American Journal of Plant Sciences
Vol.07 No.07(2016), Article ID:66341,12 pages
10.4236/ajps.2016.77097
Role of Silver Nitrate on in Vitro Flowering and Shoot Regeneration of Solanum nigrum (L.)―An Important Multipurpose Medicinal Plant
G. Geetha, K. Harathi, C. V. Naidu*
Department of Biotechnology, Dravidian University, Kuppam, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 February 2016; accepted 8 May 2016; published 11 May 2016
ABSTRACT
Silver nitrate induced MS media enhanced multiple shoot regeneration and in vitro flowering from axillary bud and leaf explants of Solanum nigrum (L.) an important antiulcer medicinal plant. Healthy axillary bud and leaf explants were inoculated on MS medium supplemented with BAP/Kn (1.0 - 2.0 mg/l) in combination with IAA/NAA (0.5 mg/l) and AgNO3 (0.1 - 1.0 mg/l). The explants were responded effectively and good regeneration frequencies were observed in all the combinations of silver nitrate tested when compared to control. Maximum number of multiple shoots (34.3) was found in leaf explants cultured on MS media supplemented with BAP (2.0 mg/l), NAA (0.5 mg/l) and AgNO3 (0.4 mg/l). These regenerated shoots were sub-cultured on to the flowering media. Maximum number of in vitro flowers (12) was obtained from axillary bud explants in BAP (2.0 mg/l), Kn (1.0 mg/l), IAA (0.5 mg/l) and AgNO3 (6.0 mg/l) supplemented media when compared to leaf (4) and control. All the in vitro raised shoots were transferred to rooting medium supplemented with NAA, IBA (1.0 - 2.0 mg/l) and AgNO3 (0.1 - 0.6 mg/l). The best rooting response (24.6) was observed in 2.0 mg/l IBA + 0.4 mg/l AgNO3. The well rooted plantlets were transferred to polybags containing soil + vermiculate in 1:1 ratio for hardening and finally the hardened plantlets were transferred to field conditions.
Keywords:
In Vitro Flowering, Micropropagation, Silver Nitrate, Ethylene Inhibitor, Solanum nigrum

1. Introduction
Solanum nigrum (L.) commonly known as black night shade, an important medicinal plant of family Solanaceae. It is grown in dry parts of India up to an elevation of 2100 mts. It is an important herbaceous medicinal plant, generally used intraditional and folklore medicines. Commonly it is used as leafy vegetable in preparing traditional dishes. The herb is antiseptic, antidysentric and diuretic used in the treatment of cardiac, skin diseases, psoriasis, herpivirus and inflammation of kidney. The leaves, stems and roots are used externally as poultice; wash etc., in the treatment of cancerous soles, boils, leucoderma and wounds [1] . The fruits and leaves have been traditionally used against various nerve disorders [2] . Root bark is laxative, useful in the treatment of neck, burning of throat, inflammation of liver and chronic fever. Berries are bitter, pungent and are useful in the disease of heart, piles and dysentery [3] .
Most important aspect of this medicinal plant is that it contains two important alkaloids solamargin and solasonine which yield solasodineas glycone [4] . Solasodine has embryogenic, teratonic as well as antifungal, antiviral and molluscidal effects [5] . Solasodine has great demand in pharmaceutical industry; owing to its demand in pharma industry, the plant is extensively harvested. So it is necessary to establish an efficient protocol for in vitro propagation of this important herbaceous medicinal plant. In vitro flowering bears immense importance in selective hybridization especially in plants that use pollens from rare stocks. In vitro flowering may help in obviating the intricate interactions present in whole plants. It also facilitates in understanding the nature of factors which influences the flowering.
