Case Reports in Clinical Medicine
Vol.3 No.1(2014), Article ID:41912,3 pages DOI:10.4236/crcm.2014.31003
Sarcomatoid carcinoma of the penis
![]()
1Department of Urology, Ibaraki Medical Center, Tokyo Medical University, Ibaraki, Japan; *Corresponding Author: veq01625@nifty.ne.jp
2Department of Urology, Tokyo Medical University Hospital, Tokyo, Japan
Received 1 December 2013; revised 28 December 2013; accepted 8 January 2014
ABSTRACT
We encountered a 63-year-old male with a sarcomatoid carcinoma of the penis accompanied by metastasis to the bilateral inguinal lymph nodes and lungs. He noticed a penile mass, but neglected it. The mass rapidly increased in size, forming an ulcer, and began to disintegrate. He visited our hospital 4 months after noticing the mass. After cystostomy and blood transfusion, surgical resection was performed. A diagnosis of a squamous cell carcinoma with a sarcomatoid carcinoma of the penis was made. After the operation, best supportive care (BSC) was selected. We made efforts to maintain his quality of life (QOL), and he died 3 months after the operation.
Keywords:Penis; Sarcomatoid Carcinoma
1. INTRODUCTION
Penile carcinomas are generally squamous cell carcinomas. However, penile carcinomas such as sarcomas associated with a poor prognosis are sometimes encountered. We report a 63-year-old male with a sarcomatoid carcinoma of the penis.
2. CASE PRESENTATION
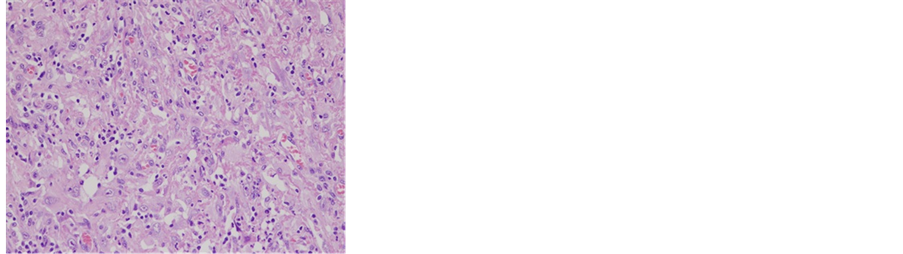
The patient was a 63-year-old male with a tumor showing marked disintegration from the penis to the bilateral inguinal regions and advanced anemia due to bleeding. In April 2012, he noticed a penile mass, but neglected it. Since the mass rapidly increased in size, forming an ulcer, and began to disintegrate, he visited our hospital in August 2012. Based on macroscopic and CT (Figures 1(a) and (b)) findings, a diagnosis of a penile carcinoma (T4N3M1) with lung metastasis was made, and treatment was immediately initiated. Blood biochemical examination at the time of the first visit showed severe anemia but a normal level of SCC as a tumor marker. After cystostomy for urination difficulty due to the tumor and blood transfusion for severe anemia, he underwent bilateral ilioinguinal node dissection, total penile amputation, and unilateral flap cover with a scrotal skin flap for local control. As a result of the operation, a pathological diagnosis of a squamous cell carcinoma with a sarcomatoid carcinoma of the penis was made (Figures 2(a) and (b)).
After the operation, we explained to the patient and his family that there is no established treatment method for sarcomatoid carcinomas of the penis, and provided information about opportunities to participate in clinical trials. However, he did not wish to undergo active treatment. Therefore, best supportive care (BSC) was selected, and we made efforts to maintain his quality of life (QOL). He died in November 2012.
3. DISCUSSION
The incidence of penile carcinomas is 0.5 - 0.9 per 100,000 males, and 95% of them are squamous cell carcinomas. Sarcomatoid carcinomas account for only 1% - 2% of penile carcinomas, and lymph node and distant metastases are often present at the time of the first consultation. Sarcomatoid carcinomas are aggressive, and their associated prognosis is extremely poor [1,2]. Lymph node or distant metastasis was present in most reported cases, suggesting that marked vascular infiltration is a cause of the poor prognosis. Since hematogenous and lymphogenous metastases readily occur, early diagnosis and treatment is the only coping method.
