American Journal of Plant Sciences
Vol.5 No.4(2014), Article ID:43251,10 pages DOI:10.4236/ajps.2014.54058
Effects of Bradyrhizobium japonicum Inoculation and Supplementation with Phosphorus on Macronutrients Uptake in Cowpea (Vigna unguiculata (L.) Walp)*
![]()
School of Life Sciences and Bioengineering, The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania.
Email: #ndakidemipa@gmail.com
Copyright © 2014 Daniel Nyoki, Patrick A. Ndakidemi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Daniel Nyoki, Patrick A. Ndakidemi. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received October 17th, 2013; revised January 7th, 2014; accepted January 17th, 2014
KEYWORDS
Mineral Elements; Bioavailability; Calcium; Magnesium; Sodium; NPK; Rhizosphere
ABSTRACT
The current study was conducted to assess the effects of phosphorus supplementation and Bradyrhizobium japonicum inoculation on the availability and uptake of N, P, K, Mg, Ca and Na on cowpea. The experiment was laid out in a split plot design where the main plots comprised two inoculation levels (with and without inoculation of B. japonicum ) and sub plots contained four different levels of phosphorus (0, 20, 40, and 80 kg P/ha). The results indicated that B. japonicum inoculation and phosphorus supplementation significantly improved the uptake of N, P, K, Mg, Ca and Na in different cowpea tissues such as roots, pods, shoots, and whole plant relative to the control. The results also indicated that there was a significant interaction of B. japonicum and phosphorus on the uptake of sodium in the roots and whole cowpea plant in the screen house. Rhizobia inoculation and supplementation of phosphorus at 40 kg P/ha resulted in the improved uptake of the most elements over other treatments tested.
1. Introduction
Plants, like all other living things, need food for their growth and development. Plants require both primary and secondary macro elements such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) and sodium (Na) for their growth, development and food production. These elements are supplied either from soil minerals and soil organic matter or by organic or inorganic fertilizers [1,2]. Availability and uptake of these elements are very important for plant growth and development especially in the depleted soils of Sub Saharan Africa. According to [3] nutrient uptake by plants depends largely in the amount, concentration and activities in the rhizosphere soil as well as the capacity of soil to replenish in the soil solution.
Nitrogen is an important macro-element getting involved in metabolic activities and protein production leading to increased vegetative and reproductive growth and finally yield of the crops [4]. Soil microorganisms such as B. japonicum inoculants and other plant growth promoting rhizobacteria are reported to influence the chemistry of soils nutrients in many ways and enhance nutrients uptake by plants [5]. In the last few years, a number of rhizobium inoculants have been developed and are primarily used for supplying nitrogen to plants [1]. For example Bradyrhizobium are reported to establish symbiotic relationship with legume where they fix nitrogen that is important for the plan growth and in turn the plant provide them with carbohydrates as their source of energy [5]. On top of atmospheric nitrogen fixation, rhizobia inoculation have been reported to improve other plant nutrition such as phosphorus nutrition by mobilizing inorganic and organic phosphorus from organic and inorganic sources in the soil rhizopshere [5,6]. Rhizobium, a soil microorganism which is capable of forming a symbiotic relationship with legumes such as cowpea (Vigna unguiculata (L.) Walp.) can fix atmospheric N2 and change it into a form that can be used by the associated plants, and hence nitrogen uptake enhanced [3]. In their study conducted at glass house and field experiments [3] reported that rhizobia inoculation significantly increased the uptake of P, K, Ca, and Mg in Phaseolus vulgaris plant parts and attributed the improved uptake to increased soil pH which favored positively the availability of most mineral elements.
Phosphorus plays a major role in energy storage and transfer as ADP and ATP (adenosine diand triphosphate) and DPN and TPN (diand triphosphopyridine nucleotide). This energy (ATP) is very important in the process of nitrogen fixation as is required to break the triple bond that exists between N atoms in N2. Phosphorus is a component of the RNA and DNA structures, which are the major components of genetic information. Phosphorus also aids the plants in root development and it increases seed yields [2] and also enhances root nodulation [7]. Others workers have reported that phosphorus application influences the content of others nutrients in leaves and seeds [8]. Research done in south western Nigeria by [9] revealed that application of the two local phosphate rocks (Ogun Phosphate Rock and Crystallizer super) tested along with water soluble phosphate, both proved to be effective in enhancing the phosphorus and calcium uptake by maize and cowpea. The comparison of inoculated and un-inoculated plants also indicated significant increase (18.88%) of phosphorus contents in grains over un-inoculated plants. The interaction between phosphorus levels and inoculation was also significant [10]. Supplementation of phosphorus and organic inputs can significantly replenish phosphorus and nitrogen in the depleted soil and improve their uptake by plants [11].
Despite the research done on the macronutrient uptake by plants, there is still little literature about the role that is played by cowpea rhizobia inoculants and phosphorus on the availability of other nutrients in legume crops. Based on these facts, it is therefore important to establish the possible role which could be played by cowpea B. japonicum inoculants and phosphorus on the availability of macronutrient in legumes such as cowpea.
2. Material and Methods
2.1. Study Location
First, the field and screen house experiments were conducted at two different locations from mid March to late July 2013. The field experiment was conducted at the Tanzania Coffee Research Institute situated in an area which is 1390 m above the sea level in Kilimanjaro region, Tanzania of latitude (3˚14'44"S) and longitude (37˚14'48E). The field experiment was conducted in an area with bimodal rainfall pattern and mean annual rainfall of 1200 mm. A screen house experiment was conducted at Seliani Agricultural Research Institute (SARI), situated in an area which is 1390 m above the sea level in Arusha, Tanzania of latitude 3˚21'50.08"S and longitude 36˚38'06.29"E.
2.2. Experimental Design
The experiment was arranged in a split plot design. The main plots comprised two inoculation treatments namely. 1) Without inoculation of B. japonicum and 2) with inoculation of B. japonicum. The B. japonicum inoculants were purchased from MEA Fertilizer Company in Nairobi, Kenya. The inoculants packets were supplied with gum Arabic for sticking as many cells as possible into the seeds. Sub plots included application of phosphorus (Triple Super Phosphate 46% P2O5) at four levels (0, 20, 40, and 80 kg P∙ha−1). Both experiments were replicated four times.