Ethylene is a ubiquitous gaseous hormone which influences the growth as promoter or inhibitor based on the species used [6] . It is a simple hydrocarbon (C2H4), but has maximum impact on growth, cellular differentiation, fruit ripening and senescence in plants even at concentrations as low as 0.01 µl∙L−1 or 10−6 % v/v [7] . Wounding during explants preparation, presence of high auxins and cytokinins concentration in media further increase ethylene production in in vitro conditions. So excess accumulation of gases directly influences the success of in vitro regeneration. Influence of ethylene in tissue culture propagation had been well documented previously. Ethylene suppresses the growth and morphogenesis of explants depending on the species and stage of the culture [8] . To overcome this problem, for the first time we had made an attempt to study the effect of AgNO3, ethylene inhibitor to enhance the shoot and root proliferation and in vitro flowering in Solanum nigrum.
2. Materials and Methods
2.1. Source of Plant Material
Healthy axillary bud and leaf explants of Solanum nigrum (L.) were collected from two-month-old seed germinated field grown plants growing in the Herbal garden of Dravidian University, Kuppam, Andhra Pradesh, India.
2.2. Surface Sterilization
Explants were washed thoroughly under running tap water to remove traces of dust etc. followed by treatment with 10% teeepol or tween −20 for 5 minutes and 0.4% bavistine (fungicide) for 10 - 15 minutes. Then the explants were sterilized in 70% alcohol for a minute, and finally with 0.01% mercuric chloride for 1 - 2 minutes and washed 3 - 4 times with sterile double distilled water.
2.3. Culture Medium
The explants were inoculated on MS medium [9] containing 3% sucrose and gelled with 0.8% agar, supplemented with various concentrations of BAP, Kn in combination with IAA or NAA and AgNO3. The pH of the medium was adjusted to 5.8 before gelling with agar and autoclaved for 20 minutes at 121˚C and 15 lbs pressure.
2.4. Sub Culturing
The cultures were maintained by regular subculture at 4 week intervals on fresh MS medium.
2.5. Culture Conditions
All cultures of Solanum nigrum were maintained in a culture room at temperature of 24˚C ± 2˚C and 55% - 65% RH with 16 h/8h photoperiod at a photon flux density of 3000 lux or 50 - 70 Em−2∙s−1 provided by cool white fluorescent tubes.
2.6. Data Collection and Statistical Analysis
Visual observations were recorded on the frequency in terms of number of cultures responding for axillary shoot proliferation, shoot development, number of shoots per explant, average length of the regenerated shoots, number of flowers and number of roots per shoot and average root length.
Despite scarcity and limitations encountered with the plant material, for most of the treatment a minimum of 10 replicates were used. All the experiments were repeated at least twice/thrice and the cultures were observed at regular intervals. The qualitative data were subjected to statistical analysis by using standard error (SE±) for shoot length, rate of shoot multiplication and then number of roots per shoot.
3. Results and Discussion
3.1. Effect of Silver Nitrate on Axillary Bud Induction and Multiplication
Axillary buds excised from field grown plants of two month old have shown shoot proliferation response. Efforts have been made to study the synergetic effect of AgNO3 along with various concentrations of cytokinins such as BAP, Kn in combination with auxins such as NAA and IAA. In axillary bud explants shoot buds initiated after 7 - 8 days of inoculation on MS medium supplemented with various plant growth regulators along with silver nitrate with different concentration of BAP (1.0 - 2.0 mg/l) and Kn (1.0 - 2.0 mg/l). Observations recorded in Table 1 and Table 2 signifies, 0.4 mg/l AgNO3 at all cytokinin and auxin concentrations initiated better response in relation to shoot regeneration and also induced higher number of shoots per explants. Significantly very high shoot regeneration frequency (95%) was observed in 0.4 mg/l AgNO3 supplemented media along with 2.0 mg/l BAP and 0.5 mg/l NAA and the highest number of multiple shoots (29.5 ± 0.40), whereas further increase in the concentration of AgNO3 supplementation in the media had resulted in poor regeneration frequency (60% at 1 mg/l AgNO3, BAP 1.0 mg/l and NAA 0.5 mg/l). The frequency, number of multiple shoots induced in this combination were less when compared to BAP (2.0 mg/l), IAA (0.5 mg/l) and AgNO3 (0.4 mg/l) (20.7 ± 0.70) respectively. Results considerably enlighten the fact that presence of silver nitrate in the media has increased number of shoots per explants over three folds as compared to those formed on control. Similar type of enhancement was observed in Coffea arabica [10] [11] and Vanilla planifolia [12] .
