Journal of Biomaterials and Nanobiotechnology
Vol.3 No.1(2012), Article ID:16695,6 pages DOI:10.4236/jbnb.2012.31003
Soybean (Glycine max) Leaf Extract Based Green Synthesis of Palladium Nanoparticles
![]()
1Department of Physics, Pondicherry University, Pondicherry, India; 2School of Engineering, University of Guelph, Guelph, Canada; 3Bioproducts Discovery and Development Centre, Department of Plant Agriculture, University of Guelph, Guelph, Canada.
Email: #mmisra@uoguelph.ca
Received October 20th, 2011; revised November 29th, 2011; accepted December 10th, 2011
Keywords: Palladium nanoparticles; Leaf extract; Bioreduction; Catalysis
ABSTRACT
Palladium (Pd) nanoparticles were synthesized using protein rich soybean leaf extract based biological process. Reduction of palladium ions by soybean leaf extract was examined by UV-visible spectroscopic technique. It was believed that the proteins and some of the amino acids that are exist in soybean leaf extracts were actively involved in the reducetion of palladium ions. Further it was confirmed by Fourier transformations infrared spectroscopic (FTIR) analysis. These amino acids are not only involving in the reduction of palladium ions but also acting as surfactants that inhibits the rapid agglomeration. The phase purity of the synthesized palladium nanoparticles was investigated through X-Ray Diffraction (XRD) analysis and the obtained pattern was compared with JCPDS data. Transmission electron microscopic (TEM) images of the palladium particles were recorded and the particle size was found to be ~15 nm.
1. Introduction
Nobel metal nanoparticles (like silver, platinum, gold and palladium) exhibits unique physical, chemical, optical and thermo dynamical properties at nano regime [1,2], which lead them in to many applications such as catalysis [2], sensors [3], magnetic recordings [4], biotechnology [5], as well as drug delivery [6]. Especially palladium nanoparticles are having an extensive application in heterogeneous and homogeneous catalysis due to their high surface to volume ratio [2,7,8]. Surface plasma resonance (SPR) is another important feature in palladium nanoparticles which is useful in sensing, chemo-optical transducers, plasmonic wave guiding [9-11]. In general, a wide range of wet chemical processes including, chemical reduction [12], sonochemical [13], electrochemical [14] as well as polyol [15] has been investigated for the structure controlled synthesis of metal nanoparticles. The challenging tasks in palladium nanoparticle synthesis are size control and inhibit the agglomeration during synthesis as well as storage [12-15].
The synthesis of metal nanoparticles using bio inspired, eco friendly greener methods is one of the most attractive aspects of current nanoscience and nanotechnology [16- 18]. Extensive research effort has been made in utilizing various biological systems such as bacteria, fungus and plant extracts for the synthesis of metal nanoparticles [19-22]. Among them plant extract mediated biological process is found to be simple and versatile process for the synthesis of different types of metal nanoparticles such as silver, gold and palladium, which has emerged as an alternate to conventional physical and chemical methods [22-30]. There are few reports available for the synthesis of palladium nanoparticles that effectively utilise Diopyros kaki. leaf [30], Cinnamom zeylanicum bark [31], C. Camphora leaf [32], Curcuma longa tuber [33] and banana peal [34] extracts, which act as reducing as well as stabilizing agents. However, synthesis of palladium nanoparticles using plant extract mediated biological process has not established as much as for silver and gold nanoparticles.
Recently, our group has successfully demonstrated the rapid synthesis of silver nanoparticles in aqueous solutions using soybean leaf extract [35]. This is motivated us to explore the possible bioreduction of palladium ions into nanoparticles. Hence, the present work deals with biological (green) synthesis of palladium nanoparticles by Glycine max leaf extract at ambient conditions. The bioreduction process was monitored by the UV-visible spectroscopy and the crystalline structure was investigated using X-ray Diffraction (XRD) technique. Microstructure of the synthesized palladium nanoparticles were characterized by transmission microscopy (TEM), and Fourier transformation infrared spectroscopy (FTIR) was used to understand the amino acid-palladium (II) ions interactions.
2. Materials and Methods
Palladium (II) chloride (PdCl2) as the source of palladium ions was received from Sigma Aldrich and used without further purification. The Glycine max (soybean) leaves of DKB 0099 variety were used to make extract. The soy leaf extract was prepared by the following procedure: 20 gm of soybean leaves were thoroughly washed with deionized water and cut in to small pieces. Further they were boiled with in 100 ml of deionized water in conical flask for 3 min. Obtained soybean leaf extract was filtered and stored in refrigerator. Figures 1(a) and (b) show the photograph of soybean leaves and their extract. In order to synthesize palladium nanoparticles, 10 ml of filtered soybean leaf extract was added into 200 ml of (0.1 × 10–3 M) palladium ion aqueous solution. The bioreduction of palladium ions and their nucleation into nanoparticles was monitored by the UV-visible spectral analysis.
The optical absorption of biosynthesized palladium nanoparticles was investigated by the Varian UV-Vis Spectrophotometer (300 UV-Vis) with using wavelength between 300 to 800 nm at a resolution of 2 nm. Powder XRD pattern of palladium nanoparticles was recorded by using Regaku multiflux X-ray powder diffractometer with Cu Kα (1.5418 Å) radiation. The crystallite size of the palladium nanoparticles was calculated using line broadening information and Scherrer’s formula. Purity of the palladium nanoparticles and reactive substance in leaf extract was investigated using Thermo Scientific’s FTIR instrument in the transmittance mode between 400 and 4000 cm–1. Microstructure of the synthesized palladium nanoparticles was characterized by the high resolution transmission electron microscopy (HRTEM). The palladium nanoparticles were placed on carbon coated copper grid and allowed the solvent to evaporate. Further, the grid was loaded in to the JEOL 2010F HRTEM instrument and the image was recorded with 200 KV operating voltage.
3. Results and Discussion
3.1. UV-Vis Spectra Analysis
Addition of soybean leaf extract in to palladium ion solution exhibits the gradual change in colour from transparent orange to dark brown over the time as shown in figure 1(c). The observed appearance of brown colour, which is due to the excitations of surface plasmon vibrations, indicates the formation of palladium nanoparticles and it was proved by UV-Vis analysis. Figure 2 shows the UV-Vis spectra of reaction mixture (PdCl2 solution and soybean leaf extract) with respect to time. The observed peaks at 420 nm at one min indicate the presence of the Pd2+ ions in reaction mixture. Over the time the peaks represent the Pd2+ ions begin to disappear, which indicates the formation of palladium nanoparticles and the completely vanished after 2 days. Obtained palladium nanoparticles were collected by centrifugation for further analysis.
