American Journal of Plant Sciences
Vol.4 No.7A2(2013), Article ID:34565,5 pages DOI:10.4236/ajps.2013.47A2003
Interactions between a Root Knot Nematode (Meloidogyne exigua) and Arbuscular Mycorrhizae in Coffee Plant Development (Coffea arabica)
![]()
1Postgrado en Zoología, Universidad Central de Venezuela, Caracas, Venezuela; 2Laboratorio de Biología de Vectores y Parásitos, Universidad Central de Venezuela, Caracas, Venezuela; 3Laboratorio de Agroecosistemas, Instituto de Zoología y Ecología Tropical, Universidad Central de Venezuela, Caracas, Venezuela.
Email: marcia.toro@ciens.ucv.ve
Copyright © 2013 Raúl Alban et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 29th, 2013; revised June 1st, 2013; accepted July 1st, 2013
Keywords: Arbuscular Mycorrhizae; Meloidogyne exigua; Coffee Plant; Ecological Interactions; Biotic Stress
ABSTRACT
This paper focuses on parasitic root knot nematodes (Meloidogyne exigua) and how to decrease their pathogenic effect on coffee plants (Coffea arabica), by examining the behaviour of and the interactions between nematodes, coffee plant and arbuscular mycorrhizae (AM). The experiment was carried out at the seedling stage, with six (6) treatments (plants with M. exigua, plants with arbuscular mycorrhizae, plants with both organisms, and the same time, first mycorrhizae plants, then nematodes were inoculated and vice versa). After 5 months the measured variables were: dry biomass (roots and shoot), nematode knots caused by M. exigua in root, nematode juvenile (J2) found in 100.0 g of soil, and mycorrhizal percentage. Plant nutrients (P and N) contents were analysed. Significant differences were found in all the variables, but concentration N content in plants. Plants with mycorrhizae and plants with mycorrhizae and then inoculated with nematodes have the same behaviour. Control plants and plants with nematode and then inoculated with mycorrhizae behave similarly. It is thought that arbuscular mycorrhizae are formed before the nematode infestation, allowing coffee plants to regain the energy lost by the parasitic interaction. AM may help coffee plants with lignifications of the plant cell wall cuticle. As the cuticle thickens it is more difficult for nematodes to penetrate and enter into plant roots. Therefore, arbuscular mycorrhizae help coffee plants to uptake and transport nutrients, improving its nutritional status and stabilizing nematode attacks. It is suggested that symbiotic interactions help neutralize parasitic interactions.
1. Introduction
In Venezuela, the harvesting and cultivation of coffee plants have a strategic importance in agriculture, due to the unique characteristics such as flavour and acidity of Venezuelan coffee [1]. The cultivation of coffee has much historical significance in this country since it was the main product of the economy during the XVIII and XIX centuries [2]. This crop also has an environmental impact due to the way it is cultivated (usually it is planted in a vertical layers multi-crop system that could include a series of crops such as citrus, plantain, papaya trees, among others, and in the same space). Also, coffee plants are used to keep water resource structures well preserved. Therefore, it is said to be a water conservation plant [3]. It is important to identify the wide range of organisms that can interact with coffee plants. In these interactions, there are a variety of parasitic ones that can damage coffee plants. Nematodes are among the most biodiverse organisms in the planet and can live in any ecosystem [4]. The Meloidogyne genus, also known as root knot nematodes, can cause great agronomical problems such as chlorosis, dwarf shoot development, leaf loss, root damage and decline in crop production of coffee plants [5]. The nematode parasitic interaction occurs at the root level, causing knots that decrease root length and nutrient absorption capacity. These root knots become nematodes’ food resources [2]. These nematodes are well distributed in all coffee growing areas in Venezuela, but Meloidogyne exigua in particular has been identified as the most abundant in coffee plants [6].
In addition, it is well known that arbuscular mycorrhizae (AM) are highly developed in coffee plant roots [7]. In this case, AM fungi create symbiotic-mutualistic interactions among plants roots and Glomeromycota fungi [8].
