Food and Nutrition Sciences
Vol.07 No.06(2016), Article ID:66694,12 pages
10.4236/fns.2016.76043
Influence of Polyphenolic Contents on the Antioxidant Properties of Hibiscus sabdariffa Extract (HSE), Aged Garlic Extract (AGE) and Garlic Tablet (GT) in Vitro
Abiodun Olusoji Owoade*, Adewale Adetutu, Olubukola Sinbad Olorunnisola
Department of Biochemistry, Ladoke Akintola University of Technology, Ogbomosho, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 April 2016; accepted 21 May 2016; published 24 May 2016
ABSTRACT
This study compared the antioxidant properties of Hibiscus sabdariffa extract (HSE), commercial aged garlic extract (
Keywords:
Hibiscus sabdariffa, Aged Garlic Extract, Antioxidants, Phenolic Compounds

1. Introduction
Reactive oxygen and nitrogen species are generated both endogenously and in response to external factors, such as diet and lifestyle, which play a major role in the etiology of several degenerative diseases [1] [2] . The effect of reactive oxygen species (
This has triggered the growing market for nutraceutical and functional foods with emphasis on the study of natural sources of antioxidants, their potential as nutraceutical and functional foods [5] . Such plants that have attracted much attention over the years are roselle (Hibiscus sabdariffa) and garlic (Allium sativum), and many studies on these two plants, have focused on the antioxidant properties of their numerous preparation and constituents. Garlic powder extract and
The human diet contains an array of different compounds that possess antioxidant activities and their ROS scavenging abilities have been suggested to be due to their structural properties [17] . Such compounds include vitamin E, vitamin C, carotenoids and phenolics (flavonoids and phenolic acids). These compounds are reported to play preventing role in the development of various pathological diseases [18] . A high flavonoid consumption has particularly been associated with a decreased risk of cardiovascular disease [19] and lower rates of stomach, pancreatic, lung and possibly breast cancer [20] . It has been identified that Allium sativum is a rich source of antioxidant flavonoids and phenolic acids such as quercetin, apigenin, myricetin, caffeic acid, vanillic acid, salicylic acid [21] [22] , while Hibiscus sabdariffa is also rich in phenolic compounds such as quercetin, luteolin, chlorogenic acid, protocatechuic acid, catechin, epigallocatechin, epigallocatechingallate and caffeic acid [23] [24] . However, in spite of this varied phenolic content of Allium sativum and Hibiscus sabdariffa, the contributory role played by these phenolic compounds as an antioxidant is sparingly evaluated.
The aim of this study was to investigate the in vitro antioxidant potential of Hibiscus sabdariffa extract (
2. Materials and Methods
2.1. Chemicals
All chemical used were of analytical grade. Special reagents were cytochrome C, xanthine, xanthine oxidase (Grade
2.2. Materials
The following natural products were investigated in this study:
1) Hibiscus sabdariffa (Malvaceae): Flowers of this plant was bought at a market in Nigeria. The identification and authentication of the plant was done by Professor A.J. Ogunkunle at Department of Pure and Applied Biology, LadokeAkintola University of Technology, Ogbomoso.
2) Kyolic, aged garlic extract is a product of Wakunaga of America Company Ltd (Mission Viejo, CA, USA). The extract is formulated by soaking sliced raw garlic (Allium sativum) in 15% - 20% aqueous ethanol for up to 20 months at room temperature. The extract is filtered and concentrated under reduced pressure at low temperature.
3) Garlic Care Tablets―Allium sativum (standardized-300 mg), is a product of Quarshi Industries (PVT) Ltd, Hattar-Pakistan.
2.3. Preparation of Hibiscus sabdariffa Extract (
Hibiscus sabdariffa was dried at room temperature and grounded with mortar and pestle. Weighed samples of this powdered material (20 g) were loaded into extraction thimbles of Soxhlet extractor and were then extracted with methanol for 16 hours. The pooled methanolic solution of the extracts was thereafter concentrated in-vacuo by distillation at 50˚C to recover most of the methanol.
2.4. Preparation of a Diethyl Ether Extract of
Due to intense colouring of extracts employed in this study (
2.5. Preparation of Aqueous Extracts of HSE,
1 g of each samples were mixed with 10 ml of phosphate buffered saline (PBS), this was centrifuged at 2000 rpm for 10 minute and the supernatant was collected for trolox equivalent antioxidant capacity (TEAC) analysis.