3.2. X-Ray Diffraction (XRD)
The XRD pattern of palladium nanoparticles is shown in the figure 3. The observed intense peaks 40, 46, 68, 82 and 87 deg. are respectively representing the (111), (200), (220), (311) and (222) Bragg reflection. Further the XRD pattern was compared with JCPDS standard (#05-0681) and confirmed the formation of palladium nanoparticles
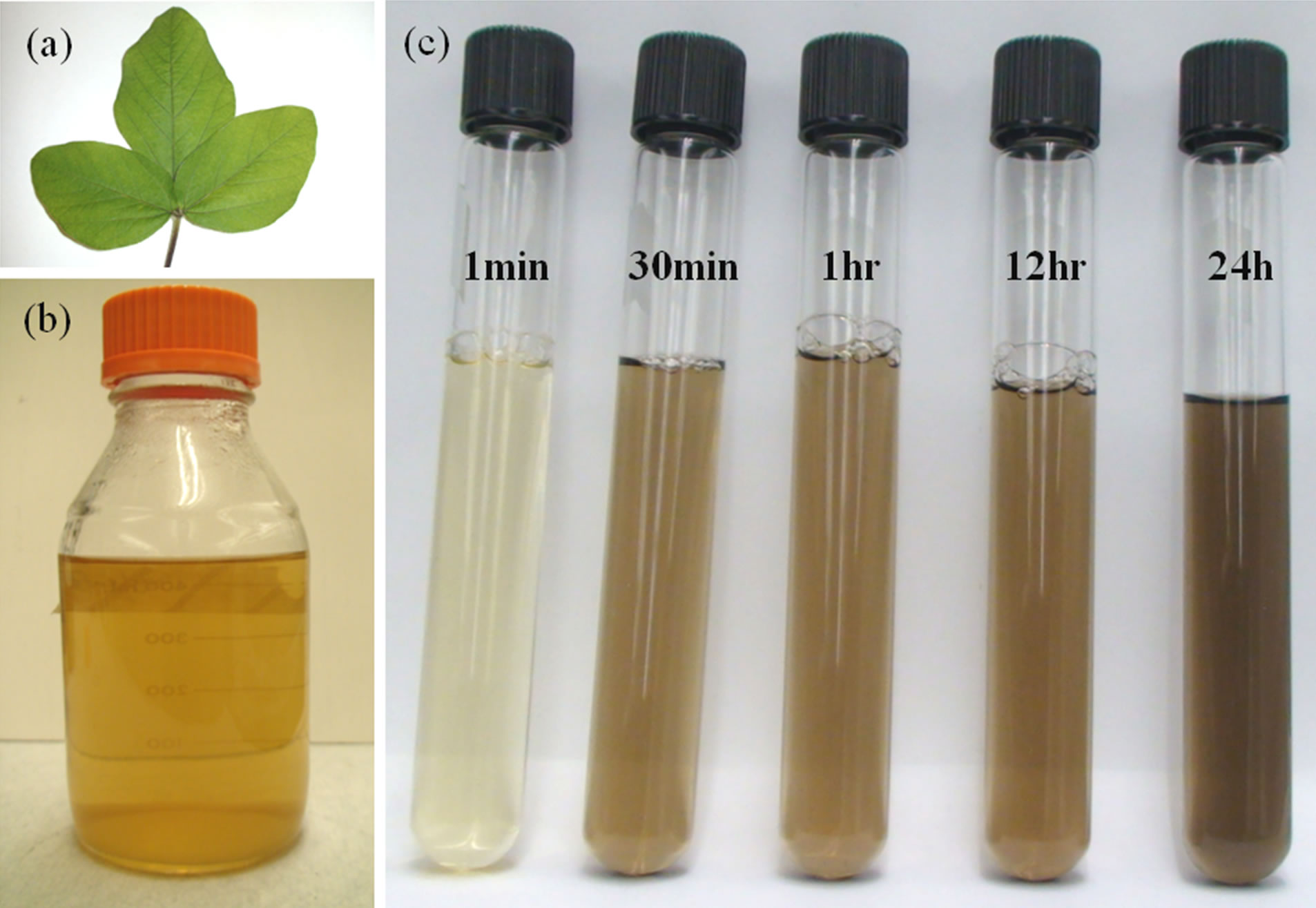
Figure 1. Photograph of (a) Soy leaf; (b) Soy leaf extract; and (c) Reaction mixture at different time intervals.
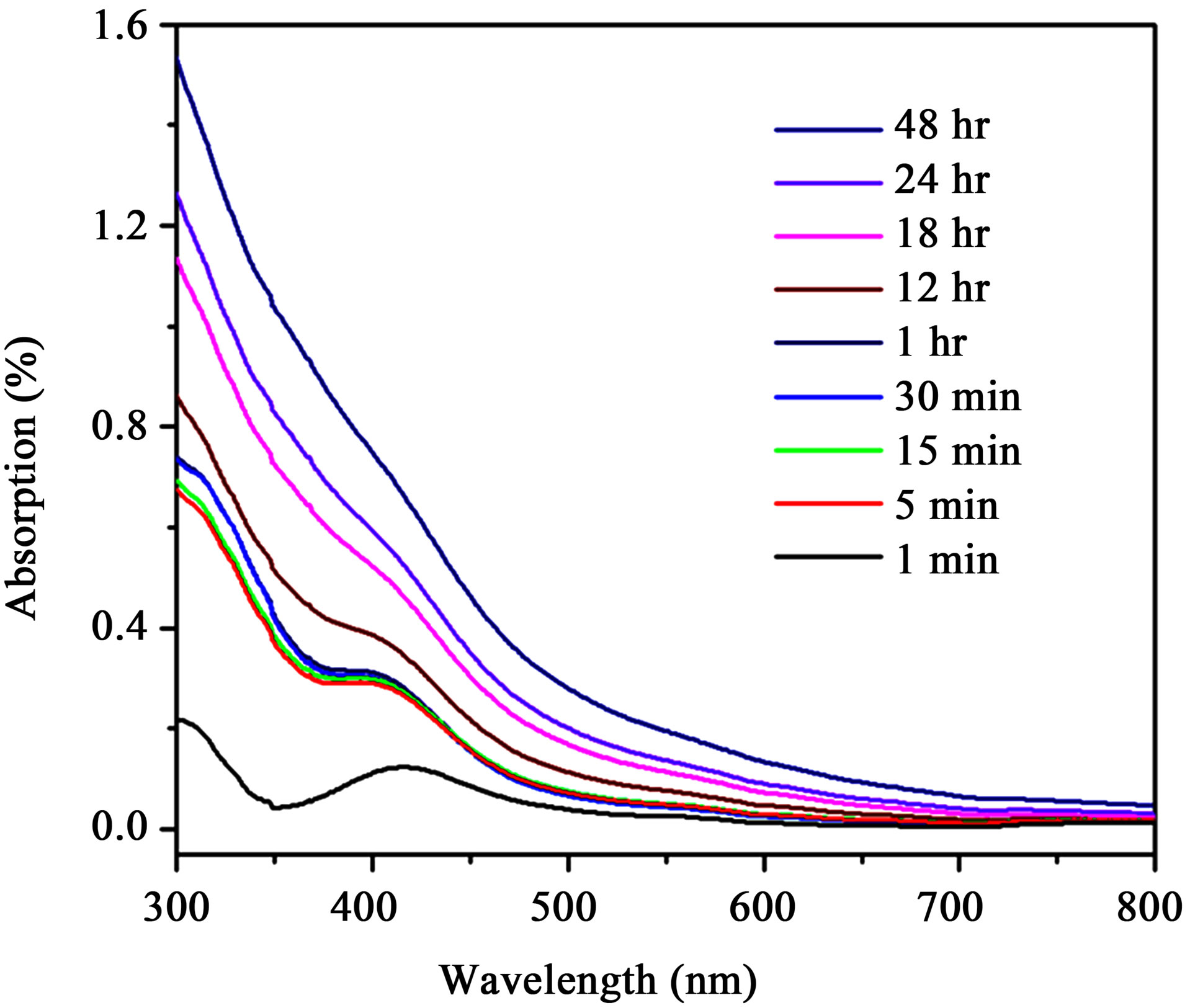
Figure 2. UV-visible spectra of reaction mixture (PdCl2 solution and soy leaf broth) at different time intervals.