A review of 46 cases of soft tissue tumors of the penis showed 22 malignant tumors, including 4 Kaposi sarco-
 (a)
(a) (b)
(b)
Figure 1. Advanced penile tumor with massive lymph node metastasis can be recognized. (a) Macroscopic finding; (b) CT.
 (a)
(a) (b)
(b)
Figure 2. Microscopic photo. Sarcomatoid component was stained by HE and CD31. (a) HE × 200; (b) CD31 × 200.
mas, 3 hemangiosarcomas, 3 leiomyosarcomas, and 2 fibrosarcomas. Thus, there are various types of tumor developing in the penis. The biologic behavior and clinical management markedly differ among these types of tumor. Therefore, differential diagnosis is also important for their early diagnosis and treatment [3,4].
In general, carcinomas coexisting with sarcomas are called carcinosarcomas. Carcinosarcomas develop by the following mechanisms: 1) sarcomatous changes of a carcinoma; 2) secondary mixture of a carcinoma and mesenchymal tumor; 3) epithelial atypia developing in the process of sarcoma infiltration; and 4) enhancement of stromal reactions due to carcinoma infiltration, resulting in a change to a sarcoma. Most carcinosarcomas are considered to be due to sarcomatous changes of carcinomas [5].
In this patient, the tumor was composed of squamous cell carcinoma elements and sarcomatous elements (spindle cells). Immunostaining revealed that both carcinomatous and sarcomatous elements were positive for AE1/AE3, CAM5.2, ck5/6, 34βE12, and p63. Based on these findings, a diagnosis of a sarcomatoid carcinoma of the penis with remaining epithelial characteristics was made.
There is no standard curative therapy for patients with advanced or metastatic disease, and treatment is directed at palliation. Palliative surgery may be considered for the control of local penile lesions in patients with advanced, ulcerated or infiltrating tumors, providing temporary tumor regression and decreasing pain and bleeding. The role of chemotherapy has not been extensively explored, and is limited to small series based on a type 3 level of evidence. In this patient, since only sarcomatous components were observed in the lymph node metastasis areas, we considered that an additional chemotherapy regimen that is effective against sarcomas should be selected. However, finally, BSC was selected because maintaining the QOL was considered to be more beneficial than the indiscriminate use of chemotherapy.
The following can be learned from this case. Sarcomatoid carcinomas of the penis rapidly grow, and early diagnosis is necessary. Therefore, efforts should be made to disseminate methods for an early diagnosis. Concerning treatment, this carcinoma should be regarded as an orphan disease, and even negative data should be disclosed in the form of case reports to share information among more clinicians and develop more effective treatment.
REFERENCES
- Cubilla, A.L., Reuter, V., Velazquez, E., Piris, A., Saito, S. and Young, R.H. (2001) Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. International Journal of Surgical Pathology, 9, 111-120. http://dx.doi.org/10.1177/106689690100900204
- Velazquez, E.F., Melamed, J., Barreto, J.E., Aguero, F. and Cubilla, A.L. (2005) Sarcomatoid carcinoma of the penis: A clinicopathologic study of 15 cases. American Journal of Surgical Pathology, 29, 1152-1158. http://dx.doi.org/10.1097/01.pas.0000160440.46394.a8
- Katona, T.M., Lopez-Beltran, A., MacLennan, G.T., Cheng, L., Montironi, R. and Cheng, L. (2006) Soft tissue tumors of the penis: A review. Analytical and Quantitative Cytology and Histology, 28, 193-206.
- Ranganath, R., Singh, S.S. and Sateeshan, B. (2008) Sarcomatoid carcinoma of the penis: Clinicopathologic features Indian Journal of Urology, 24, 267-268.
- Lont, A.P., Gallee M.P., Snijders, P. and Horenblas, S. (2004) Sarcomatoid squamous cell carcinoma of the penis: A clinical and pathological study of 5 cases. Journal of Urology, 172, 932-935. http://dx.doi.org/10.1097/01.ju.0000136363.90911.e5