2.6. Superoxide and Xanthine Oxidase Activity
This was carried out as reported by Dillion et al., [22] with slight modifications. Briefly superoxide production and xanthine oxidase activity were measured as cytochrome C reduction and uric acid production, respectively. Xanthine oxidase was prepared to a concentration of 53.5 mU/ml in Phosphate Buffered Saline (PBS), pH 7.2 and xanthine was prepared as a 0.8 mM solution also in PBS. Superoxide ions were generated in a reaction volume of 1 ml containing 80 µM xanthine and 0.625 mg cytochrome C. The reaction was initiated by the addition of 5.35 mU xanthine oxidase, and superoxide ion production was monitored at 550 nm [25] . In a series of separate experiments, xanthine oxidase activity was monitored as the production of uric acid at 284 nm. Generation of superoxide ions was confirmed by the addition of 50 U superoxide dismutase (SOD), which inhibited the reduction of cytochrome C without affecting xanthine oxidase activity. Extracts were added at 0% - 20% (v/v). Results for superoxide production are expressed as ΔA550 nm/minute whilst, result for uric acid production are expressed as ∆A284 nm/minute.
2.7. Trolox Equivalent Antioxidant Capacity (TEAC) with Manganese Dioxide
The assay was performed as previously described by Schelesier et al., [18] with slight modifications. Briefly, the ABTS radical cation was prepared by filtering a solution of ABTS (in PBS) through manganese dioxide powder. Excess manganese dioxide was removed from the filtrate by passing it through a 0.2 µm syringe filter. This solution was diluted in 5 mM PBS pH 7.4, adjusted to an absorbance of 0.700 ± 0.020 at 734 mm and pre-incu- bated at room temperature prior to use for 2 hours. 1 ml of ABTS•+ solution and various concentrations of the extracts (diluted with water) were vortexed for 45 seconds in reaction tubes, and the absorbance (734 nm) was taken exactly 2 minutes after initiation of mixing. PBS blanks were run with each assay. The antioxidant activity of the extracts was calculated by determining the decrease in absorbance at different concentrations by using the following equation:
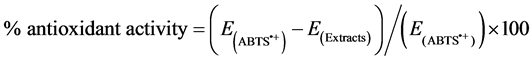 .
.
where E is the extinction.
2.8. Trolox Equivalent Antioxidant Capacity with Potassium Persulfate
The assay was performed as described by Re et al., [26] with modifications. Briefly ABTS radical cation was produced by reacting 3.5 mM ABTS stock solution with 1.225 mM potassium persulphate and allowing the mixture to stand in the dark at room temperature for 12 - 24 h before use. The ABTS•+ solution was diluted with water for the hydrophilic assay and with ethanol for the lipophilic assay and adjusted to an absorbance of 0.700 ± 0.020 at 734 nm. For the photometric assay, 1 ml of the ABTS•+ solution and various concentrations of the extracts were mixed for 45 seconds and measured immediately after 1 minute at 734 nm. The antioxidant activity of the extracts was calculated by determining the decrease in absorbance at different concentrations by using the following equation:
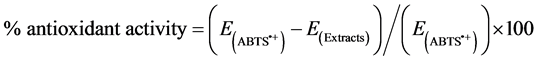
2.9. Determination of Total Phenolic Compounds in HSE,
The amount of total phenolic was determined using Folin-Ciocalteu’s reagent, as described by Ragazzi and Veronese, [27] . One ml of
2.10. Thin Layer Chromatography (TLC) Analysis of HSE,
Diethyl ether extracts of HSE,
2.11. High Performance Liquid Chromatography (HPLC) Analysis of
The HPLC method employed a 5 µ RP-18 column, Hibiscus sabdariffa extract (
2.12. Statistical Analysis
Results are expressed as means ±
3. Results
3.1. Superoxide Scavenging Ability of HSE, AGE and GT
Superoxide production by xanthine-xanthine oxidase gave a reaction rate of 0.108 ± 0.001 ∆A550 nm/min while xanthine oxidase activity gave a reaction rate of 0.106 ± 0.001 ∆A284 nm/minutes. At 20% (v/v) of the reaction volume, diethyl ether extract of HSE, AGE and GT significantly inhibited superoxide production i.e. the reduction of cytochrome C by 100%, 64.81% and 16.67% respectively (Table 1). Superoxide production was inversely related to the concentrations of diethyl ether extract of HSE, AGE and GT. Uric acid production was not
Table 1. The effect of SOD, diethyl ether extracts of HSE, AGE and GT on superoxide and uric acid produc- tion by xanthine/xanthine oxidase.