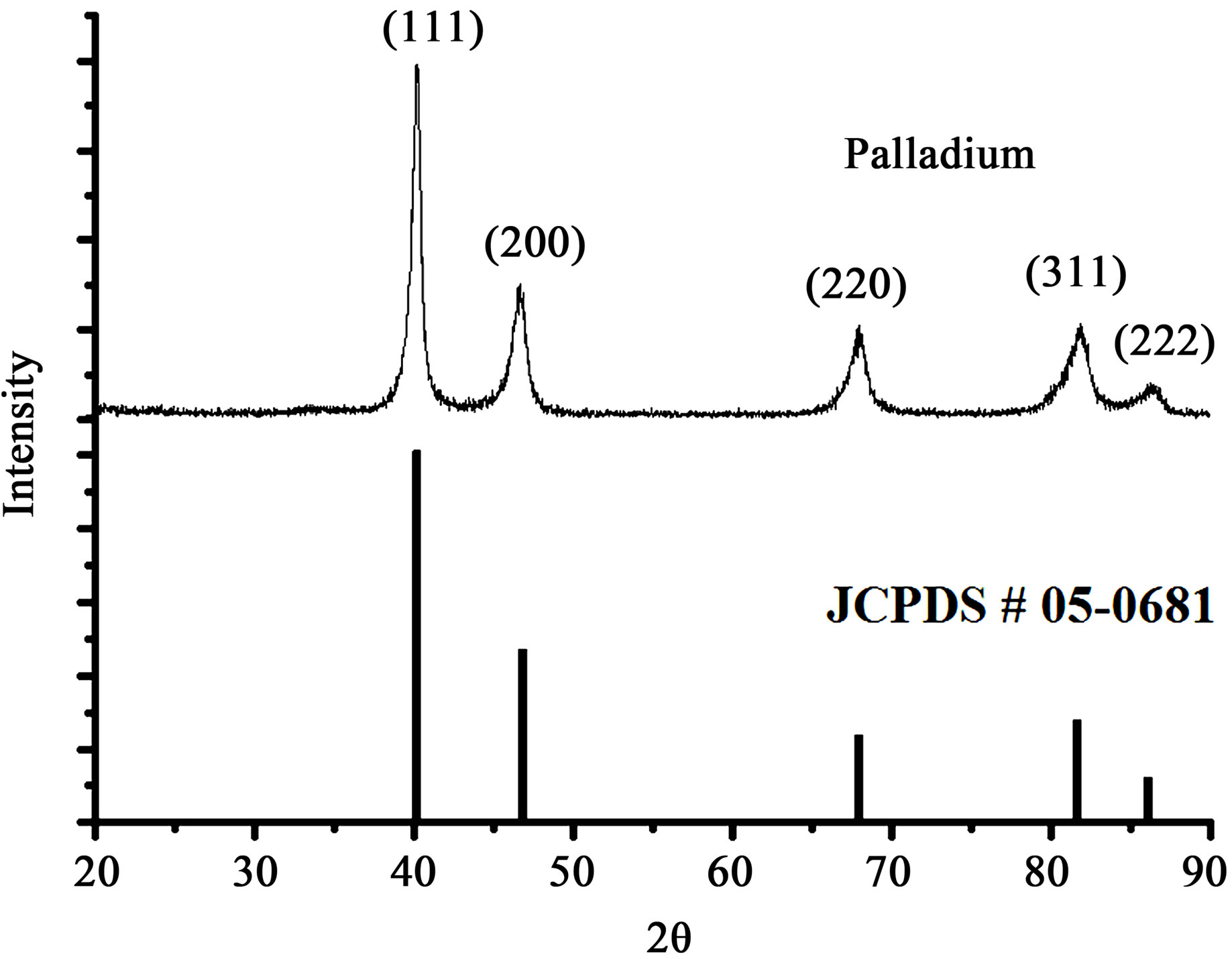
Figure 3. XRD patterns of palladium nanoparticles synthesized by plant leaf extract mediated biological process with JCPDS (05-0681) data.
with cubic (fcc) crystal structure, which is also consistence with the earlier reports [32-34]. The crystallite size of the palladium was calculated using peak broadening profile of (111) peak at 40˚ and Sherrer’s formula as follows,
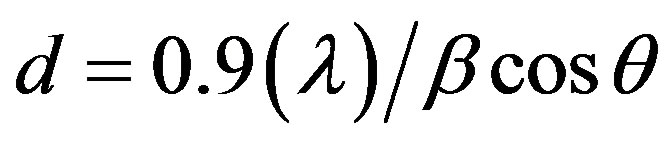
where λ is wavelength (1.5418 Å) and β is full width half maximum (FWHM) of corresponding peek. The calculated crystallite size of the synthesized palladium nanoparticles is 13 nm.
