Food and Nutrition Sciences
Vol.3 No.12(2012), Article ID:25265,5 pages DOI:10.4236/fns.2012.312213
Effect of Cooking on the Oxalate Content of Selected Thai Vegetables
![]()
1Agro-Industry Department, Faculty of Agriculture and Technology, Rajamangala University of Technology Isan, Surin Campus, Nakhon Ratchasima, Thailand; 2Food Group, Agriculture and Life Sciences, Lincoln University, Canterbury, New Zealand.
Email: *savage@lincoln.ac.nz
Received September 26th, 2012; revised October 26th, 2012; accepted November 4th, 2012
Keywords: Total Oxalate; Soluble Oxalate; Insoluble Oxalate; Thai Vegetables
ABSTRACT
Total and soluble oxalate content levels were measured in thirteen selected vegetables purchased from a local market in Surin Province in the northeast of Thailand. Total oxalate contents of the leaves, shoots and roots of the fresh vegetables ranged from 249.5 ± 12.1 to 7597.9 ± 77.6 mg oxalate/100g dry matter (DM) while soluble oxalate content ranged from 205.0 ± 2.3 to 2677.6 ± 19.0 mg oxalate/100g DM. Very high levels of total oxalates were found in three of the selected Thai vegetables, Polygonum odoratum (7597.9 ± 77.6 mg/100g DM), Piper aurantaucum (7026.6 ± 76.9 mg/100g DM) and Limnophila aromatica (6179.0 ± 23.6 mg/100g DM). However, the soluble oxalate content of L. aromatica was low and the highest soluble oxalate contents of fresh vegetables were found in P. odoratum, P. aurantuacum and Neptunia oleracea at 2677.6 ± 19.0, 2152.2 ± 65.3 and 1640.8 ± 3.4 mg/100g DM, respectively. Boiling the vegetables reduced the soluble oxalate content between 30.4 and 65.0%. The insoluble oxalate levels increased in eleven of the cooked vegetables while small decreases were observed in L. aromatica and N. oleracea.
1. Introduction
Vegetables are an important part of the Thai diet. Some vegetables are commercially grown, some are grown in family gardens and some are only found in the forest. Vegetables are important sources of vitamins, minerals, dietary fibre, and antioxidants. However, some plants are well known to contain oxalates and these tend to occur in higher concentrations in the leafy parts of vegetables rather than in the roots or stalks [1,2]. Oxalic acid and its salts occur as end products of metabolism in a number of plant tissues. The consumption of high oxalate containing foods promotes oxaluria which leads to an increased risk of kidney stone formation [3] with the predominate type being composed mainly of crystals of calcium oxalate [3]. Reductions in the oxalate concentration in the urine can be achieved by avoiding foods which are known to contain high levels of oxalates [3], and by consuming a higher liquid intake to reduce the oxalate concentration of the urine [2,3]. In addition, soaking and cooking food leads to losses of soluble oxalates into the cooking water [1,4] resulting in less oxalates being absorbed.
Oxalic acid forms water-soluble salts with Na+, K+ and 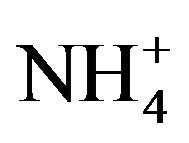 ions, it can also form insoluble oxalates by binding with Ca2+, Fe2+ and Mg2+ rendering these minerals unavailable [1]. A diet high in soluble oxalates is widely known to be associated with an increased risk of developing kidney stones the predominant type being composed mainly of crystals of calcium oxalate [5].
ions, it can also form insoluble oxalates by binding with Ca2+, Fe2+ and Mg2+ rendering these minerals unavailable [1]. A diet high in soluble oxalates is widely known to be associated with an increased risk of developing kidney stones the predominant type being composed mainly of crystals of calcium oxalate [5].