N.D: not determined. Values are means of three experiments ± SEM, each experiment comprised of three observations (n = 9). Significant differences (p < 0.05) from control1 are indicated by*.
significantly affected in the presence of diethyl ether extracts of
3.2. ABTS Radical Cation Scavenging Ability of HSE, AGE and GT
In the three versions of TEAC assay, TEAC II and TEAC
3.3. The Phenolic Nature of
The phenolic content of diethyl ether extracts of
3.4. Analysis of Phenolic Compounds in
Thin-layer chromatography (TLC) analysis was performed to compare the properties of the phenolic compounds present in
3.5. Analysis of Phenolic Compounds in
High performance liquid chromatography (HPLC) analysis is a useful technique for investigating complex mix-
Figure 1. The effects of different concentrations of
Figure 2. The effects of different concentrations of
Table 2. Trolox equivalent antioxidant capacities (TEAC) (mmol/L) of trolox, HSE, AGE and GT.
Values are means of three experiments ± SEM.
Table 3. The total phenolic content of
Figure 3. The effects of different concentrations of
Table 4. The Rf values for standard phenolic compounds and Diethyl Ether Extract of
tures of phenolic compounds. The methanol/water gradient system used allowed separation of phenolic compounds present in Hibiscus sabdariffa by polarity where the most polar compounds eluted first. A Shimadzu detection system was employed, eight peaks were obtained when
possible presence of protocatechuic acid, chlorogenic acid, p-coumaric and caffeic acid in
4. Discussion
Oxidative stress occurs when there is imbalance between free radical generating and scavenging systems. It has
Table 5. The Rf values (in millimetres) for standard phenolic compounds and Diethyl Ether Extract of
Table 6. The retention times for standard phenolic compounds and HSE extract in HPLC. All experiments were repeated three times and values shown are typical of the results obtained.
been implicated in the pathogenesis of wide range of disorders, which include neurodegerative disorders, cardiovascular diseases, cancer, and ageing [28] . This is based on evidence obtained from diseased tissues such as increased levels of free radical and, free radical-induced products of
secondary plant products widely distributed in fruit and vegetables in ameliorating the effects of oxidative stress [18] . This has been attributed to the antioxidant properties of these substances which include scavenging of free radical such as superoxide anion, hydroxyl and nitric oxide radicals [30] .
In cellular oxidation reactions, superoxide are normally formed first, and their effects can be magnified because they produce other kinds of cell-damaging free radicals and oxidizing agents [3] . Xanthine oxidase is one of the main enzymatic sources of ROS in vivo. It-mediated the breakdown of hypoxanthine to xanthine and then to uric acid which is a key source of the
Generation of the ABTS (2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical cation forms the basis of one of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity of solutions of pure substances [26] [33] , aqueous mixtures and beverages [18] [34] . The abilities of antioxidants to scavenge the pre-formed ABTS•+ radical cation measured in TEAC value are influenced by the presence of functional groups and number of conjugated double bonds in carotenoids [33] , as well as polyphenols and ascorbic acid content in the beverages [18] . To further evaluate the antioxidant potential of
Phenolic compounds are commonly found in the plant kingdom, and they have been reported to have multiple biological effects, including antioxidant activity [35] [36] . Hibiscus sabdariffa and Garlic are rich source of plant polyphenols such as flavonoids and phenolic acids [21] - [24] . Using numerous methods of analysis the current study, examined the content of phenolic compounds for the
The beneficial health properties of garlic (Allium sativum L.), and its antioxidant activities, are accredited to the biologically active lipophilic sulfur-bearing compounds like allicin, S-allylycysteine (SAC), diallyl-di-sulf- ide (DADS) and diallylsulfide (DAS) [37] - [39] , although the contributory role play by phenolic content observed in this study to the health benefit of garlic may be highly significant. However, since polyphenols are by far the major antioxidant constituents of Hibiscus sabdariffa, this class of compounds appears to be of major relevance for the observed antioxidant properties of this plant, although other antioxidant compounds such as ascorbic acid may also contribute.