3.3. Transmission Electron Microscopy (TEM)
Size and selected area electron diffraction (SAED) patterns of the palladium nanoparticles synthesized using soybean leaf extract were investigated using HRTEM. The HRTEM images of palladium nanoparticles that are shown in the figures 4(a) and (b) indicate the formation of uniformly distributed spherical structure with ~15 nm in diameter. The HRTEM high magnification image of palladium nanoparticles (figure 4(c)) recorded along the (111) axis shows the lattice structure, which are separated by the 2.24 Å. The Selected area electron diffraction (SAED) patterns were recorded and the obtained Debye-Sherrer rings are show in figure 4(d), which are represented to (111), (200), (220), (311) and (222) planes and its supported by the XRD analysis. Energy dispersion X-ray spectroscopy (EDS) was recorded and shown in figure 5. The observed characteristic peak assigned to palladium metal in EDS spectrum, also evident the successful formation of palladium nanoparticles.
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
The bioreduction mechanism of plant leaf extract is unclear, however it has been strongly believed that the portions, polyols, terpenoids, as well as reducing sugar in the leaf extract causes the metal ion reduction. In present investigation, it was understood that the metal ion reducetion was predominantly performed by various amino acids, which are present in Glycine max (soybean) leaf. Hence, FTIR analysis was carried out to investigate the interactions of amino acids with metal ions. Among the 20 essential amino acids, only 8 are infrared active and they are Glutamic, Aspartic, Arginine, Lysine, Tyrosine, Histidine, Asparagine and Glutamine. Table 1 shows their respective active IR vibrations along with band positions [36].
Figure 6(a) shows the FTIR spectrum of soybean leaf extract at room temperature. The observed peaks at 1041
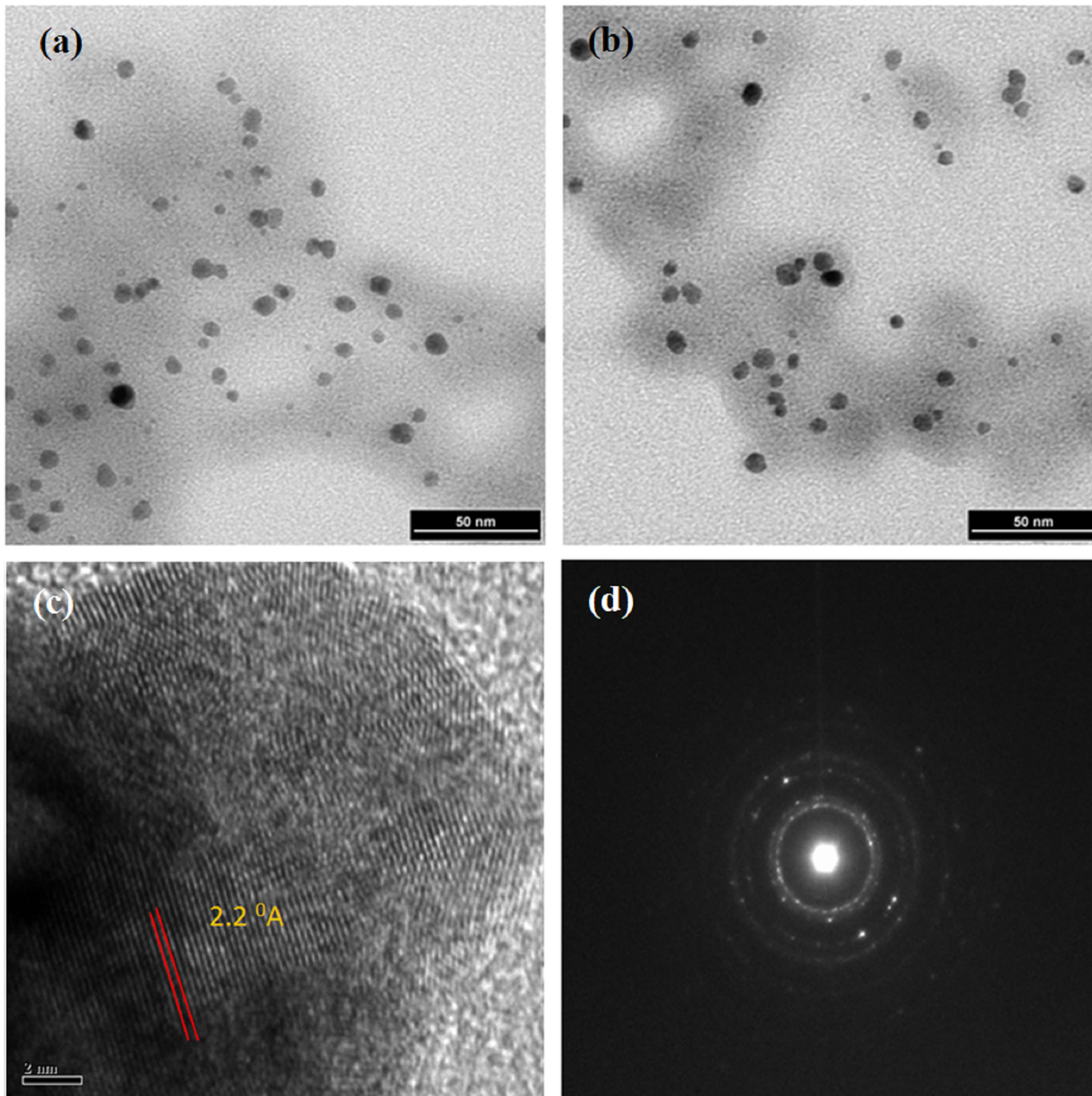
Figure 4. TEM images of synthesized palladium nanoparticles (a)-(c) with selected area electron diffraction (d).