Supplementation of 0.4 mg/l AgNO3 in the media had effectively enhanced the shoot length similar to multiple shoot regeneration. The maximum shoot length was observed in AgNO3 (0.4 mg/l), BAP (2.0 mg/l) and NAA (0.5 mg/l) (12.7 ± 0.85 cm) and the same concentration in case of IAA (0.5 mg/l) the shoot length was (8.46 ± 0.05 cm) respectively. Multiple shoots can be sub cultured repeatedly by excising the shoots from the cluster and transferring them on to fresh medium with four weeks passage time. Large clumps of multiple shoots were separated and sub cultured as small clusters for further proliferation in nutrient media supplemented with different concentrations and combinations of AgNO3, cytokinins (BAP, Kn) and auxins (IAA, NAA). Between the two cytokinins tested, BAP was found to be more effective than Kn in the induction of multiple shoots from the axillary bud explants. AgNO3 at the concentration of 0.4 mg/l in the media had efficiently improved the shoot regeneration in axillary bud cultures, hence further experiments were conducted with the same concentration (0.4 mg/l) of AgNO3 (Figure 1).
3.2. Effect of Silver Nitrate on Direct Organogenesis from Leaf Explants
Effect of two different cytokinins (BAP, Kn) in combination with auxins (NAA, IAA) along with ethylene inhibitor AgNO3 on direct shoot induction was examined (Table 3, Figure 2). In leaf explants after three weeks of inoculation leaf folding, leaf enlargement were noticed. Shoot bud primordia were emerged at the cut portions and midrib regions of young leaf explants, later they were developed as shoots. Silver nitrate is added to the culture medium with growth regulators significantly improved the initial shoot regeneration. In BAP (1.0 - 3.0 mg/l), NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) combinations multiple shoots were induced with mean shoot number in the range of (17.3 - 34.3) and the mean shoot length of (4.3 - 10.3 cms) respectively. The highest number of shoots (34.3 ± 0.25) were obtained in BAP (2.0 mg/l), AgNO3 (0.4 mg/l) and NAA (0.5 mg/l) supplemented medium. Similar results were observed in Brassica napus [13] , Gloxinia (Sinnigia speciosa) [14] .
Table 1. Effect of different concentrations of BAP, NAA and IAA in combination with AgNO3 on multiple shoot regeneration from axillary bud explants of field grown Solanum nigrum plants. Observation: After 8 weeks, values are mean ± S.E. of 20 independent determinants; Intensity of callus: C+ = very low, C++ = low.
In Kn-AgNO3-NAA/IAA combination less number of multiple shoots formed when compared to BAP combination. Silver nitrate at 0.4 mg/l significantly enhanced shoot regeneration compared with higher concentrations. Chraibi et al. [15] have shown similar results from cotyledons of Sunflower. Most of the plant tissues have to produce ethylene. The effect of ethylene on in vitro culture, as with other hormones, depends on its concentration in and around the cultured tissues, as well as their sensitivity to it [16] . Silver nitrate is usually employed in in vitro studies for inhibiting ethylene action. In silver nitrate, Ag+ ions can prevent a wide diversity of ethylene induced plant responses, the growth inhibition the effect was assumed to mediate the inhibition of the physiological action of ethylene [17] , a potential inhibitor of many plant regeneration systems.
Table 2. Effect of different concentrations of Kn, NAA and IAA in combination with ethylene inhibitor AgNO3 on multiple shoot regeneration from axillary bud explants of field grown Solanum nigrum plants. Observation: After 8 weeks, values are mean ± S.E. of 20 independent determinants; Intensity of callus: C+ = very low, C++ = low.
