Sheppard Jr., “Conferrig Biospecificity to Electroconductive Polymer-Based Biosensors Devices,” ACS Northeast Regional Meeting (NERM), Rochester, October 1995, pp. 22-25.
- C. J. Small, C. O. Too and G. G. Wallace, “Responsive Conducting Polymer-Hydrogel Composites,” Polymer Gels and Network, Vol. 5, No. 3, 1997, pp. 251-265. doi:10.1016/S0966-7822(96)00044-5
- R. A. Green and S. Baek, L. A. Poole-Warren and P. J. Martens, “Conducting Polymer-Hydrogels for Medical Electrode Applications,” Science and Technology of Advanced Materials, Vol. 11, No. 1, 2010, pp. 1-13. doi:10.1088/1468-6996/11/1/014107
- A. Guiseppi-Elie, “Electroconductive Hydrogels: Synthesis, Characterization and Biomedical Applications,” Biomaterials, Vol. 31, No. 10, 2010, pp. 2701-2716. doi:10.1016/j.biomaterials.2009.12.052
- R. C. Barthus, L. M. Lira and S. I. Córdoba de Torresi, “Conducting Polymer-Hydrogel Blends for Electrochemically Controlled Drug Release Devices,” Journal of the Brazilian Chemical Society, Vol. 19, No. 4, 2008, pp. 630-636. doi:10.1590/S0103-50532008000400004
- L. M. Lira and S. I. Córdoba de Torresi, “Polymeric Electro-Mechanic Devices Applied to Antibiotic-Controlled Release,” Sensors and Actuators B, Vol. 130, No. 2, 2008, pp. 638-644. doi:10.1016/j.snb.2007.10.020
- L. M. Lira and S. I. Córdoba de Torresi, “Conducting Polymer-Hydrogel Composites for Electrochemical Release Devices: Synthesis and Characterization of SemiInterpenetrating Polyaniline-Polyacrylamide Networks,” Electrochemistry Communications, Vol. 7, No. 7, 2005, pp. 717-723. doi:10.1016/j.elecom.2005.04.027
- P. J. Sinko, “Martin’s Physical Pharmacy and Pharmaceutical Sciences,” Lippincott Williams & Wilkins, Baltmore, 2008.
- A. Safavi and H. Abdollahi, “Optical Sensor for High pH Values,” Analytica Chimica Acta, Vol. 367, No. 1-3, 1998, pp. 167-173. doi:10.1016/S0003-2670(98)00079-8
- G. Maia, R. M. Torresi, E. A. Ticianelli and F. C. Nart, “Charge Compensation Dynamics in the Redox Processes of Polypyrrole-Modified Electrodes,” Journal of Physical Chemistry, Vol. 100, No. 39, 1996, pp. 15910-15916. doi:10.1021/jp9607780
- P. L. Ritger and N. A. Peppas, “A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs,” Journal of Controlled Release, Vol. 5, No. 1, 1987, pp. 23-36. doi:10.1016/0168-3659(87)90034-4
NOTES
*Corresponding author.