2.3. Inoculation Procedure
The crop plant used for this experiment was Cowpea (Vigna unguiculata (L.) Walp.) supplied by the breeder from Sokoine University of Agriculture, Morogoro, Tanzania. The B. japonicum inoculants were inoculated according to manufacturers’ guidelines as follows: three (3) gram of gum Arabic was added to two tablespoonful of water and mixed to form a solution. 1 kg of cowpea seeds was weighed and 2 tablespoonful of gum Arabic solution was added and mixed well. 10 gm of legume inoculants was added and mixed well so that all seeds were coated. The inoculated seeds were put under shade to avoid drying, and the seeds were then sown immediately in a moist soil.
2.4. Field and Screen House Preparation and Management
Prior to the experiment, the soil was sampled and analyzed to identify the macronutrients available in the soil and also to determine the soil pH. The field was ploughed and harrowed by using tractor before planting. The crop was seeded at a spacing of 50 cm by 20 cm, where the plot size was 4 m by 3 m. In the field trial, three seeds were seeded per hill and then thinned to two plants. The plots were weeded twice where the first weeding was done two weeks after emergence and the second weeding was done just before flowering. Each plot comprised of six rows. Data were collected from the four middle rows. The soil for screen house experiment was collected from the site where field experiment was conducted. The soil was packed into 4 kg pots where four seeds were germinated in each pot, and later thinned to two after germination and uniformly established. Both experiments were planted at the mid of March 2013, and closely monitored from this point until physiological maturity for field, and pod formation for screen house experiment.
2.5. Plant Harvest and Sample Preparation
At 50% pod formation, the cowpea (Vigna unguiculata (L.) Walp.) was sampled for dry matter and nutrient determination. Plants were excavated carefully from the soil with their entire root system, washed, and separated into roots, shoots and pods. The plant organs were ovendried at 60˚C for 48 hrs, weighed and ground into a fine powder for nutrient analysis.
2.6. Measurement of Macro Elements in Plant Tissues
Total N was determined by the micro-Kjeldahl method [12]. Phosphorus was determined by the molybdenum blue method as described by [13]. Ca, Mg, K and Na concentrations in plant extracts were determined by method described in [14].
2.7. Statistical Analysis
The statistical analysis was performed using the 2-way analysis of variance (ANOVA) in factorial arrangement, with the computations being performed with the software program STATISTICA. The fisher’s least significance difference (L.S.D.) was used to compare treatment means at p = 0.05 level of significance [15].
3. Results
3.1. Soil Analysis Results
The results presented in Table 1 shows the physical and chemical properties of the soil. It is evident that soil nutrients were low to support proper growth of the crops (Table 1).
3.2. Effects of B. japonicum Inoculation on Macronutrient Uptake in Different Plant Tissues
This study showed a significant differences on the uptake of nutrients such as N, P, K, Ca, Mg and Na in the roots, pods and shoots of V. unguiculata following B. japonicum inoculation in both screen house and field experiments over the control treatment. Inoculation of cowpea with B. japonicum significantly increased the amount of N, P, Mg and Na in the roots of cowpea grown under screen house (Table 2). Furthermore, the amount of N, P, K and Na was increased in the roots of cowpea grown under field condition by inoculating with B. japonicum relative to the un-inoculated treatments (Table 2). However, B. japonicum inoculation had no significant effects on the uptake of K and Ca in the rots of cowpea grown under screen house and uptake of Mg and Ca in the roots of cowpea grown in the field condition (Table 2). The results in Tables 2-4 indicated that inoculation with B. japonicum significantly increased the uptake of N, P, K, Mg, Ca and Na in the pods, shoots and in whole cowpea plant grown in both screen house and field condition relative to the control treatment.
3.3. Effects of Phosphorus on Macronutrient Uptake in Different Plant Tissues
Supplementation of phosphorus at different levels had significant effects on the uptake of N, P, K, Mg, Ca and Na in different tissues of cowpea grown under the screen house and field condition over the control. For example, the results in Table 2 showed that the uptake of N, P, K and Na in the roots of cowpea grown under both screen house and field condition was significantly enhanced by supplementation of phosphorus at different levels relative to the control. Supplementation of phosphorus had no significant difference in the uptake of Mg and Ca in the roots of cowpea in both the screen house and field experiment compared with the control. The results in Table 2 indicated that most nutrients were enhanced by supplementation of phosphorus at 40 kg P/ha. Supplementing phosphorus at 80 kg P/ha numerically but not statistically decreased the uptake of most macro elements in the roots of cowpea relative to 40 kg P/ha which showed significant effects in enhancing the macro-element uptake in
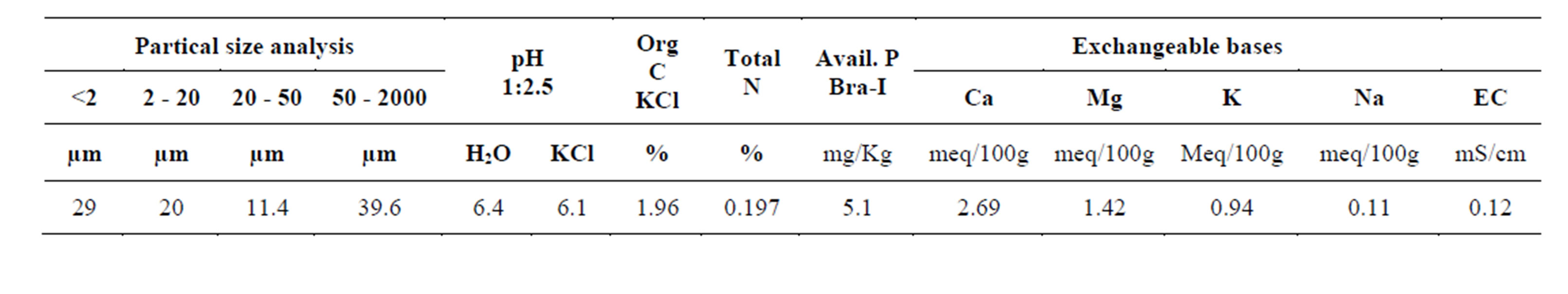
Table 1. Physical and chemical properties of the soil.