This interaction plants supply fungi with essential carbohydrate nutrients while the Glomeromycota fungi uptake and transport essential nutrients such as phosphorus and slow diffusion microelements (Zn, Cu) to the plant root system [9,10]. Therefore, this interaction occurs at the cellular level where plants allow Glomeromycota fungi to enter the root tissues via inter and intra cellular [10]. Moreover, this symbiosis is well-known to work as a biofertilizer [10,11] and can also help improve the tolerance of biotic stress caused by any other pathogenic organisms [12].
Coffee plants (Coffea arabica) were chosen due to the economical importance this agricultural product has in Venezuela and the South American region, and because of the economic impact nematode infestations have in coffee productivity [13]. The objective of this study is to observe the interaction between root knot nematodes (M. exigua) and Glomeromycota fungi in coffee plants, in order to establish whether the arbuscular mycorrhizae can prevent plant damage caused by parasitic nematode infestation, or to at least verify whether damage levels decreased or not, by changing fungi and nematode inoculation times. Furthermore, this study can show different behavioral responses ways by which these organisms may affect the plant.
2. Materials and Methods
2.1. Area of Study
This research was carried out in the Experimental Station Jaime Henao Jaramillo, which is part of the Agronomy Faculty of the Central University of Venezuela, located at the Laurel sector, Guaicaipuro municipality, Miranda State (10˚22'24"N 66˚54'04"W). This station has 369 acres and specializes in the cultivation of coffee, both with organic and conventional management. The station is situated in a mountain landscape, at an altitude of 1230 meters above sea level. The experimental station has a combination of acrisol and humic cambisol soils, with a clay loam texture, a 6.40 pH and organic matter percentage of 5.34 [14]. The annual temperature is about 19.2˚C and an average precipitation of 1.282 mm [15]. The soil used for this study was taken from the organic coffee plantation sector; five samples were randomly collected following a zigzag pattern [14]. It was sterilized by applying vapour fluent technique (1hour for 3 days), in order to avoid any microorganism presence [16]. Then, the soil was put in plastic bags containing two Caturra Amarilla variety coffee seedlings on each bag.
2.2. M. exigua Nematodes Extraction and Identification
Several coffee plant seedlings were collected from the experimental station; to obtain M. exigua juvenile nematodes, roots and rhizosphere of seedling were extensively revised. Female nematodes were identified as M. exigua by comparing morphometrical relations such as stylet, body longitude and using the perianal pattern [17]. Egg masses were collected from root knots when positive for M. exigua [5] and were preserved in a mixture of tap water and 5% lactic acid solution. After 4 egg masses were collected, these were put in a 10.0 ml aliquot to be used as the treatment procedure. Some authors indicate that every egg mass may have about 450 to 500 nematode second stage juvenile (J2) [18]. Therefore each treatment application contained about 2000 J2 M. exigua nematodes.
2.3. Reproduction and Cultivation of Arbuscular Mycorrhizae
To obtain Glomeromycota fungi, a soil sample was taken from the experimental station, and then it was air dried during 4 days to control nematode presence. Spore count was done using the wet sieving and decanting metho dology [16,19] giving a total of 56 spores in 100.0 g of soil. Air dried soil was used pot tramp, [11] with onion (Allium cepa) and sorghum (Sorghum bicolour) as symbiotic hosts. After five months the Glomeromycota fungi community was analyzed to check fungal spore reproduction, displaying an increase of 543 spores in 100.0 g of soil as suggested by several authors [20,21]. A Glomeromycota consortia consisting of spores, roots and mycelium was used as AM inocula for this study. 30.0 g of the former inocula were used in each treatment required.