5. Conclusion
The result of this study indicates that garlic extracts (AGE and GT) contain components that actively scavenge superoxide ions while Hibiscus sabdariffa extract (HSE) is more efficient in inhibiting superoxide ion production. The three extracts (HSE, AGE and GT) also scavenge ABTS radical cations and these abilities are found to be dose-dependent. In all antioxidant assays, HSE is found to be more effective as an antioxidant when compared with antioxidant ability displayed by AGE and GT. The same extract also has higher amount of phenolic compounds than
Cite this paper
Abiodun Olusoji Owoade,Adewale Adetutu,Olubukola Sinbad Olorunnisola, (2016) Influence of Polyphenolic Contents on the Antioxidant Properties of Hibiscus sabdariffa Extract (HSE), Aged Garlic Extract (AGE) and Garlic Tablet (GT) in Vitro. Food and Nutrition Sciences,07,417-428. doi: 10.4236/fns.2016.76043
References
- 1. Weisburger, J.H. (2001) Antimutagenesis and Anticarcinogenesis, from the Past to the Future. Mutation Research, 480-481, 23-35.
http://dx.doi.org/10.1016/S0027-5107(01)00166-X - 2. Huang, D.J., Chen, H.J., Lin, C.D. and Lin, Y.H. (2005) Antioxidant and Antiproliferative Activities of Water Spinach (Ipomoea Aquatica Forsk) Constituents. Botanical Bulletin of Academia Sinica, 46, 99-106.
- 3. Valko, M., Rhodes, C.J., Moncol, J., Izakovic, M. and Mazur, M. (2006) Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chemico-Biological Interactions, 6, 1-40.
http://dx.doi.org/10.1016/j.cbi.2005.12.009 - 4. Mantle, D., Wilkins, R.M. and Gok, M.A. (2003) Comparison of Antioxidant Activity in Commercial Ginkgo Biloba Preparations. The Journal of Alternative and Complementary Medicine, 9, 625-629.
http://dx.doi.org/10.1089/107555303322524472 - 5. Cevalls-Casals, B.A. and Cisneros-Zevallos, L. (2003) Stoichimetric and Kinetic Studies of Antioxidants from Audean Purple Corn and Red—Fleshed. Chemistry, 51, 3313-3319.
- 6. Imai, J., Ide, N., Nagae, S., Moriguchi, T. and Itakura, Y. (1994) Antioxidant and Radical Scavenging Effects of Aged Garlic Extract and Its Constituents. Planta Medica, 60, 417-420.
http://dx.doi.org/10.1055/s-2006-959522 - 7. Aruoma, O.I., Spencer, J.P.E., Warren, D., Jenner, P., Butler, J. and Haliwell, B. (1997) Characterisation of Food Antioxidants, Illustrated Using Commercial Garlic and Ginger Preparations. Food Chemistry, 60, 149-156.
http://dx.doi.org/10.1016/S0308-8146(95)00254-5 - 8. Medina-Campos, O.N., Barrera, D., Segoviano-Murillo, S., Rocha, D., Maldonado, P.D., Mendoza-Patino, N. and Pedraza-Chaverri, J.S. (2007) Allylcysteine Scavenges Singlet Oxygen and Hypochlorous Acid and Protects LLC-PK (1) Cells of Potassium Dichromate-Induced Toxicity. Food and Chemical Toxicology, 45, 2030-2039.
http://dx.doi.org/10.1016/j.fct.2007.05.002 - 9. Wang, C.J., Wang, J.M., Lin, W.L., et al. (2000) Protective Effect of Hibiscus anthocyanins against Tert-Butyl Hydroperoxide-Induced Hepatic Toxicity in Rats. Food and Chemical Toxicology, 38, 411-416.
http://dx.doi.org/10.1016/S0278-6915(00)00011-9 - 10. Usoh, I.F., Akpan, E.J., Etim, E.O. and Farombi, E.O. (2005) Antioxidant Actions of Dried Flower Extracts of Hibiscus sabdariffa L. on Sodium Arsenite—Induced Oxidative Stress in Rats. Pakistan Journal of Nutrition, 4, 135-141.
http://dx.doi.org/10.3923/pjn.2005.135.141 - 11. Chang, Y.C., Hungang, K.X., Hungang, A.C., Ho, Y.C. and Wang, C.J. (2006) Hibiscus anthocyanins—Rich Extract Inhibited LDL Oxidation and oxLDL Mediated Macrophage Apoptosis. Food and Chemical Toxicology, 244, 1015- 1023.
http://dx.doi.org/10.1016/j.fct.2005.12.006 - 12. Adetutu, A. and Owoade, O.A. (2013) Hepatoprotective and Antioxidant Effect of Hibiscus Polyphenol Rich Extract (HPE) against Carbon Tetrachloride (CCL4)—Induced Damage in Rats. British Journal of Medicine & Medical Research, 3, 1574-1586.
http://dx.doi.org/10.9734/BJMMR/2013/3762 - 13. Owoade, O.A. and Adetutu, A. (2015) Antioxidant and Hepatoprotective Effect of Hibiscus sabdariffa Methanolic Extract (HME) against Carbon Tetrachloride (CCL4) Induced Damage in Rats. Researcher, 7, 64-72.