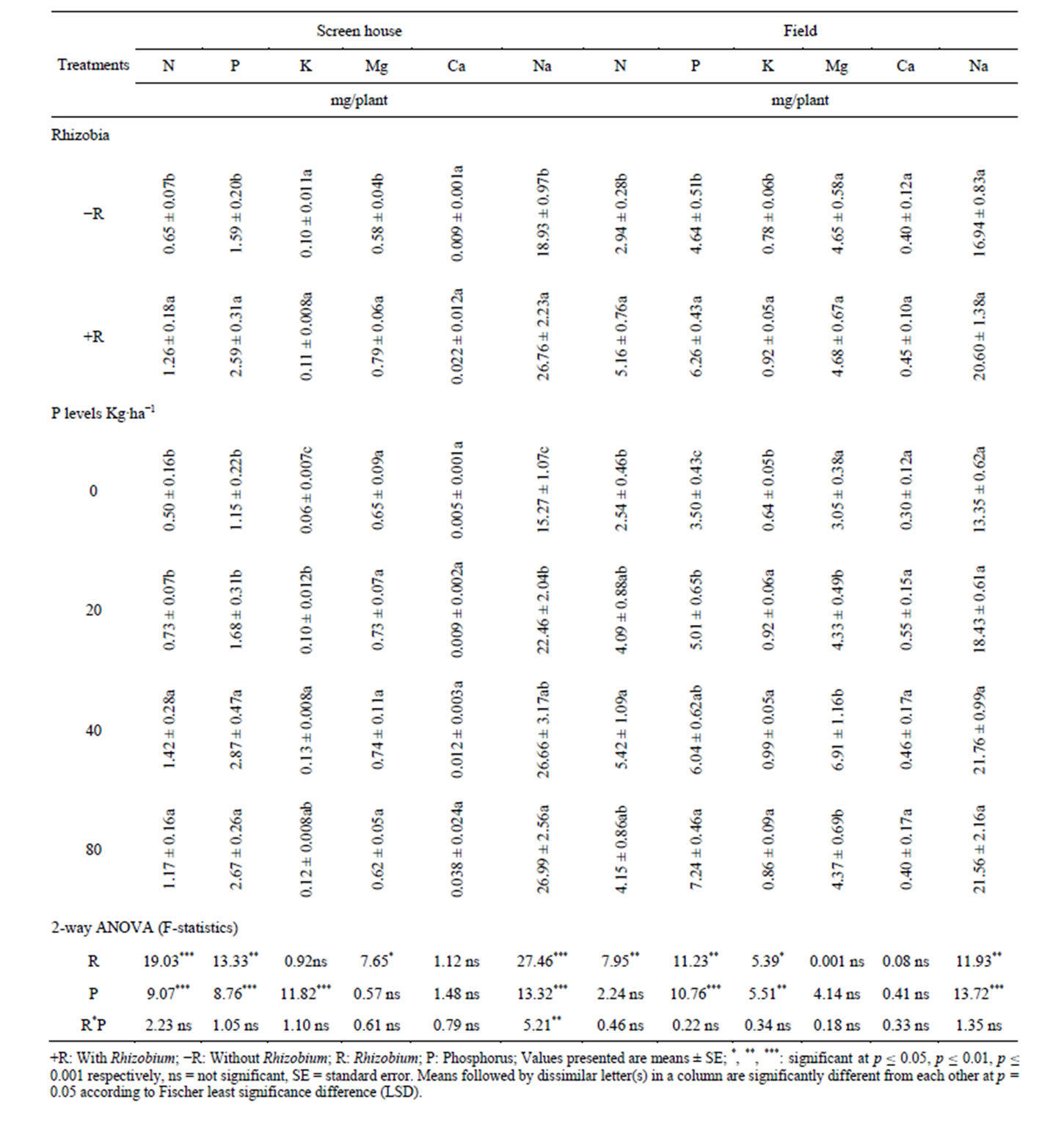
Table 2. Effects of Bradyrhizobium japonicum and phosphorus on macronutrient uptake in the roots of cowpea.
this study.
The results presented in (Tables 3-5) of this study indicated the significant uptake of N, P, K, Mg, Ca and Na in the pods, shoots and whole cowpea plant respectively in both the screen house and field experiments. For example, the uptake of K, Mg and Na in cowpea pods collected from the screen house and, Mg and Ca from pods collected from field experiment were significantly higher in the pots and plots supplied with 40 kg P/ha relative to other treatments and declined from this point to 80 kg P/ha. Furthermore, the results in Table 4 indicated that phosphorus supplementation significantly improved the
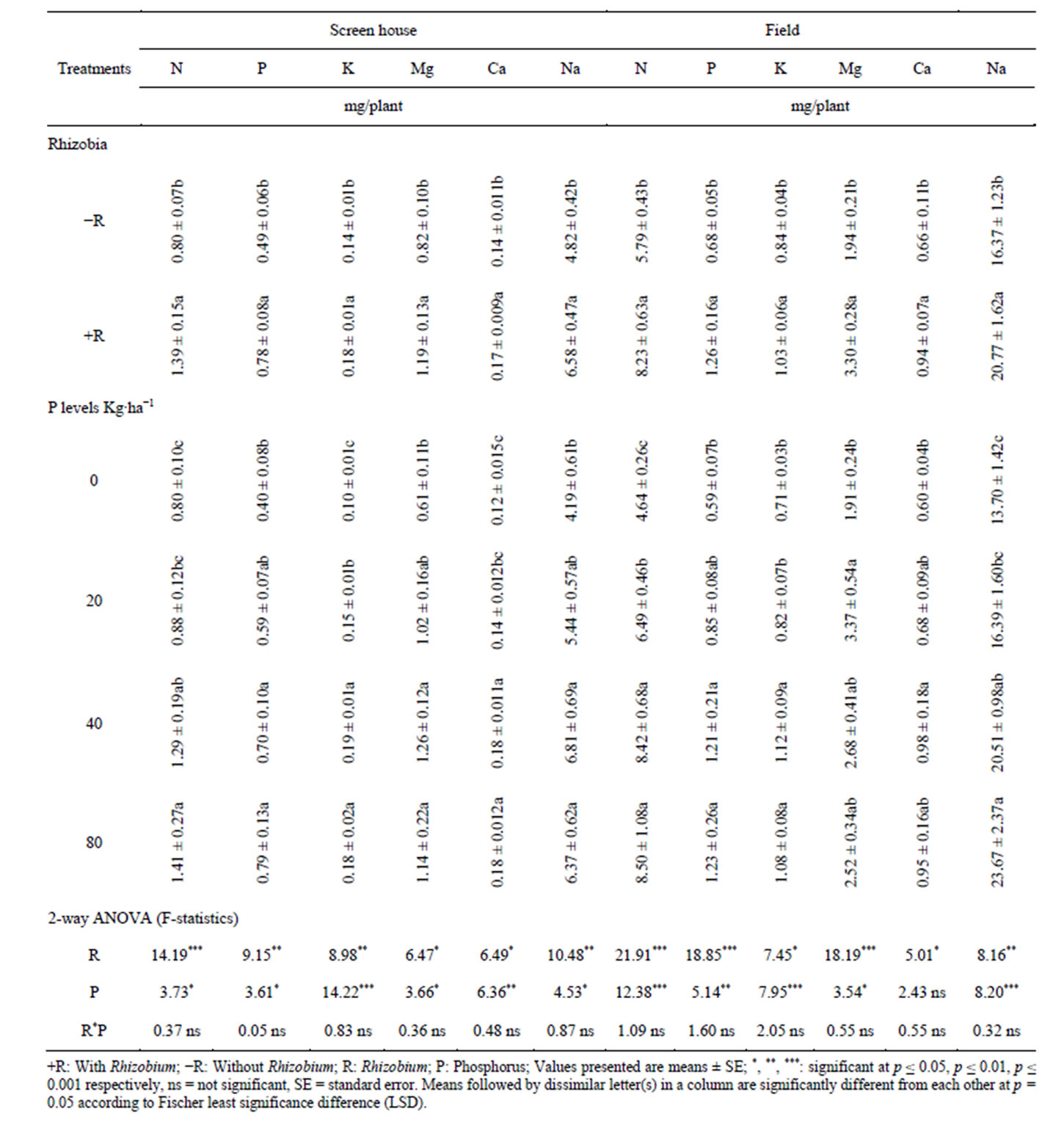