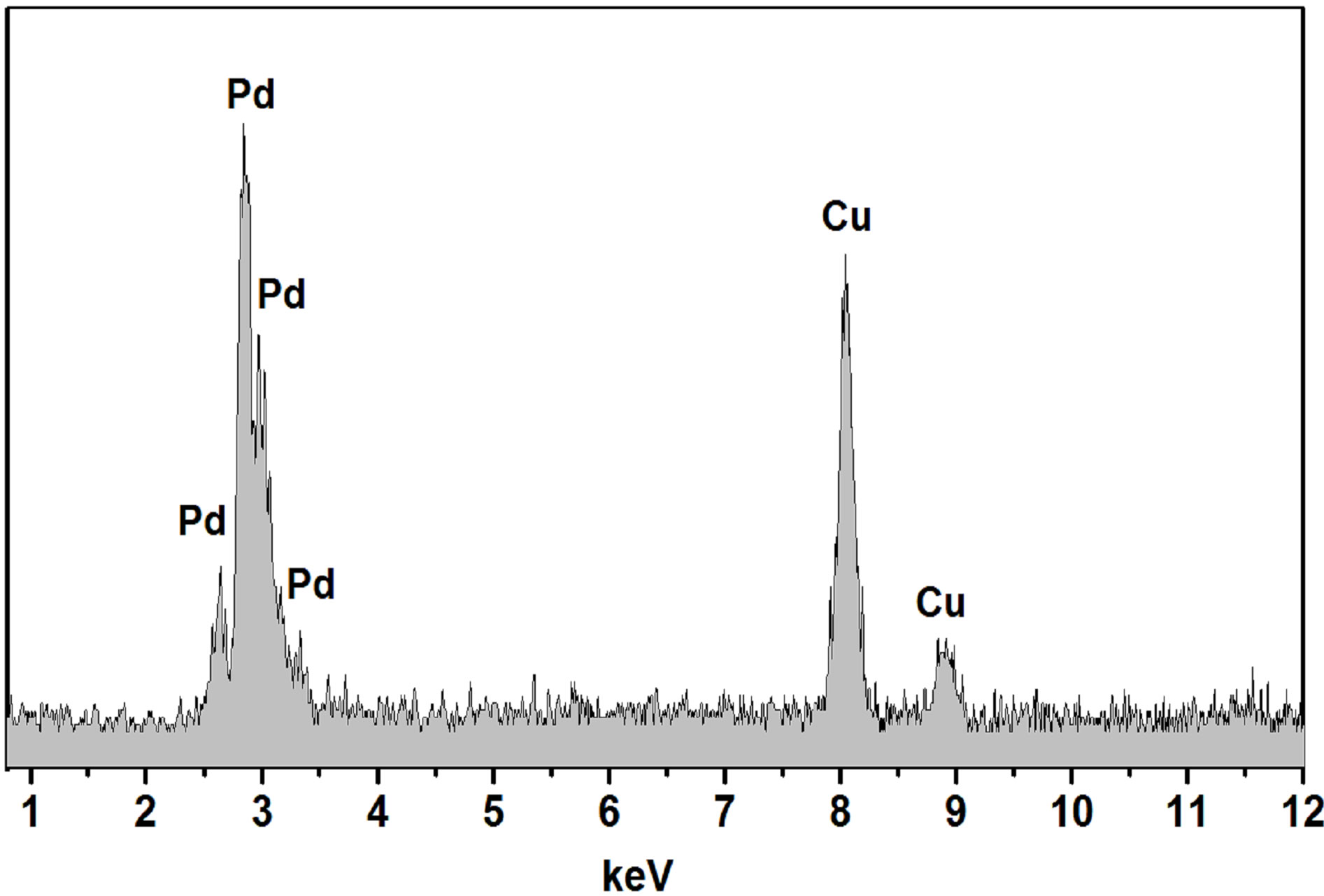
Figure 5. EDS spectrum of synthesized palladium nanoparticles using soy leaf extract.
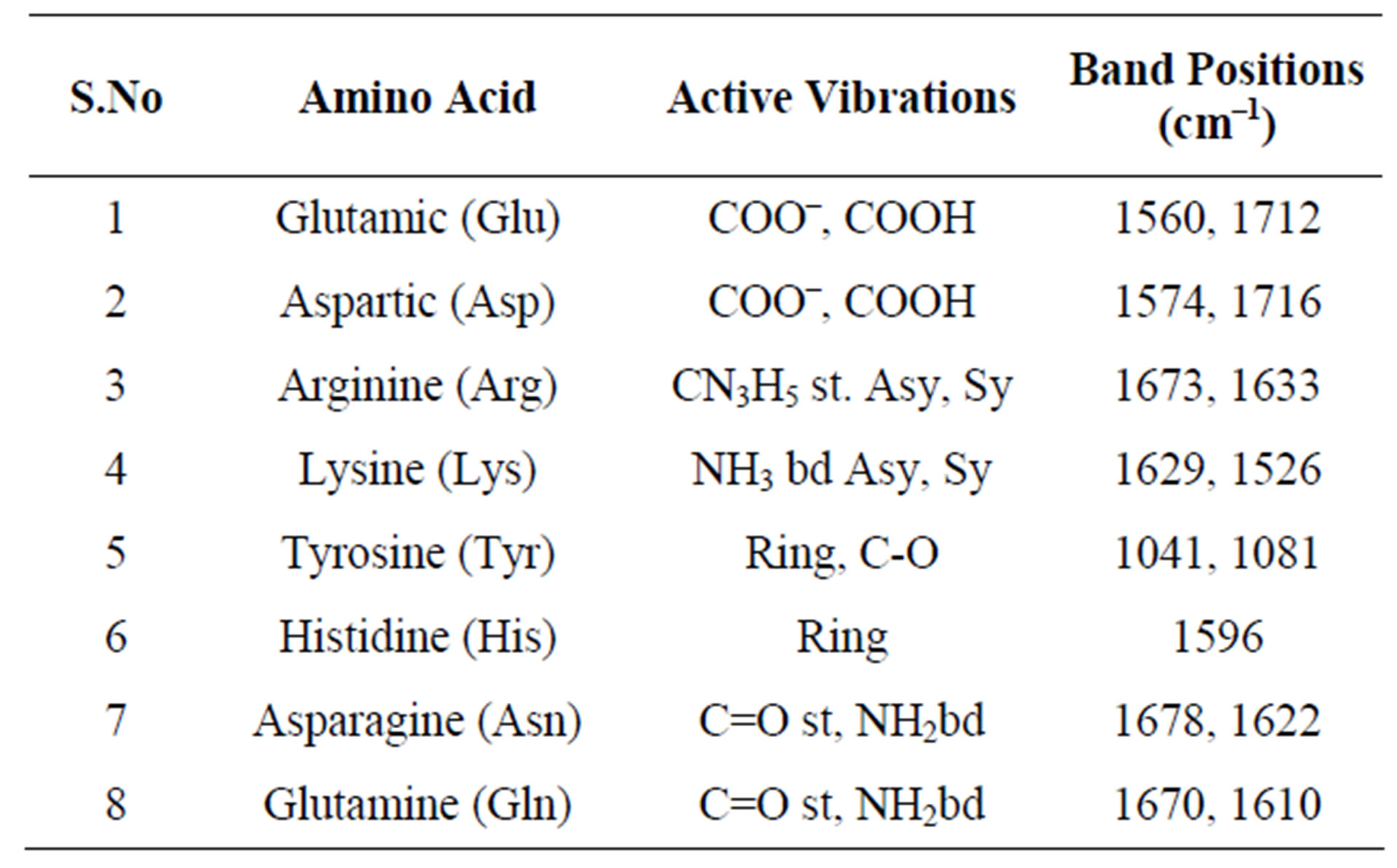
Table 1. Active vibrations and respective band positions of IR active amino acids in soybean.