Commercially grown Thai vegetables such as Chinese convolvulus (Ipomoea reptans), Acacia pennata (Acacia pennata), and cultivated bamboo shoot (Bambusa spp.), contain more than 150 mg total oxalate/100g FW, while legume seeds such as soybeans (Glycine max) and peanuts (Arachis hypogaea) contain highest and moderate amounts of total oxalate, 204 ± 14 mg and 142 ± 35 mg/ 100g fresh weight (FW), respectively [6]. The soluble oxalate levels of leafy Thai vegetables ranged from < 3 to 110 mg/100g FW [6]. The total oxalate levels in some indigenous Thai fruits and vegetables such as Phyllanthus embrica, Musa sapientum, Careya sphaerica and Eugenai grata were 2056.4 ± 27.5, 421.1 ± 2.9, 226.7 ± 2.7 and 151 ± 2.5 mg/100g FW, respectively, while the soluble oxalate content ranged from 51.3 to 1238.1 mg/ 100g FW [7]. The distribution of oxalates in plants is also very variable. Some green leafy vegetables such as spinach, purple and green amaranth and colocasia contain very high oxalate levels [8].
Thai leafy vegetables are normally consumed in two ways, either fresh or after boiling, while taro corms are primarily consumed after being boiled, baked or fried. The water after boiling many vegetables is often discarded, thus, soluble oxalates that can be leached from the vegetable into the cooking water will be lost during cooking [1,9,10]. Losses of soluble oxalates in Pakistan vegetables ranged from 16% - 77% (mean 64.7%), the highest loss of soluble oxalates was observed when white beans were boiled (77%) [10]. These values are similar to the losses (5% to 80%) of soluble oxalate lost following boiling a wide range of Thai vegetables [6]. In other studies insoluble oxalates are also lost after boiling of plant foods, giving mean reductions of 24.8% [1] and 43.6% [10].
High oxalate foods should always be cooked to reduce the oxalate content; soaking a food prior to cooking will also reduce the oxalate contents by leaching [2]. There is little data published on the effect of cooking of Thai vegetables, therefore, this present study focused on the effect of cooking on the total, soluble and insoluble oxalate contents of some selected Thai vegetables grown during October 2011 in the northeast of Thailand.
2. Materials and Methods
2.1. Source of Sample Materials
The 13 fresh Thai vegetables used in this study were purchased from local markets in Surin Province in the northeast of Thailand between the middle and end of October 2011; details are shown in Table 1.
2.2. Sample Preparation
The vegetables were washed with tap water and edible parts of all the vegetables were selected for oxalate analysis. The vegetables were divided into two portions (fresh and cooked). The cooked portion of each vegetable was boiled for five min and then cooled in cold water; the water was discarded. Taro corms (C. esculenta) were pealed and boiled for 30 min or baked at 100˚C with the skin on for 1 hour. The cooked and fresh vegetables were then chopped into small pieces. All of the vegetables were vacuum dried for 24 h at 60˚C and then vacuum packed until analysis. The vacuum dried material was then ground into a fine powder in a coffee grinder (Sunbeam, model: EM0400, China). The powdered material was sealed in an aluminum foil bag until analysis could commence.
2.3. Extraction of Total and Soluble Oxalates
Total and soluble oxalate contents of each finely ground sample of dried material were extracted and measured by HPLC [1]. Ground sample (0.5 g) was mixed with 40 ml of 0.2 M HCl or nanopure water (Barnstead International, Dubuque, USA) for the extraction of the total and soluble oxalates, respectively. The conical flasks were covered with aluminium foil and incubated in a water bath at 80˚C for 20 min and then allowed to cool. The mixture was quantitatively transferred into 100 ml volumetric flask and the volume made up to 100 ml with 0.2 M HCl or distilled water. Each sample was filtered through a 0.45 µm syringe filter into the HPLC vial prior to HPLC analysis. The insoluble oxalate content was calculated by difference between the total and the soluble oxalate content [11]. Each sample was analysed in triplicate and all data are presented as a mean of mg oxalate/100g dried weight (DM) ± SD.