Table 3. Effects of Bradyrhizobium japonicum and phosphorus on macronutrient uptake in the pods of cowpea.
shoot uptake of N, P, K, Ca, Mg and Na in the screen house experiments and N, P, K, Ca and Na in the field experiment over the control treatments. At the whole plant level, phosphorus supplementation significantly increased the uptake of N, P, K, Ca, Mg and Na above the control (Table 5).
3.4. Interactive Effects of B. japonicum and Phosphorus on the Uptake of Na in the Roots of Cowpea
The current study demonstrated the significant interactive effects between B. japonicum and phosphorus on the uptake of Na in the roots at the whole plant level in the
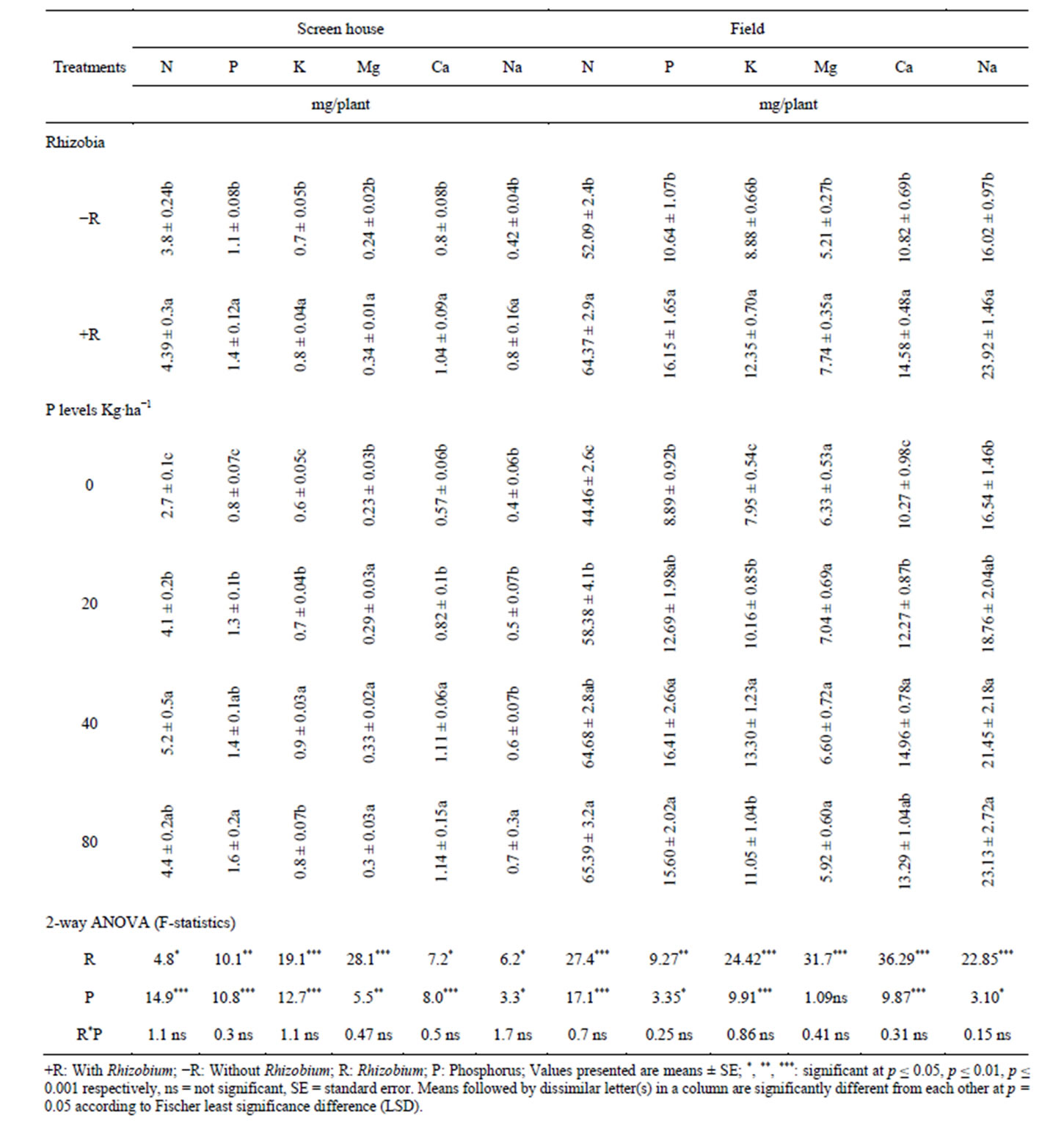
Table 4. Effects of Bradyrhizobium japonicum and phosphorus on macronutrient uptake in the shoots of cowpea.