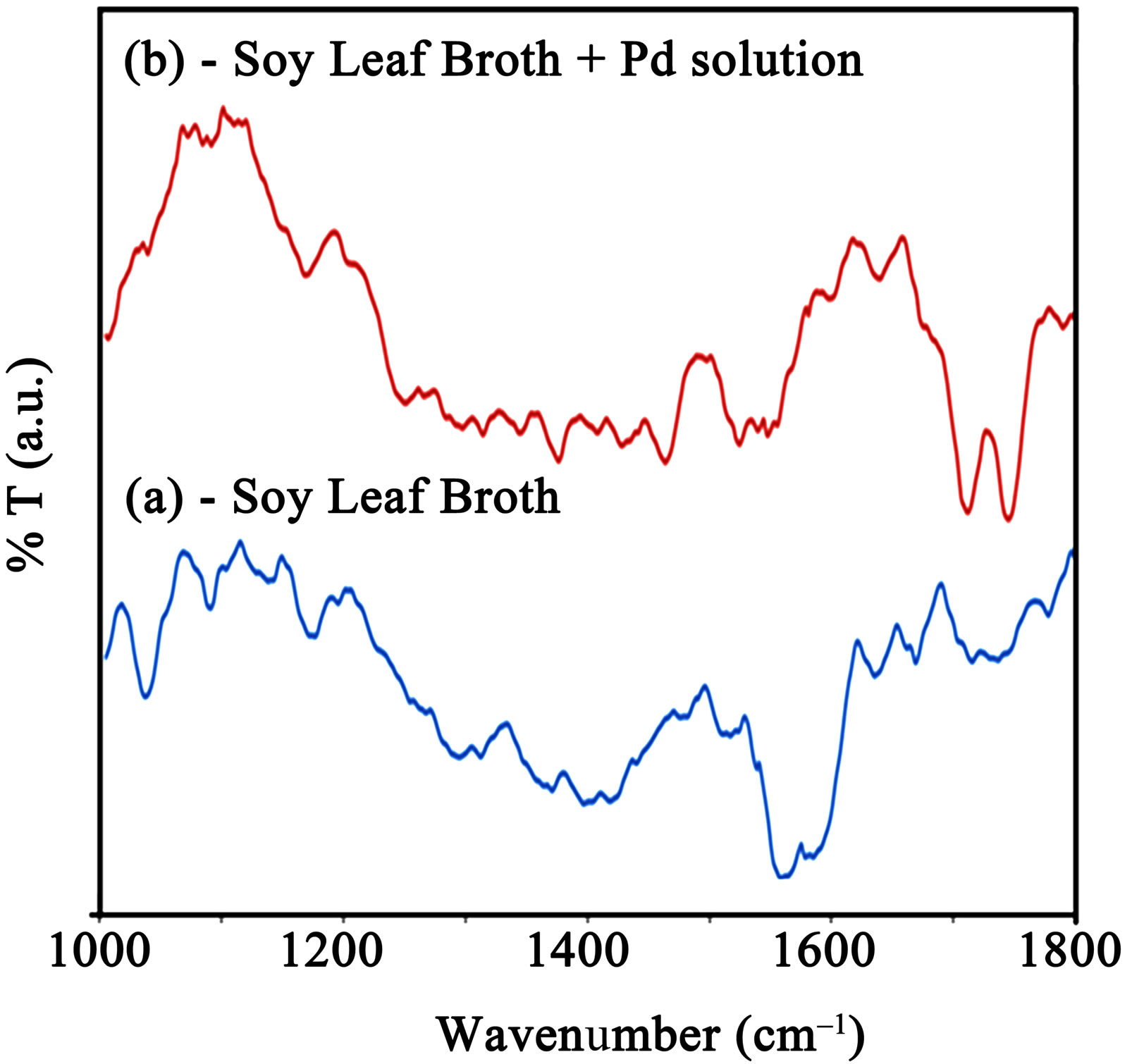
Figure 6. FTIR spectra of (a) Soy leaf broth and (b) Soy leaf broth + Pd solution.
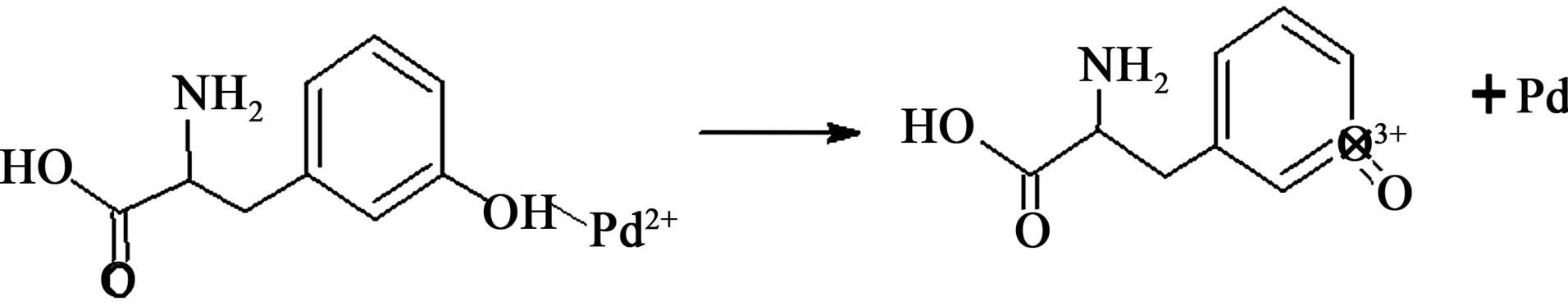
Figure 7. Predicted reduction reaction between Tyrosine and palladium ions.
cm–1 and 1081 cm–1 are assigned to coupled vibrations (δO-H + νC-O) of the hydroxyl group of the tyrosine. The possible reaction of tyrosine with palladium ions, which donates electrons and converts them in to palladium (Pd0) is schematically represented in figure 7.
During the reduction, the -OH functional group in tyrosine has converted into -COOH group and it was confirmed in the newly appeared FITR peaks in figure 6(b) at 1730 cm–1 and 1756 cm–1, which is assigned to symmetrical and asymmetrical C=O stretching [37-39]. Further investigations are in progress in order to reveal the detailed information of bioreduction mechanism for the extracellular synthesis of metal nanoparticles using plant extracts.