3.3. Effect of Silver Nitrate on in Vitro Flowering from Axillary Bud and Leaf Explants
The multiple shoots formed from different explants like axillary buds and leaves, showed in vitro flowering on MS medium supplemented with different combinations and concentration of cytokinins such as BAP/Kn (1.0 - 2.0 mg/l), AgNO3 (2.0 - 8.0 mg/l) and auxins IAA/NAA (0.5 mg/l) (Table 4, Figure 3). This exogenous hormone supply might have been added up to the endogenous contents, raising the hormonal level required for triggering the flowering. In the present investigation flowering has been induced by the presence of lower concentrations of auxin (0.5 mg/l IAA) and higher concentrations of cytokinin (1 mg/1 BAP) along with the presence of additional nitrate in the form of silver nitrate. Cytokinin promotes the transition in some higher plants to
Table 3. Effect of different concentrations of BAP, Kn, NAA and IAA in combination with AgNO3 on direct multiple shoot organogenesis from leaf explants of field grown Solanum nigrum plants. Observation: After 8 weeks, values are mean ± S.E. of 20 independent determinants; Intensity of the callus: C+ = very low, C++ = low.
their reproductive stage in vitro.
The first flower bud was observed in axillary bud culture after three weeks of sub culturing on to a fresh medium MS medium supplemented with BAP (1.0 mg/l), AgNO3 (2.0 mg/l) and NAA (0.5 mg/l). Maximum number of in vitro flowers (12) per culture were obtained in (2.0 mg/l) BAP, Kn (1.0 mg/l), AgN03 (6.0 mg/l) and IAA (0.5 mg/l) supplemented MS medium. In the present study IAA in combination with Kn also induced in vitro flowers. The flower buds were appeared after four weeks of subculture on to a fresh MS medium along with silver nitrate. Similar results were obtained in Rosa indica [18] . The addition of AgN03 in the medium along with low concentration of auxin induced in vitro flowering. The number of flowers formed in silver nitrate treated plants was more when compared to control ones (without silver nitrate).
In leaf culture in vitro flower initiation was observed after 5 - 6 weeks of culture on MS medium along with silver nitrate supplemented with BAP (1.0 m/l), AgNO3 (2.0 - 8.0 mg/l) and IAA (0.5 mg/l). Maximum number of in vitro flowers (4) per culture were obtained in BAP (1.0 mg/l), AgNO3 (6.0 mg/l) and IAA (0.5 mg/l). But the number of flowers (12) produced from axillary bud explants was more when compared to leaf (4) explants and control.
Table 4. Effect of different concentrations of BAP, Kn, NAA and IAA in combination with AgNO3 on in vitro flowering from axillary bud and leaf explants of Solanum nigrum. Observation: After 8 weeks, values are mean ± S.E. of 20 independent determinants. =No flowering.
Figure 1. Direct shoot organogenesis from axillary bud explants: (a) Shoot intiation from axillary bud explants cultured on MS media containing BAP (1.0 mg/l), NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) (b) Shoot initiation from explants on MS media with BAP (2.0 mg/l), IAA (0.5 mg/l) and AgNO3 (0.4 mg/l) (c) Formation of multiple shoots in MS + Kn (1.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.1 mg/l) supplemented media (d) Formation of multiple shoots in MS + BAP (2.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) media (e), (f) Elongation of multiple shoots on MS + Kn (2.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) media.
Figure 2. Direct shoot regeneration from leaf explants: (a) Shoot intiation from leaf explants cultured on MS media containing BAP (1.0 mg/l), NAA (0.5 mg/l) and AgNO3 (0.4 mg/l). (b) Shoot initiation from explants on MS media with BAP (2.0 mg/l), IAA (0.5 mg/l) and AgNO3 (0.4 mg/l) (c) Formation of multiple shoots in MS + Kn (1.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.1 mg/l) supplemented media. (d) Formation of multiple shoots in MS + BAP (2.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) media (e), (f) Elongation of multiple shoots on MS + Kn (2.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (0.4 mg/l) media.