screen house (Figures 1(a) and (b)). When supplied without rhizobia, phosphorus significantly increased the uptake of sodium relative to the control. However, the combination of rhizobia inoculation and phosphorus significantly increased the uptake of sodium compared with the un-inoculated control (Figure 1(a)). The results presented in Figure 1(b), indicated thatin un-inoculated control, any level of P supplementation significantly increased the uptake of sodium (Na) over the control. Furthermore, there was a highly significant effect on Na uptake when B. japonicum inoculation was supplemented with phosphorus. Highest values were recorded in plots
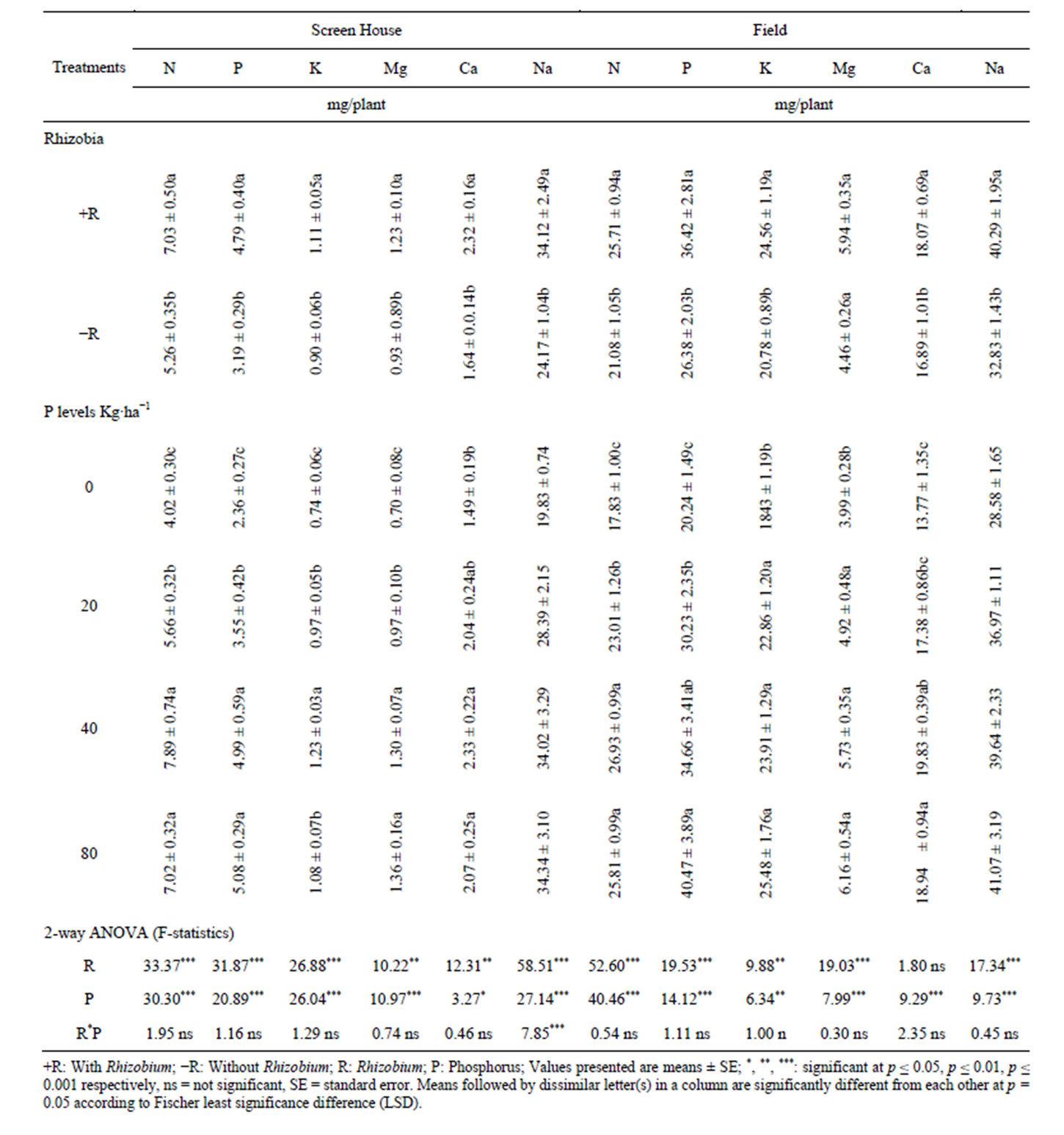
Table 5. Effects of Bradyrhizobium japonicum and phosphorus on macronutrient uptake in the whole cowpea plant.
supplied with 40 - 80 kg P/ha (Figure 1(b)).