- 14. Tseng, T.H., Wang, C.J., Kao, E.S. and Chu, C.Y. (1996) Hibiscus protocatechuic Acid Protects against Oxidative Damage Induced by Tert-Butylhydroperoxide in Rat Primary Hepatocytes. Chemi-co-Biological Interactions, 101, 137- 148.
http://dx.doi.org/10.1016/0009-2797(96)03721-0 - 15. Tseng, T.H., Kao, E.S., Chu, H.Y., et al. (1997) Protective Effects of Dried Flower Extracts of Hibiscus sabdariffa L. against Oxidative Stress in Rat Primary Hepatocytes. Food and Chemical Toxicology, 35, 1159-1164.
http://dx.doi.org/10.1016/S0278-6915(97)85468-3 - 16. Liu, C.-L., Wang, J.-M., Chu, C.-Y., Cheng, M.-T. and Tseng, T.-H. (2002) In Vivo Protective Effect of Protocatechuic Acid on tert-Butyl Hydroperoxide-Induced Rat Hepatotoxicity. Food and Chemical Toxicology, 40, 635-641.
http://dx.doi.org/10.1016/S0278-6915(02)00002-9 - 17. Liu, R.H. (2004) Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. Journal of Nutrition, 134, 3479S-3485S.
- 18. Schlesier, K., Harwat, M., Bohm, V. and Bitsch, R. (2002) Assessment of Antioxidant Activity by Using Different in Vitro Methods. Free Radical Research, 36, 177-187.
http://dx.doi.org/10.1080/10715760290006411 - 19. Mennen, L.I., Sapinho, D., Bree, A.D., Arnault, N., Bertrais, S., Galan, P. and Hercberg, S. (2004) Consumption of Foods Rich in Flavonoids Is Related to a Decreased Cardiovascular Risk in Apparently Healthy French Women. Journal of Nutrition, 134, 923-926.
- 20. Damianaki, A., Bakogeorgou, E., Kampa, M., Notas, G., Hatzoglou, A., Panagiotou, S., Gemetzi, C., Kouroumalis, E., Martin, P.M. and Castanas, E. (2000) Potent Inhibitory Action of Red Wine Polyphenols on Human Breast Cancer Cells. Journal of Cellular Biochemistry, 78, 429-441.
http://dx.doi.org/10.1002/1097-4644(20000901)78:3<429::AID-JCB8>3.0.CO;2-M - 21. Miean, K.H. and Mohamed, S. (2001) Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. Journal of Agricultural and Food Chemistry, 49, 3106-3112.
http://dx.doi.org/10.1021/jf000892m - 22. Dillon, S.A., Burmi, R.S., Lowe, G.M., Billington, D. and Rahman, K. (2003) Antioxidant Properties of Aged Garlic Extract: An in Vitro Study Incorporating Human Low Density Lipoprotein. Life Sciences, 72, 1583-1594.
http://dx.doi.org/10.1016/S0024-3205(02)02475-X - 23. Salah, A.M., Gathumbi, J. and Vierling, W. (2002) Inhibition of Intestinal Motility by Methanolic Extracts of Hibiscus sabdariffa L. (Malvaceae) in Rats. Phytotherapy Research, 16, 283-285.
http://dx.doi.org/10.1002/ptr.846 - 24. Lin, H.H., Huang, H.P., Huang, C.C., Chen, J.U.H. and Wang, C.J. (2005) Hibiscus Polyphenol-Rich Extract Induces Apoptosis Inhuman Gastric Carcinoma Cells via p53 Phosphorylation and p38 MAPK/FasL Cascade Pathway. Molecular Carcinogenesis, 43, 86-99.
http://dx.doi.org/10.1002/mc.20103 - 25. Edwards, S.W., Nurcombe, H.L. and Hart, C.A. (1987) Oxidative Inactivation of Myeloperoxidase Released from Human Neutrophils. Biochemical Journal, 245, 925-928.
http://dx.doi.org/10.1042/bj2450925 - 26. Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. and Rice-Evans, C. (1999) Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine, 26, 1231-1237.