Figure 3. In vitro flowering from axillary bud and leaf explants: In vitro flower initiation from axillary bud explants cultured on. (a) MS + BAP (1.0 mg/l) + NAA (0.5 mg/l) and AgNO3 (2.0 mg/l) (b) MS + BAP (2.0 mg/l) + IAA (0.5 mg/l) and AgNO3 (4.0 mg/l) (c) MS + BAP (2.0 mg/l) + Kn (1.0 mg/l) + IAA (0.5 mg/l) and AgNO3 (6.0 mg/l). In vitro flower initiation from leaf explants cultured on. (d), (e) MS + BAP (1.0 mg/l) + IAA (0.5 mg/l) and AgNO3 (2.0 mg/l) F) MS + BAP (1.0 mg/l) + IAA (0.5 mg/l) and AgNO3 (6.0 mg/l).
3.4. Effect of Silver Nitrate on in Vitro Rooting
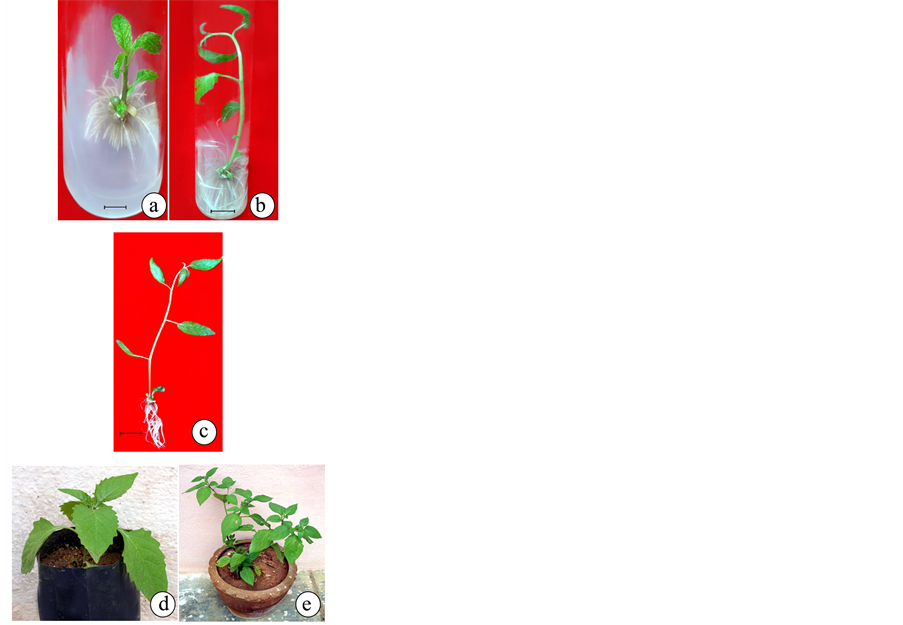
The shoots developed in vitro (3.0 - 5.0 cm) were excised from shoot clumps and transferred to MS medium supplemented with silver nitrate and different concentrations of auxins such as IBA, NAA and IAA. A marked difference with declined rate of rooting was observed (Table 5, Figure 4), when culture was supplemented with either lower or higher concentrations of auxins. Similar to the other parameters under study, rooting of in vitro grown shoots also responded well in the presence of 0.4 mg/l AgNO3 which was almost enhanced by 2 to 2.5 folds compared to control. In all the concentrations tested, root primordia appeared between 6 - 10 days after transferred in vitro raised shoots to rooting medium. When in vitro shoots transferred to MS media fortified with IBA, NAA, IAA and AgNO3 95% of rooting frequency with highest number of roots (24.6 ± 0.26) were obtained in the IBA (2.0 mg/l) and AgNO3 (0.4 mg/l) and maximum mean root length (6.5 ± 0.36 cm) was observed. But plenty of hairy root emergence was observed when auxin concentration in the media increases.