4. Discussion
Crop production depends much on the bioavailability and uptake of both primary and secondary macronutrients such as N, P, K, Ca, Mg and Na. These macronutrients are declining day to day due to diverse factors including continuous cropping without additional inputs in the soil, acidification, leaching [16] and soil erosion [17,18] causing a huge yield reduction. The current study was conducted to assess the effects of phosphorus and B. japonicum on the uptake of macronutrients in different
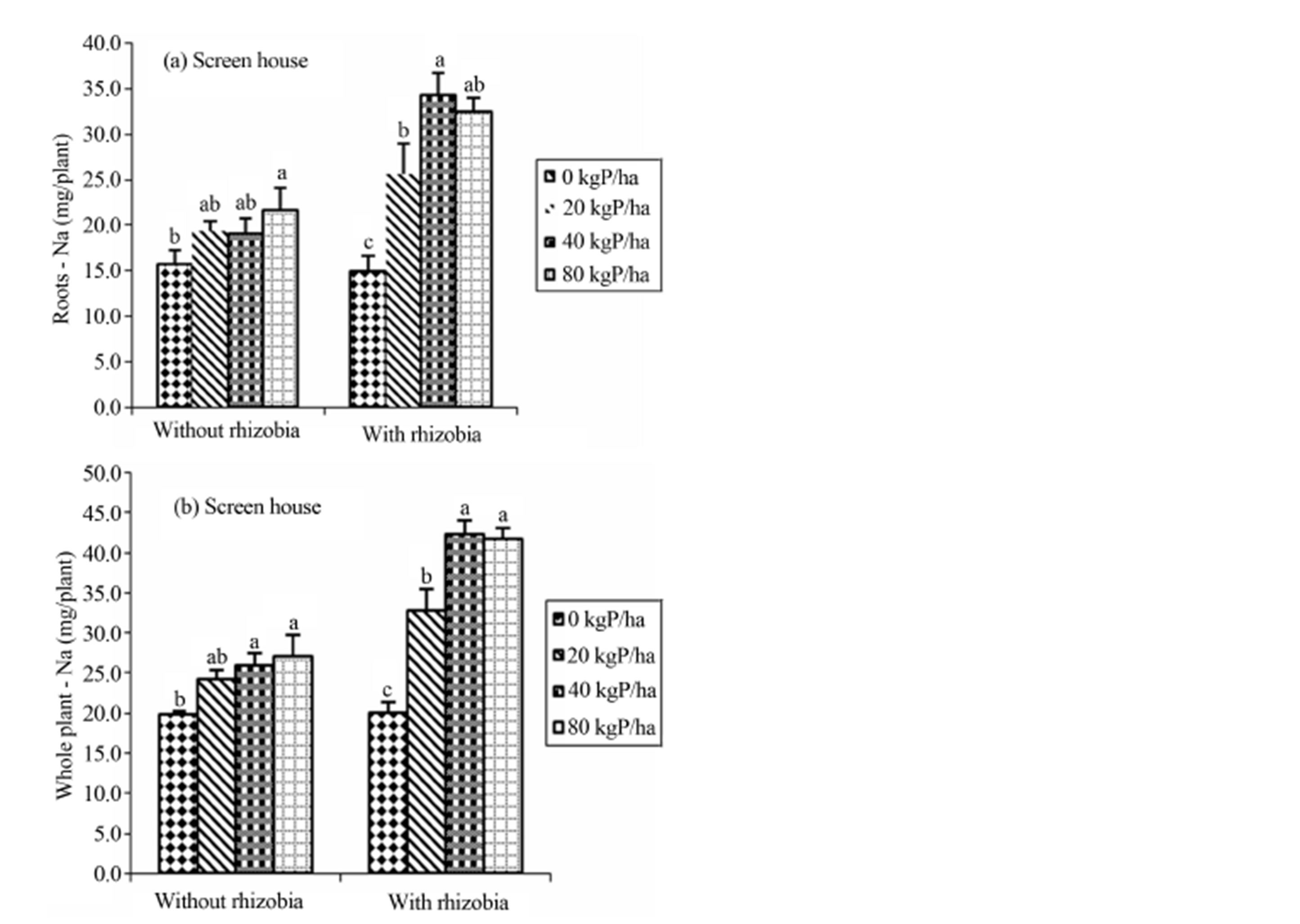
Figure 1. (a) and (b): Interactive effects of B. japonicum and phosphorus (P) on the sodium (Na) uptake in the roots and whole plant grown in the screen house. Bars followed by similar letter(s) are not significantly different from each other.
plant tissues such as roots, pods and shoots and whole cowpea plant grown at different condition in northern Tanzania. The results indicated that phosphorus supplementation and B. japonicum inoculation significantly improved the uptake of macro elements such as N, P, K, Ca, Mg and Na in different tissues of cowpea in both screen house and field experiments compared with the control treatments.
The results in Table 2 indicated that B. japonicum significantly improved the uptake of N, P, Mg and Na (screen house) and N, P, K and Na (field experiment) in the roots of cowpea. It is also indicated in Tables 3-5 that the uptake of macro elements at the pods, shoots and whole plant was improved in the pots and plots received B. japonicum inoculation relative to un-inoculated treatments. These findings are supported by other researcher [1,3] who found that rhizobium inoculation significantly improved the uptake of micro and macro elements in the roots, shoots, pods and whole plant of P. vulgaris in the glasshouse and field experiments. Other research report [19] has demonstrated that rhizobium inoculation significantly improved the uptake of N, P, and K in the leaves of mulberry plant. The improved uptake of macronutrients measured in this study as a result of B. japonicam inoculation might be due to increased soil pH in inoculated plots (data not shown), because high soil pH improves the uptake of important nutrients such as phosphorus [20]. Research evidence has shown that improved soil pH to the optimum levels may improve other soil chemical properties and the availability of some mineral nutrients in the soil [20,21] and hence improved plant growth and production. Other research findings [3,5,22, 23] have shown that rhizobium inoculation can increase availability of macro elements by releasing dead cells which may contain macro elements or biomolecules that can solubilize unavailable to available nutrients. The results in Tables 2-5 also indicated that N content was significantly increased in the rhizobia inoculated treatments in both the screen house and field experiment above the control. This might be due to the possibility of increased biological nitrogen fixation in the B. japonicum inoculated treatments over the control [5]. This argument is supported by other researchers [24] who found that rhizobial inoculation increased shoot N content for about four fold increase and concluded that all shoot N was derived from biological nitrogen fixation since soil N was immobilized by the incorporation of ground sugarcane bagasse.