2.4. HPLC Conditions
Analysis of total and soluble oxalates was carried out using a 300 × 7.8 mm Rezex ion exclusion column (Phenomenex Inc., California, USA) attached to a cation h+ guard column (Bio-Rad, Richmond, California, USA), using an isocratic elution at 0.6 mL/min with 25 mM sulphuric acid (Baker Chemicals, Phillipsburg, NJ, USA) as a mobile phase. The analytical column was held at 25˚C and the column was equilibrated at a flow rate of 0.2 mL/ min prior to use and in between sample sets. Peaks were detected at 210 nm. The oxalate peak was eluted after 8.7 min and was identified by comparison of the retention time to a range of common plant organic acid standards. Standard curves were prepared using 0.2 M HCl and nanopure water for total and soluble oxalate; the r2 for the acid and water calibration curves was 0.997 and 0.998, respectively.
3. Results
The average dry matter of the thirteen indigenous Thai vegetables ranged from 3.2% in L. begonifolia to 24.3% in P. aurantuacum and oxalate contents (total, soluble and insoluble) are shown in Table 2.
Total oxalate contents of the fresh material ranged from 249.5 ± 12.1 mg/100g DM in M. cordifolia to 7597.9 ± 77.6 mg/100g DM in P. odoratum while soluble oxalate ranged from 205.0 ± 2.3 mg/100g DM in M. cordifolia to 2677.6 ± 19.0 mg/100g DM in P. odoratum. Insoluble oxalate levels in the dry matter of fresh plant varied from 2.0 ± 28.5 mg/100g DM (I. aquatica) to 4920.4 ± 61.2 mg/100g DM (P. odoratum) and only one plant (C. esculenta) contained no detectable levels of insoluble oxalates. The result showed very high total oxalate levels of fresh leaves and shoots in the dry matter of P. odoratum (7597.9 ± 77.6 mg/100g DM), P. aurantuacum (7026.6 ± 76.9 mg/100g DM) and L. aromatica (6179 ± 23.6 mg/100 mg/100g DM).
However, levels of soluble oxalate were considerably lower in L. aromatica compared with the other two high oxalate-containing plants (Table 2).
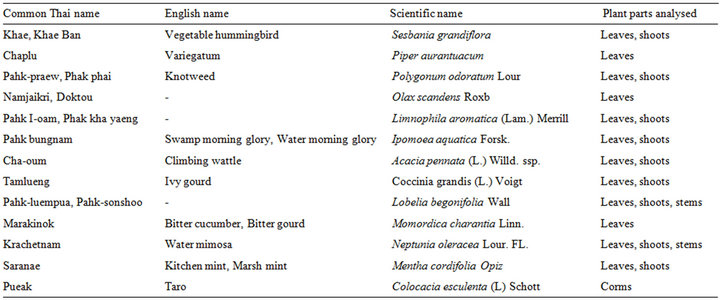
Table 1. Source of selected Thai vegetables.
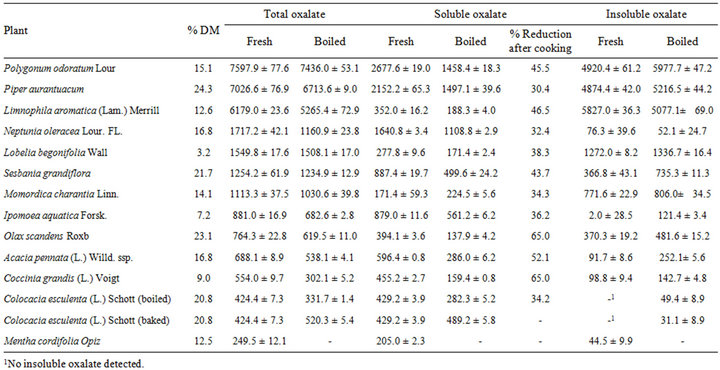
Table 2. Mean oxalate content of fresh and cooked selected Thai vegetables (mg oxalate/100g DM ± SD).