Table 5. Effect of AgNO3 on different concentrations of IBA, NAA and IAA on in vitro rooting using MS medium. Observation: After 8 weeks, values are mean ± S.E. of 20 independent determinants.
Figure 4. Rooting and acclimatization of Solanum nigrum: (a), (b) Root formation from shoot lets inoculated on MS media with IBA (1.0 mg/l) and AgNO3 (0.4 mg/l) (c) Plantlet showing elongated root system (d) Hardened plantlet in polybags containing soil and vermiculate in 1:1 ratio (e) Plantlet in field condition.
Presence of silver nitrate in in vitro propagation showed marked significance in almost all parameters under present investigation. Media without silver nitrate showed lesser response. This may be due to presence of silver nitrate, which might have suppressed the activity of excess of ethylene present in the in vitro culture tubes and further promoted for easy translocation and assimilation of these energy sources available in the media by the explants resulting in cell division and leading to vigorous growth. Similar type of results was documented in Decalepsis hamiltonii [19] , Vanilla planifolia [20] .
3.5. Acclimatization and Hardening
The well rooted shoots were removed from the culture tubes and washed thoroughly to remove the traces of agar. Acclimatization of the regenerated plants to the external environment is the last and very important stage of micropropagation and its success depends upon different factors as suggested by various researchers [21] [22] . The plantlets of in vitro grown Sphaeranthus indicus with well developed roots and shoots were transplanted to plastic cups containing autoclaved vermiculite and soil (1:1). About 90% of the transplanted plantlets survived after acclimatization and showed healthy growth without any morphological variations. Finally after one month the hardened plants were transferred to pots containing garden soil and sand (2:1) and were allowed to grow under nursery shade conditions. These plants were watered at 3 days intervals and were finally planted in field condition. All the plantlets were phenotypically indistinguishable from the parent plants.
4. Conclusion
In the present investigation, an attempt has been made to study the effect of AgNO3 on in vitro flowering and multiple shoot regeneration for the first time in medicinal plants like Solanum nigrum. Addition of AgNO3 along with low concentrations of cytokinins and auxins in the medium induced in vitro flowering. The present study may offer to achieve better genetic varieties of plants which fail to produce seeds.
Cite this paper
G. Geetha,K. Harathi,C. V. Naidu, (2016) Role of Silver Nitrate on in Vitro Flowering and Shoot Regeneration of Solanum nigrum (L.)—An Important Multipurpose Medicinal Plant. American Journal of Plant Sciences,07,1021-1032. doi: 10.4236/ajps.2016.77097
References
- 1. Moerman. (1998) Native American Ethano Botany. Timber Press, Oregon.
- 2. Perez, G.R.M., Perez, L.A., Garcia, D.L.M. and Sossa, M.H. (1998) Neuropharmacological Activity of Solanum nigrum (L.) Fruit. Journal of Ethanopharmacology, 62, 43-48.
http://dx.doi.org/10.1016/S0378-8741(98)00059-2 - 3. Kritikar, K.R. and Basu, B.S. (1987). Indian Medicinal Plants, 3, 1781-1784.
- 4. Anonymous (1972) Wealth of India. Vol.9, CSIR, New Delhi, India.
- 5. Kim, Y.C., Che, Q.M., Gunatilaka, A.A. and Kingston, D.G. (1996) Bioactive Steroidal Alkaloids from Solanum umbelliferum. Journal of Natural Products, 59, 283-285.
http://dx.doi.org/10.1021/np960125a - 6. Biddington, N.L. (1992) The Influence of Ethylene in Plant Tissue Culture. Plant Growth Regulation, 11, 173-178.
http://dx.doi.org/10.1007/BF00024072 - 7. Reid, M.S. (1995) Ethylene in Plant Growth, Development, and Senescence. In: Davis, P.J., Ed., Plant Hormones, Kluwer-Academic Publishers, Dordrecht, 486-508.