http://dx.doi.org/10.1016/S0891-5849(98)00315-3 - 27. Ragazzi, E. and Veronese, G. (1973) Quantitative Analysis of Phenolic Compounds after Thin-Layer Chromatographic Separation. Journal of Chromatography A, 77, 369-375.
http://dx.doi.org/10.1016/S0021-9673(00)92204-0 - 28. Halliwell, B. and Gutteridge, J.M.C. (1999) Oxidative Stress: Adaptation, Damage, Repair and Death. In: Halliwell, B. and Gutteridge, J.M.C, Eds., Free Radicals in Biology and Medicine, 3rd Edition, Oxford University Press, Oxford, 246-350.
- 29. Thérond, P., Bonnefont-Rousselot, D., Davit-Spraul, A., Conti, M. and Legrand, A. (2000) Biomarkers of Oxidative Stress: An Analytical Approach. Current Opinion in Clinical Nutrition and Metabolic Care, 3, 373-384.
http://dx.doi.org/10.1097/00075197-200009000-00009 - 30. Farombi, E.O. and Fakoya, A. (2005) Free Radical Scavenging and Antigenotoxic Activities of Natural Phenolic Compounds in Dried Flowers of Hibiscus sabdariffa L. Molecular Nutrition & Food Research, 49, 1120-1128.
http://dx.doi.org/10.1002/mnfr.200500084 - 31. Valko, M., Izakovic, M., Mazur, M., Rhodes, C.J. and Telser, J. (2004) Role of Oxygen Radicals in DNA Damage and Cancer Incidence. Molecular and Cellular Biochemistry, 266, 37-56.
http://dx.doi.org/10.1023/B:MCBI.0000049134.69131.89 - 32. Kovacic, P., Pozos, R.S., Somanathan, R., Shangari, N. and O’Brien, P.J. (2005) Mechanism of Mitochondrial Uncouplers, Inhibitors, and Toxins: Focus on Electron Transfer, Free Radicals, and Structure-Activity Relationships. Current Medicinal Chemistry, 12, 2601-2623.
http://dx.doi.org/10.2174/092986705774370646 - 33. Miller, N.J., Sampson, J., Vandeias, L.P., Bramley, P.M. and Rice-Evans, C.A. (1996) Antioxidant Activities of Carotenes and Xanthophylls. FEBS Letters, 384, 240-242.
http://dx.doi.org/10.1016/0014-5793(96)00323-7 - 34. Rice-Evans, C.A. and Miller, N.J. (1995) Antioxidant—The Case for Fruit and Vegetables in the Diet. British Food Journal, 97, 35-40.
http://dx.doi.org/10.1108/00070709510100163 - 35. Schroeter, H., Boyd, C., Spencer, J.P.E., Williams, R.J., Cadenas, E. and Rice-Evans, C. (2002) MAPK Signaling in Neurodegeneration: Influences of Flavonoids and of Nitric Oxide. Neurobiology of Aging, 23, 861-880.
- 36. Fraga, C.G. (2007) Plant Polyphenols: How to Translate Their in Vitro Antioxidant Actions to in Vivo Conditions. International Union of Biochemistry and Molecular Biology Life (IUBMB LIFE), 59, 308-315.
http://dx.doi.org/10.1080/15216540701230529 - 37. Kodera, Y., Suzuki, A., Imada, O., Kasuga, S., Sumioka, I., Kanezawa, A., Taru, N., Fujikawa, M., Nagae, S., Masamoto, K., Maeshige, K. and Ono, K. (2002) Physical, Chemical, and Biological Properties of S-Allylcysteine, an Amino Acid Derived from Garlic. Journal of Agricultural and Food Chemistry, 50, 622-632.
http://dx.doi.org/10.1021/jf0106648 - 38. Okada, Y., Tanaka, K., Fujita, I., Sato, E. and Okajima, H. (2005) Antioxidant Activity of Thiosulfinates Derived from Garlic. Redox Report: Communications in Free Radical Research, 10, 96-102.
http://dx.doi.org/10.1179/135100005X38851 - 39. Chung, L.Y. (2006) The Antioxidant Properties of Garlic Compounds: Allyl Cysteine, Alliin, Allicin, and Allyl Disulfide. Journal of Medicinal Food, 9, 205-213.
http://dx.doi.org/10.1089/jmf.2006.9.205
NOTES
*Corresponding author.