4. Conclusion
Palladium nanoparticles were successfully synthesized using Glycine max (soybean) leaf extract mediated biosynthesis process. Protein rich soybean leaf extract is acting as an effective reducing agent for palladium ions, which was proved by our FTIR analysis. The synthesized palladium nanoparticles were characterized using XRD and confirmed the fcc phase. HRTEM investigation results that the average size of synthesized palladium nanoparticles is around 15 nm. From this investigation we found that the soybean leaf mediated biosynthesis process can be cost effective and also alternate for conventional chemical and physical processes for the synthesis of palladium nanoparticles. Also these bio synthesized palladium nanoparticles can be used as catalysis especially in the degradation of azo dyes.
5. Acknowledgements
Authors are thankful to the 2009, Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)—New Directions & Alternative Renewable Fuels Research Program Project number SR9225 for the financial support to carry out this research. The authors are grateful to Canadian Bureau for International Education, on behalf of Foreign Affairs and International Trade Canada (DFAIT) for providing Graduate Student Exchange Program (GSEP) fellowship to RP. Also, NS is grateful to CSIR, DST and DRDO, Govt. of India for their research funding.
REFERENCES
- K. Watanabe, D. Menzel, N. Nilius and H. J. Freund, “Photochemistry on Metal Nanoparticles,” Chemical Reviews, Vol. 106, No. 10, 2006, pp. 4301-4320. doi:10.1021/cr050167g
- S. Cheong, J. D. Watt and R. D. Tilley, “Shape Control of Platinum and Palladium Nanoparticles for Catalysis,” Nanoscale, Vol. 2, No. 10, 2010, pp. 2045-2053. doi:10.1039/c0nr00276c
- Z. Li, X. Wang, G. Wen, S. Shuang, C. Dong, M. C. Paau and M. M. F. Choi, “Application of Hydrophobic Palladium Nanoparticles for the Development of Electrochemical Glucose Biosensor,” Biosensors and Bioelectronics, Vol. 26, No. 11, 2011, pp. 4619-4623. doi:10.1016/j.bios.2011.04.057
- B. Aktaş, F. Mikailov and L. Tagirov, “Self-Assembled FePt Nanoparticle Arrays as Potential High-Density Recording Media,” Magnetic Nanostructures, Springer Series in Materials Science, Vol. 94, No. 1, 2007, pp. 15-28. doi:10.1007/978-3-540-49336-5_3
- A. G. Tkachenko, H. Xie, D. Coleman, W. Glomm, J. Ryan, M. F. Anderson, S. Franzen and D. L. Feldheim, “Multifunctional Gold Nanoparticle-Peptide Complexes for Nuclear Targeting,” Journal of the American Chemical Society, Vol. 125, No.16, 2003, pp. 4700-4701. doi:10.1021/ja0296935
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, “Gold Nanoparticles in Delivery Applications”, Advanced Drug Delivery Reviews, Vol. 60, No. 11, 2008, pp. 1307- 1315. doi:10.1016/j.addr.2008.03.016
- H. Chen, G. Wei, A. Ispas, S. G. Hickey and A. E. Iler, “Synthesis of Palladium Nanoparticles and Their Applications for Surface-Enhanced Raman Scattering and Electrocatalysis,” The Journal of Physical Chemistry C, Vol. 114, No. 50, 2010, pp. 21976-21981. doi:10.1021/jp106623y
- K. R. Gopidas, J. K. Whitesell and M. A. Fox, “Synthesis, Characterization, and Catalytic Applications of a Palladium-Nanoparticle-Cored Dendrimer,” Nano Letters, Vol. 3, No. 12, 2003, pp. 1757-1760. doi:10.1021/nl0348490
- P. TobisÏka, O. Hugon, A. Trouillet and H. Gagnaire, “An Integrated Optic Hydrogen Sensor Based on SPR on Palladium,” Sensors and Actuators B, Vol. 74, No. 1-3, 2001, pp. 168-172. doi:10.1016/S0925-4005(00)00728-0
- Z. H. Chen, J. S. Jie, L. B. Luo, H. Wang, C. S. Lee and S. T. Lee, “Applications of Silicon Nanowires Functionalized with Palladium Nanoparticles in Hydrogen Sensors,” Nanotechnology, Vol. 18, No. 34, 2007, p. 345502. doi:10.1088/0957-4484/18/34/345502
- P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, “Searching for Better Plasmonic Materials,” Laser & Photonics Reviews, Vol. 4, No. 6, 2010, pp. 795-808. doi:10.1002/lpor.200900055
- V. L. Nguyen, D. C. Nguyen, H. Hirata, M. Ohtaki, T. Hayakawa and M. Nogami, “Chemical Synthesis and Characterization of Palladium Nanoparticles,” Advances in Natural Sciences: Nanoscience and Nanotechnology, Vol. 1, No. 3, 2010, p. 035012.
- A. Nemamcha, J. L. Rehspringer and D. Khatmi, “Synthesis of Palladium Nanoparticles by Sonochemical Reduction of Palladium(II) Nitrate in Aqueous Solution,” The Journal of Physical Chemistry B, Vol. 110, No. 1, 2006, pp. 383-387. doi:10.1021/jp0535801
- J. H. Cha, K. S. Kim, S. Choi, S. H. Yeon, H. Lee, C. S. Lee and J. J. Shim, “Size-Controlled Electrochemical Synthesis of Palladium Nanoparticles Using Morpholinium Ionic Liquid,” Korean Journal of Chemical Engineering, Vol. 24, No. 6, 2007, pp. 1089-1094. doi:10.1007/s11814-007-0126-3
- Y. Xiong, J. Chen, B. Wiley and Y. Xia, “Understanding the Role of Oxidative Etching in the Polyol Synthesis of Pd Nanoparticles with Uniform Shape and Size,” Journal of the American Chemical Society, Vol. 127, No. 20, 2005, pp. 7332-7333. doi:10.1021/ja0513741
- K. Badri Narayanan and N. Sakthivel, “Biological Synthesis of Metal Nanoparticles by Microbes,” Advances in Colloid and Interface Science, Vol. 156, No. 1-2, 2010, pp. 1-13. doi:10.1016/j.cis.2010.02.001
- M. Gericke and A. Pinches, “Biological Synthesis of Metal Nanoparticles,” Hydrometallurgy, Vol. 83, No. 1-4, 2006, pp. 132-140. doi:10.1016/j.hydromet.2006.03.019