2.4. Greenhouse Assay and Experimental Design
Coffee plants were planted in 2 Kg plastic bags. These were placed in the greenhouse for 5 months Plants were randomised and 6 treatments were applied using presence and non-presence of Glomeromycota fungi and M. exigua at day zero; then they were inoculated again with the same organisms depending on the treatment at day twelve as seen on Table 1. 10 repetitions per inoculation treatment were planted; after 5 months coffee plant seedlings were cut. Root and shoot dry weight (g), AM colonization %, root knot numbers were measured, and presence of M. exigua J2 in rhizospheric soil of coffee seedlings was quantified. In addition, P and N shoot content (mg/g) were determined using binary and Kendalh techniques. A one way ANOVA test was applied to each variable (parametrical o non-parametrical). If significant differences were found, a square test (Tukey test for parametrical or Fisher test if non-parametrical with p < 0.05) was applied. Microsoft Excel © (2007), Statistical programs (Statistix 9.0 and R) were used.

Table 1. Treatment used in the greenhouse study with coffee seedlings.
3. Results and Discussion (Table 2)
3.1. Dry Biomass (Roots and Shoots)
At the root level, it is found that MYCO and NEM0MYC0 plants behave similarly by having a significant root biomass and having a heavier root system. Glomeromycota fungi may allow plant roots to develop faster than nematode infested plants, since root hair production is stimulated by the AM interaction with plants allowing more efficient nutrient uptake [22].
MYCO plants showed the biggest shoot biomass. Thus it can be argued that the symbiotic interaction allowed plant development, since it generated intake the uptake of essential nutrients for plants to grow as a good biofertilizer will do [23]. In another group CONTROL, MYC0NEM0 and NEM1MYC0 and as nematodes are inoculated, plant development was poor since nematodes used plant energy to survive.
3.2. Biological Variables, Presence of Root Knots, J2 and Mycorrhizal Percentage
Two well defined groups were found at the variables knot number in roots: NEMA and NEM0MYC1 have the biggest amount of root knots while NEM0MYC0 and NEM1MYC0 have the fewest root knot number. It is proposed that Glomeromycota fungi prevent nematode infestation in the second group. Therefore a lower number of knots is found. It is suggested that as AM is formed faster due to the coffee mycotrophic properties [7], and because it is difficult for nematodes to enter plant cuticles. For its entrance mechanism is to use its style, in order to penetrate plant cuticle and cells. But when plant cell walls are thickening by the AM symbiotic interaction, this entrance way becomes harder to pass through for the J2 nematode and it dies outside the plant [24]. The presence of J2 nematode in soil variable, the same groups were formed in a more limited extension than with the last variable, where NEMA and NEM0MYC1 group is more defined than at the root knot variable.
Two groups with significant differences were formed which were found at root MA colonization % and the first group is formed with NEM0MYC1 with more roots MA colonization percentage and the other treatments with less percentage. It is thought that once the nematode infestation is under way, Glomeromycota fungi interaction speeds up in order to stabilize the parasitic energy loss, preventing the decrease of plant development. It is assumed that the symbiotic interaction absorbed the energy levels needed to support the parasite survival [22]. In other treatments there is a smaller root MA colonization percentage since the Glomeromycota fungi which enter coffee plants, first developed the symbiotic interaction that in some ways acts as a barrier to prevent nematode entrance to the plant
3.3. Plant P and N Shoot Coffee Plant Content
A significant difference was found at the P shoot content, where three groups were formed, first MYCO treatment, second NEM0MYC1 and third CONTROL, NEMA, NEM0MYC0 and NEM1MYC0. It is known that Glomeromycota fungi are able to uptake and transport P effectively [25]. Therefore, AM plants have more P in their structure.