The percentage reduction in soluble oxalate content after cooking 12 of the vegetables ranged from 30.4 to 65.0% (mean 43.6%). The soluble oxalates in taro corms were reduced by 34.2% after boiling for 30 min. However, the soluble oxalate levels in taro coms that were then baked at 100˚C for 1 hour only showed a final loss of 21.8%. High soluble oxalate losses were observed in O. scandens (65.0%), C. grandis (65.0%), A. pennata (52.1%) followed by L. aromatica (46.5%) and P. odoratum (45.5%). These data suggest that household cooking is a very effective method to reduced soluble oxalate levels in almost all of the selected Thai vegetables. However, boiling for 5 min only reduced total oxalate in P. odoratum by 2.1%. No reduction of the insoluble oxalates in the selected Thai vegetables after boiling was observed except for L. aromatica and N. oleracea where, respectively, 12.9 and 31.7% reductions of insoluble oxalates occurred.
4. Discussion
Losses of total oxalates ranging from 22.4% to 70.3% after boiling for 12 - 15 min from vegetables such as spinach, carrots, beetroot, white bean, red bean and soy bean and losses of soluble oxalates following boiling for the same foods ranging from 15.6% to 76.9% have been reported from Pakistan [10]. Similar results have been reported previously for Thai, New Zealand and Wyoming, USA grown vegetables [1,4,6,12]. Boiling had a variable effect on Japanese grown vegetables, especially spinach [13].
The ratio of soluble oxalate to total oxalate of Chaoum (A. pinnata) after boiling (53.1%) was similar (55%) to an earlier report [6]. Our results are in keeping with those of other studies of Thai vegetables. This most recent study [7] went on to report that the high levels of soluble oxalate found in the fruits and juice of Indian gooseberry (P. emblica) could be reduced by soaking and cooking [7]. The measurement of oxalate contents in vegetables commonly consumed in New Zealand revealed that boiling reduces the oxalate contents of food by leaching losses into the cooking water [1].
The total oxalate contents of taro corms in this study falls within the range reported (278 - 574 mg/100g FW) for taro grown in a number of different Pacific Islands. [11]. .In our study boiling taro corms grown in Thailand for 30 min reduced the level of soluble oxalate by 34.2%, while in contrast, the soluble oxalate level of Japanese taro grown in New Zealand was reduced to below detectable levels after boiling for 40 min [14]. In our study the taro corms were boiled or baked which resulted in a final loss of only 21.8% total oxalates from the cooked corms even though no insoluble oxalate was found in the corms. The loss of moisture during baking may have concentrated the remaining oxalate in the cooked tissue. Boiling, therefore, might be a better way of cooking taro corms than boiling followed by baking [15,16]. L. aromatica was one of three vegetables found to contain high levels of total oxalates in our study (Table 2). However, the soluble oxalate content of the uncooked vegetable was lower than that of the other two vegetables and furthermore there was a 46.5% loss during boiling. Thus, L. aromatica is likely to be less important in terms of kidney stone formation and mineral availability than the other two high oxalate-containing vegetables P. odoratum and P. aurantuacum and the high soluble oxalatecontaining vegetable N. oleracea.
5. Conclusion
In this study two Thai vegetables (P. odoratum and L. aromatic) contained high levels of total and soluble oxalates and one (N. oleracea) a high level of soluble oxalates. These would be classified into Group 1 following the classification suggested earlier [2] even after reduction of oxalate levels in the foods following boiling. Foods in this group should be consumed in moderation and should not be consumed on a regular basis. All the remaining foods analysed in this study would be classified in Group 2 and after light cooking would pose no significant problems even if consumed regularly in larger amounts.
6. Acknowledgements
The authors wish to thank Rajamangala University of Technology Isan, Nakhon Ratchasima and Surin Campus, Thailand for its financial support.
REFERENCES
- G. P. Savage, L. Vanhanen, S. L. Mason and A. B. Ross, “Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods,” Journal of Food Composition and Analysis, Vol. 13, No. 3, 2000, pp. 201- 206. doi:10.1006/jfca.2000.0879
- S. Noonan and G. P. Savage, “Oxalate Content of Foods and Its Effect on Humans,” Asia Pacific Journal of Clinical Nutrition, Vol. 8, No. 1, 1999, pp. 64-74. doi:10.1046/j.1440-6047.1999.00038.x
- L. Massey, “Dietary Influences on Urinary Oxalate and Risk of Kidney Stones,” Frontiers in Bioscienes, Vol. 8, 2003, pp. 584-594. doi:10.2741/1082
- W. Chai and M. Liebman, “Effect of Different Cooking Methods on Vegetable Oxalate Content,” Journal of Agricultural Food Chemistry, Vol. 53, No. 8, 2005, pp. 3027- 3030.