http://dx.doi.org/10.1007/978-94-011-0473-9_23 - 8. Kumar, P.P., Lakshmanan, P. and Thorpe, T.A. (1998) Regulation of Morphogenesis in Plant Tissue Culture by Ethylene. In Vitro Cellular and Developmental Biology-Plant, 34, 94-103.
http://dx.doi.org/10.1007/BF02822771 - 9. Murashige, T. and Skoog, F. (1962) A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Cultures. Plant Physiology, 15, 473-497.
http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x - 10. Ganesh, D.S. (2000) Standardization of Micropropagation Techniques I Some Selected Varieties of Coffee Using Apical Bud and Auxiliary Bud Cultures. PhD Thesis, University of Mysore, India.
- 11. Ganesh, D. and Sreenath, H.L. (2008) Micropropagation of Coffea arabica Using Apical Buds of Mature Field Grown Plants. Journal of Plant Crops, 36, 1-7.
- 12. Sankar, A., Libinmary, S., Vijaykumar, A., Karthi Rani, R., Raja Selvam, J., Kohila, R., Liby, I., Vadivukarasi, S. and Ganesh, D. (2008) Phloroglucinol Enhances Shoot Proliferation in Nodal Explants of Vanila planifolia Andr. Journal of Plant Crops, 36, 127-131.
- 13. Akasaka-Kennedy, Y., Yoshida, H. and Takahata, Y. (2005) Efficient Plant Regeneration from Leaves of Rapeseed (Brassica napus L.): The Influence of AgNO3 and Genotype. Plant Cell Reports, 24, 649-654.
http://dx.doi.org/10.1007/s00299-005-0010-8 - 14. Park, E.H., Bae, H., Park, W.T., Kim, Y.B., Chae, S.C. and Park, S.U. (2012) Improved Shoot Organogenesis of Gloxinia (Sinningia speciosa) Using Silver Nitrate and Putrescine Treatment. Plant Omics, 5, 6-9.
- 15. Chraibi, B.K.M., Latche, A., Raustan, J.P. and Fallot, J. (1991) Stimulation of Shoot Regeneration from Cotyledons of Helianthus annuus by Ethylene Inhibitors Silver and Cobalt. Plant Cell Reports, 10, 204-207.
- 16. Thorpe, T.A. (1994) Morphogenesis and Regeneration, ln: Vasil, I.K. and Thorpe, T.A., Eds., Plant Cell and Tissue Culture, Kluwer Academic Publishers, Dordrecht, 17-36.
- 17. Beyer, E.M., Page, Y.M. and Yang, S.F. (1984) Ethylene. In: Wilkins, M.B. Ed., Advanced Plant Physiology, Pitman, London, 111-115.
- 18. Pratheesh, P.T. and Kumar, M.A. (2012) In Vitro Flowering in Rosa indica L. International Journal of Pharma and Bio Sciences, 2, 196-200.
- 19. Bais, H.P., Sudha, G., Suresh, B. and Ravishankar, G.A. (2000) Silver Nitrate Influences in Vitro Root Formation in Decalepis hamiltonii Wight & Arn. Current Science, 79, 894-898.
- 20. Giridhar, P., Reddy, B.O. and Ravishankar, G.A. (2001) Silver Nitrate Influences in Vitro Shoot Multiplication and Root Formation in Vanilia planifolia Andr. Current Science, 81, 1166-1170.
- 21. Bhojwani, S.S. and Razdan, M.K. (1983) Plant Tissue Culture: Theory and Practice. Elsevier Science Publishing, Amsterdam, 194.
- 22. George, E.F. and Sherrington, P.D. (1984) Plant Propagation by Tissue Culture: A Hand Book and Dictionary of Commercial Laboratories. Exgetics Ltd., England.
Abbreviations
BAP 6-benzyl amino purine
Kn Kinetin
AgNO3 Silver nitrate
NAA α-naphthalene acetic acid
IAA Indole-3-acetic acid
IBA Indole-3-butyric acid
MS Murashige and Skoog
NOTES
*Corresponding author.