Phosphorus supplementation significantly improved the uptake of some macro elements in different tissues (roots, pods, shoots and whole plant) of the cowpea in both screen house and field experiments. For example, the results of this study indicated that supplementation of phosphorus significantly increased the root uptake of N, P, K, and Na (screen house) and P, K and Na (field experiment) (Table 2); pod uptake of N, P, K, Mg, Ca and Na (screen house) and N, P, K, Mg and Na (field experiment) (Table 3); shoot uptake of N, P, K, Mg, Ca and Na (screen house) and N, P, K, Ca and Na (field experiment) (Table 4); and whole plant uptake of N, P, K, Mg, Ca and Na in the screen house and field experiments (Table 4). In both the screen house and field experiments, phosphorus supplementation at 40 kg P/ha produced greater values of most macro elements such as N, P, K, Mg, Ca and Na in all the plant tissues measured (roots, pods and shoots and whole plant) which was statistically at par with 80 kg P/ha (Tables 2-4). However, supplementation of phosphorus at 80 kg/ha numerically but not statistically decreased the content of most macro elements in the roots, pods, shoots and whole plant (Tables 2-4). The increased uptake of phosphorus in all plant organs could be due to the increased availability phosphorus in the treatments supplemented with different levels of phosphorus [8,22,25]. These results are in agreements with [26] who reported that phosphorus addition increased the content of phosphorus in the fruits of black pepper. N uptake in all organs of cowpea may be attributed to the nodulation enhanced by phosphorus which increases biological nitrogen fixation, the process which accounts for 65% of the nitrogen currently utilized in agriculture [27], and hence N uptake [5,28,29]. Similar to our findings, other research findings reported by [9] indicated that P and Ca uptake by maize and cowpea was markedly increased by phosphorus application from three different phosphate sources. However, in our current study, Ca uptake was only significant in the pods (screen house), shoots and whole plant (screen house and field experiment), while it was not significant in the roots (screen house and field experiments) and pods (field).
The results also showed a significant interaction between phosphorus and B. japonicum on the uptake of Na in the roots and at whole plant level in the screen house. When the crop was treated with B. japonicum and supplemented with phosphorus it resulted in high uptake of the sodium (Na) especially at the rate of 40 kg P/ha which was statistically the same as 80 kg P/ha when compared with other treatments. The control (0 kg P/ha) treatments resulted in a significantly lower uptake of Na in all treatments with and without inoculation. Similarly un-inoculated treatments significantly resulted in lower uptake of Na (Figures 1(a) and (b)) indicating that for Na uptake, treatment of legumes with rhizobia and phosphorus is a good approach.
5. Conclusion
In conclusion, B. japonicum inoculation and supplementation of phosphorus on cowpea, resulted into increased uptake of macronutrients in different plant tissues such as roots, pods, and shoots in both the screen house and field experiment. The increased macro nutrients uptake following inoculation and P fertilization is reflected to the increased crop yield [30] and is advantageous to small scale farmers in Tanzania who may practice this technology to improve their production. The results of the current study also indicated that supplementation of phosphorus at the highest level (80 kg P/ha) numerically resulted in decreased uptake of N, P, K, Mg and Ca in different plant tissues under different growth condition (screen house and field experiment). Therefore, the results of this study suggest that supplementation of phosphorus at 40 kg P/ha is ideal for the uptake of most macro elements in cowpea and may improve crop production and give reasonable profit to the producers [30].
Acknowledgements
Special thanks to the Nelson Mandela African Institution of Science and Technology (NM-AIST) and the Commission for Science and Technology (COSTECH) of Tanzania that supported this study, Tanzania Coffee Research Institute (TaCRI) and Seliani Agricultural Research Institute (SARI) for providing study sites. Lastly special thanks to Melchior Tesha, from Tanzania Coffee Research Institute for his great assistance for the entire period of field study.
REFERENCES
- P. A. Ndakidemi, S. Bambara and J. H. J. R. Makoi, “Micronutrient Uptake in Common Bean (“Phaseolus vulgaris” L.) as Affected by Rhizobium Inoculation, and the Supply of Molybdenum and Lime,” Plant Omics, Vol. 4, No. 1, 2011, pp. 40-52.
- R. Uchida, “Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms, Plant Nutrient Management in Hawaii’s Soils,” College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, 2000, pp. 31-55.
- J. H. J. R. Makoi, S. Bambara and P. A. Ndakidemi, “Rhizobium Inoculation and the Supply of Molybdenum and Lime Affect the Uptake of Macroelements in Common Bean (P. vulgaris L.) Plants,” Australian Journal of Crop Science, Vol. 7, No. 6, 2013, pp. 784-794.
- I. Cechin and T. de F. Fumis, “Effect of Nitrogen Supply on Growth and Photosynthesis of Sunflower Plants Grown in the Greenhouse,” Plant Science, Vol. 166, No. 5, 2004, pp. 1379-1385. http://dx.doi.org/10.1016/j.plantsci.2004.01.020
- B. Saharan and V. Nehra, “Plant Growth Promoting Rhizobacteria: A Critical Review,” Life Sciences and Medical Research, Vol. 2011, LSMR-21, 2011, pp. 1-30.
- V. N. Matiru and F. D. Dakora, “Potential Use of Rhizobial Bacteria as Promoters of Plant Growth for Increased Yield in Landraces of African Cereal Crops,” African Journal of Biotechnology, Vol. 3, No. 1, 2004, pp. 1-7.
- C. Tang, P. Hinsinger, J. J. Drevon and B. Jaillard, “Phosphorus Deficiency Impairs Early Nodule Functioning and Enhances Proton Release in Roots of Medicago truncatula L,” Annals of Botany, Vol. 88, No. 1, 2001, pp. 131-138. http://dx.doi.org/10.1006/anbo.2001.1440
- A. Singh, A. Baoule, H. Ahmed, A. Dikko, U. Aliyu, M. Sokoto, J. Alhassan, M. Musa and B. Haliru, “Influence of Phosphorus on the Performance of Cowpea (Vigna unguiculata (L) Walp.) Varieties in the Sudan Savanna of Nigeria,” Agricultural Sciences, Vol. 2, No. 3, 2011, pp. 313-317. http://dx.doi.org/10.4236/as.2011.23042
- M. O. Akande, “Effect of Phosphate Rock on Selected Chemical Properties and Nutrient Uptake of Maize and Cowpea Grown Sequentially on Three Soil Types in South Western Nigeria,” International Research Journal of Agricultural Science and Soil Science, Vol. 1, No. 11, 2011, pp. 471-480.