- T. S. Simpson, G. P. Savage, R. Sherlock and L. P. Vanhanen, “Oxalate Content of Silver Beet Leaves (Beta vulgaris var. cicla) at Different Stages of Maturation and the Effect of Cooking with Different Milk Sources,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 22, 2009, pp. 10804-10808. doi:10.1021/jf902124w
- K. Judprasong, S. Charoenkiatkul, P. Sungpuag, K. Vasanachitt and Y. Nakjamanong, “Total and Soluble Oxalate Contents in Thai Vegetables, Cereal Grains and Legume Seeds and Their Changes after Cooking,” Journal of Food Composition and Analysis, Vol. 19, No. 4, 2006, pp. 340- 347. doi: org/10.1016/j.jfca.2005.04.002
- L. Vanhanen, G. P. Savage and C. Sangketkit, “Oxalate Content of Eleven Indigenous Thai Plants,” Journal of Food, Agriculture & Environment, Vol. 9, 2011, pp. 7-9.
- M. Brogren and G. P. Savage, “Bioavailability of Soluble Oxalate from Spinach Eaten with and without Milk Products,” Asia Pacific Journal of Clinical Nutrition, Vol. 12, No. 2, 2003, pp. 219-224.
- G. P. Savage, V. Nilzen, K. Osterberg and L. Vanhanen, “Soluble and Insoluble Oxalate Content of Mushrooms,” International Journal of Food Sciences and Nutrition, Vol. 53, No. 4, 2002, pp. 293-296.
- M. S. Akhtar, B. Israr, N. Bhatty and A. Ali, “Effect of Cooking on Soluble and Insoluble Oxalate Contents in Selected Pakistani Vegetables and Beans,” International Journal of Food Properties, Vol. 14, 2010, pp. 241-249.
- W. D. Holloway, M. E. Argall, W. T. Jealous, J. A. Lee and J. H. Bradbury, “Organic Acids and Calcium Oxalate in Tropical Root Crops,” Journal of Agricultural Food Chemistry, Vol. 37, No. 2, 1989, pp. 337-341. doi:10.1021/jf00086a014
- G. Jaworska, “Nitrates, Nitrites, and Oxalates in Products of Spinach and New Zealand Spinach,” Food Chemistry, Vol. 89, No. 2, 2005, pp. 395-401. doi:org/10.1016/j.foodchem.2004.02.030
- H. Ohkawa, “Gas Chromatographic Determination of Oxalic Acid in Foods,” Journal of the Association of Official Analytical Chemists, Vol. 68, No. 1, 1985, pp.108-111.
- D. J. Catherwood, G. P. Savage, S. M. Mason, J. J. C. Scheffer and J. A. Douglas, “Oxalate Contents of Cormels of Japanese Taro (Colocasia esculenta L. Schott) and the Effect of Cooking,” Journal of Food Composition and Analysis, Vol. 20, No. 3, 2007, pp. 147-151. doi:org/10.1016/j.jfca.2005.12.012
- P. E. Albihn and G. P. Savage, “The Effect of Cooking on the Location and Concentration of Oxalate in Three Cultivars of New Zealand-Grown Oca (Oxalis tuberosa Mol.),” Journal of the Science of Food and Agriculture, Vol. 81, No. 10, 2001, pp. 1027-1033.
- A. Albihn and G. P. Savage, “Effect of Cooking on the Soluble Oxalate Content of Three Cultivars of Oca (Oxalis tuberosa),” Proceedings of the Nutrition Society of New Zealand, Vol. 25, 2000, pp. 66-70.
NOTES
*Corresponding author.