- P. Mohanpuria, N. K. Rana and S. K. Yadav, “Biosynthesis of Nanoparticles: Technological Boncepts and Future Applications,” Journal of Nanoparticle Research, Vol. 10, No. 3, 2008, pp. 507-517. doi:10.1007/s11051-007-9275-x
- Y. Konishi, K. Ohno, N. Saitoh, T. Nomura, S. Nagamine, H. Hishida, Y. Takahashi and T. Uruga, “Bioreductive Deposition of Platinum Nanoparticles on the Bacterium Shewanella Algae,” Journal of Biotechnology, Vol. 128, No. 3, 2007, pp. 648-653. doi:10.1016/j.jbiotec.2006.11.014
- N. Pugazhenthiran, S. Anandan, G. Kathiravan, N. Kannaian, U. Prakash, S. Crawford and M. Ashokkumar, “Microbial Synthesis of Silver Nanoparticles by Bacillus sp.,” Journal of Nanoparticle Research, Vol. 11, No. 7, 2009, pp. 1811-1815. doi:10.1007/s11051-009-9621-2
- A. Mohammed Fayaz, K. Balaji, P. T. Kalaichelvan and R. Venkatesan, “Fungal Based Synthesis of Silver Nanoparticles—An Effect of Temperature on the Size of Particles,” Colloids and Surfaces B: Biointerfaces, Vol. 74, No. 1, 2009, pp. 123-126. doi:10.1016/j.colsurfb.2009.07.002
- M. Rai, A. Yadav and A. Cade, “Current Trends in Phytosynthesis of Metal Nanoparticles,” Critical Reviews in Biotechnology, Vol. 28, No. 4, 2008, pp. 277-284. doi:10.1080/07388550802368903
- V. Kumar, S. C. Yadav and S. K. Yadav, “Syzygium Cumini Leaf and Seed Extract Mediated Biosynthesis of Silver Nanoparticles and Their Characterization,” Journal of Chemical Technology & Biotechnology, Vol. 85, No. 10, 2010, pp. 1301-1309. doi:10.1002/jctb.2427
- G. Zhan, J. Huang, M. Du, I. Abdul-Rauf, Y. Ma and Q. Li, “Green Synthesis of Au-Pd Bimetallic Nanoparticles: Single-Step Bioreduction Method with Plant Extract,” Materials Letters, Vol. 65, No. 19-20, 2011, pp 2989- 2991. doi:10.1016/j.matlet.2011.06.079
- M. N. Nadagouda and R. S. Varma, “Green Synthesis of Silver and Palladium Nanoparticles at Room Temperature Using Coffee and Tea Extract,” Green Chemistry, Vol. 10, No. 8, 2008, pp. 859-862. doi:10.1039/b804703k
- D. Philip, “Green Synthesis of Gold and Silver Nanoparticles Using Hibiscus Rosa Sinensis,” Physica E, Vol. 42, No. 5, 2010, pp. 1417-1424. doi:10.1016/j.physe.2009.11.081
- R. K. Das, B. B. Borthakur and U. Bora, “Green Synthesis of Gold Nanoparticles Using Ethanolic Leaf Extract of Centella Asiatica,” Materials Letters, Vol. 64, No. 13, 2010, pp 1445-1447. doi:10.1016/j.matlet.2010.03.051
- S. Kaviya, J. Santhanalakshmi, B. Viswanathan, J. Muthumary and K. Srinivasan, “Biosynthesis of Silver Nanoparticles Using Citrus Sinensis Peel Extract and its Antibacterial activity,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 79, No. 3, 2011, 594-598. doi:10.1016/j.saa.2011.03.040
- J. Y. Song and B. S. Kim, “Biological Synthesis of Bimetallic Au/Ag Nanoparticles Using Persimmon (Diopyros kaki) Leaf Extract,” Korean Journal of Chemical Engineering, Vol. 25, No. 4, 2008, pp. 808-811. doi:10.1007/s11814-008-0133-z
- J. Y. Song, E. Y. Kwon and B. S. Kim, “Biological Synthesis of Platinum Nanoparticles Using Diopyros Kaki Leaf Extract,” Bioprocess and Biosystems Engineering, Vol. 33, No. 1, 2010, pp. 159-164. doi:10.1007/s00449-009-0373-2
- M. Sathishkumar, K. Sneha, I. S. Kwak, J. Mao, S. J. Tripathy and Y. S. Yun, “Phyto-Crystallization of Palladium Through Reduction Process Using Cinnamom Zeylanicum Bark Extract,” Journal of Hazardous Materirals, Vol. 171, No. 1-3, 2009, pp 400-404. doi:10.1016/j.jhazmat.2009.06.014
- X. Yang, Q. Li, H. Wang, J. Huang, L. Lin, W. Wang, D. Sun, Y. Su, J. B. Opiyo, L. Hong, Y. Wang, N. He and L. Jia, “Green Synthesis of Palladium Nanoparticles Using Broth of Cinnamomum Camphora Leaf,” Journal of Nanoparticle Research, Vol. 12, No. 5, 2010, pp. 1589-1598. doi:10.1007/s11051-009-9675-1
- M. Sathishkumar, K. Sneha and Y. S. Yun, “Palladium Nanocrystal Synthesis Using Curcuma longa Tuber Extract,” International Journal of Materials Sciences, Vol. 4, No. 1, 2009, pp. 11-17.
- A. Bankar, B. Joshi, A. R. Kumar and S. Zinjarde, “Banana Peel Extract Mediated Novel Route for the Synthesis of Palladium Nanoparticles,” Materials Letters, Vol. 64, No. 18, 2010, pp. 1951-1953. doi:10.1016/j.matlet.2010.06.021
- S. Vivekanandhan, M. Misra and A. K. Mohanty, “Biological Synthesis of Silver Nanoparticles Using Glycine Max (Soybean) Leaf Extract: An Investigation on Diffe0 rent Soybean Varieties,” Journal of Nanoscience and Nanotechnology, Vol. 9, No. 12, 2009, pp. 6828-6833. doi:10.1166/jnn.2009.2201
- F. De Lange, C. H. W. Klaassen, S. E. Wallace-Williams, P. H. M. Bovee-Geurts, X. M. Liu, W. J. DeGrip and K. J. Rothschild, “Tyrosine Structural Changes Detected during the Photoactivation of Rhodopsin,” The Journal of Biological Chemistry, Vol. 273, No. 37, 1998, pp. 23735- 23739.
- S. Yu. Venyaminov and N. N. Kalnin, “Quantitative IR Spectrophotometry of Peptide Compounds in Water (H2O) Solutions. 1. Spectral Parameters of Amino Acid Residue Absorption Bands,” Biopolymers, Vol. 30, No. 13-14, 1990, pp. 1243-1257. doi:10.1002/bip.360301309
- E. S. Bonwell and D. L.Wetzel, “Innovative FT-IR Imaging of Protein Film Secondary Structure before and after Heat Treatment,” Journal of Agricultural and Food Che0 mistry, Vol. 57, No. 21, 2009, pp. 10067-10072.
- G. Socrates, “Infrared and Raman Characteristic Group Frequencies,” John Wiley and Sons, New York, 2001.
NOTES
*Both authors equally contributed to this work.
#Corresponding author.