- A. Hussain, A. Ali and I. R. Noorka, “Effect of Phosphorus with and without Rhizobium Inoculation in Nitrogen and Phosphorus Concentration and Uptake by Mungbean (Vigna radiata L),” Journal of Agricultural Research, Vol. 50, No. 1, 2012, pp. 49-57.
- R. J. Buresh, P. A. Sanchez and F. Calhoun, “Replenishing Soil Fertility in Africa,” SSSA Special Publication No. 51, Soil Science Society of America, Madison, Wisconsin, 1997, p. 251.
- J. M. Bremner, “Total Nitrogen,” In: C. A. Black, Ed., Methods of Soil Analysis, Part 2, American Society of Agronomy, Madison, 1965, pp. 1149-1178.
- J. Murphy and J. P. Riley, “A Modified Single Solution Method for the Determination of Phosphate in Natural Water,” Analytica Chimica Acta, Vol. 27, 1962, pp. 31-36. http://dx.doi.org/10.1016/S0003-2670(00)88444-5
- P. R. Hesse, “A Textbook of Soil Chemical Analysis,” Murray, London, 1971, pp. 120-309.
- R. G. D. Steel and J. H. Torrie, “Principles and Procedures of Statistics: A Biometrical Approach,” 2nd Edition, McGraw-Hill Kogakusha, New York, 1980.
- S. B. G. Christoph, W. G. Teixeira, J. Lehmann, W. E. H. Blum and W. Zech, “Nitrogen Retention and Plant Uptake on a Highly Weathered Central Amazonian Ferralsol Amended with Compost and Charcoal,” Journal of Plant Nutrition and Soil Sciences, Vol. 171, No. 6, 2008, pp. 893-899. http://dx.doi.org/10.1002/jpln.200625199
- J. O. Achieng and G. Odhiambo, “Use of Organic Inputs in Management of Alfisols and Ultisols for Sustainable Maize Production in Western Kenya,” American Journal of Experimental Agriculture, Vol. 3, No. 4, 2013, pp. 884-895.
- A. Bationo, B. Ntare, S. Tarawali and R. Tabo, “Soil Fertility Management and Cowpea Production in the Semiarid Tropics,” Challenges and Opportunities for Enhancing Sustainable Cowpea Production, 2002, pp. 301- 318.
- M. F. Baqual and P. K. Das, “Influence of Biofertilizers on Macronutrient Uptake by the Mulberry Plant and Its Impact on Silkworm Bioassay,” Caspian Journal of Environmental Sciences, Vol. 4, No. 2, 2006, pp. 98-109.
- S. Bambara and P. A. Ndakidemi, “Changes in Selected Soil Chemical Properties in the Rhizosphere of Phaseolus vulgaris L. Supplied with Rhizobium Inoculants, Molybdenum and Lime,” Scientific Research and Essays, Vol. 5, No. 7, 2010, pp. 679-684.
- M. Bagayoko, A. Buerkert, G. Lung, A. Bationo and V. Romheld, “Cereal/Legume Rotation Effects on Cereal Growth in Sudano-Sahelian West Africa: Soil Mineral Nitrogen, Mycorrhizae and Nematodes,” Plant and Soil, Vol. 218, No. 1-2, 2000, pp. 103-116. http://dx.doi.org/10.1023/A:1014957605852
- M. H. Abd-alla, “Phosphatases and the Utilization of Organic P by Rhizobium leguminosarum Biovarviceae,” Letters in Applied Microbiology, Vol. 18, No. 5, 1994, pp. 294-296. http://dx.doi.org/10.1111/j.1472-765X.1994.tb00873.x
- A. Halder and P. K. Chakrabartty, “Solubilization of Inorganic Phosphate by Rhizobium,” Folia Microbiologica, Vol. 38, No. 4, 1993, pp. 325-330. http://dx.doi.org/10.1007/BF02898602
- P. Singleton and J. Tavares, “Inoculation Response of Legumes in Relation to the Number and Effectiveness of Indigenous Rhizobium Populations,” Applied and Environmental Microbiology, Vol. 51, No. 5, 1986, pp. 1013- 1018.
- I. Magani and C. Kuchinda, “Effect of Phosphorus Fertilizer on Growth, Yield and Crude Protein Content of Cowpea (Vigna unguiculata (L.) Walp) in Nigeria,” Journal of Applied Biosciences, Vol. 23, 2009, pp. 1387-1393.
- Y. C. Ann, “Determination of Nutrient Uptake Characteristic of Black Pepper (Piper nigrum L.),” Journal of Agricultural Science and Technology, Vol. 2, No. 10B, 2012, pp. 1091-1099.
- R. Hayat, S. Ali, U. Amara, R. Khalid and I. Ahmed, “Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review,” Annals of Microbiology, Vol. 60, No. 4, 2010, pp. 579-598. http://dx.doi.org/10.1007/s13213-010-0117-1
- P. A. Ndakidemi, “Nutritional Characterization of the Rhizosphere of Symbiotic Cowpea and Maize Plants in Different Cropping Systems,” Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, 2005, p. 150.
- N. Stamford, C. Santos and S. Dias, “Phosphate Rock Biofertiliser with Acidithiobacillus and Rhizobia Improves Nodulation and Yield of Cowpea (Vigna unguiculata) in Greenhouse and Field Conditions,” Tropical Grasslands, Vol. 40, No. 4, 2006, pp. 222-230.
- D. Nyoki and P. A. Ndakidemi, “Economic Benefits of Bradyrhizobium japonicum Inoculation and Phosphorus Supplementation in Cowpea (Vigna unguiculata (L) Walp) Grown in Northern Tanzania,” American Journal of Research Communication, Vol. 1, No. 11, 2013, pp. 173- 189.
NOTES
*Effects of B. japonicum and phosphorus on macro nutrients uptake in cowpea.
#Corresponding author.