On the other hand, there was no significant difference at the N shoot content. It is thought that even though AM
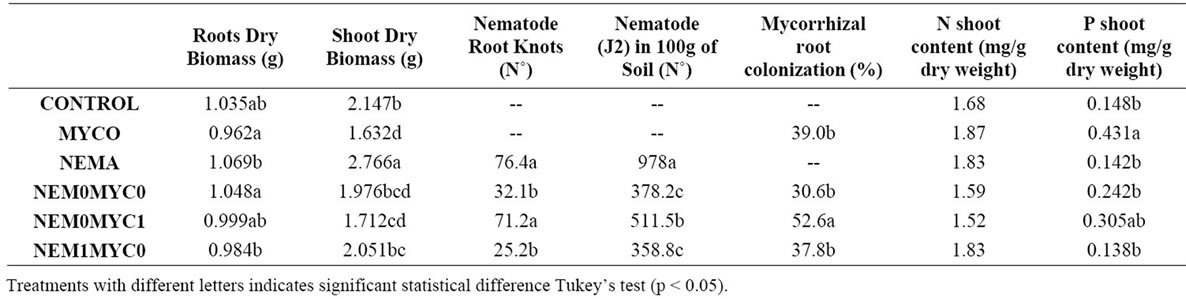
Table 2. Results of Root Dry Biomass (g), Shoot Dry Biomass (g), Nematode Root knot (number), M. exigua J2 presence in soil (number), mycorrhizal root colonization percentage and P and N shoot content (mg/g) found at the greenhouse study.
do uptake and transport N, this interaction focuses on P and other micronutrients such as Zn and Cu, and that it may need more time to stabilize and produce a different result.
However, some authors [22,26] reported that nematodes parasite and grow in plants faster than Glomeromycota fungi; this is because the nematode entrance is physical and not biochemical as in the case of AM fungi. In this case, it is thought that the coffee high mycotropy allows AM fungi to enter faster than M. exigua in order to obtain nutrients through the symbiosis interaction needed to defend themselves from parasitic attacks [7,27].
4. Conclusions
The findings reported here show first, that the AM fungi interact faster with the coffee plant than do nematode parasites. Second, AM fungi increase root and shoot biomass, while M. exigua decreases such variables. Third, AM fungi increase P shoot content in coffee plants. Finally, based on these findings, it is possible to propose a model looking at plant response as a bio-indicator, considering the complex interactions between these organisms.
Case I: If Glomeromycota fungi are inoculated earlier than M. exigua, coffee plants will be healthy and strong and could resist nematode infestation, since these plants developed a thick root cuticle. Therefore, the nematode infestation rate is low.
Case II: If coffee seedlings are attacked by M. exigua it is possible to use AM fungi in order to prevent productivity loss. This occurs because the symbiosis interaction speeds up. It aims to maintain plant growth similar to non-infected plants. In this case nematode infestation rate is medium.
Case III: If Glomeromycota fungi and M. exigua are found at the same time in the soil or greenhouse subtracts, it is thought that AM colonizes coffee plants first, giving it a head start in its development while nematode infestation occurs. This will lead to a low to medium nematode infestation rate.
5. Acknowledgements
The authors wish to thank Dr. Marcia Escala and Msc. Luis Hermoso (IBE, Central University of Venezuela) for the plant morphological cuts and identifications, and Dr. Renato Crozolli for the nematode identifications, as well as Lic. Rosas for her assistance in the laboratory.
REFERENCES
- J. Jaramillo, “El Café en Venezuela,” Ediciones Biblioteca Universidad Central de Venezuela (UCV), Caracas, 1982, 291 Pages.
- I. Ferreira, “Aspectos Biológicos y Efecto de Diferentes Densidades Poblacionales de Meloidogyne exigua GöEDI, 1887 en Tres Variedades de Café,” Comisión de Estudios de Postgrado UCV FAGRO Postgrado en Agronomía, 1995, 243 Pages.
- Instituto de Salud Agrícola Integral (INSAI), “Manejo Integrado del Cultivo de Café,” 2nd Edition, Ministerio del Poder Popular para la Agricultura y Tierra, Caracas, 2010, 82 Pages.
- G. Agrios, “Fitopatología,” 3rd Edition, Noriega Editores, México, 1998, 838 Pages.
- D. Barbosa, H. Viera, R. Souza and C. Silva, “Field Estimates of Coffee Yield Losses and Damage Threshold by Meloidogyne exigua,” Nematología Brasileira, Vol. 28, No. 1, 2004, pp. 49-54.
- R. Crozzoli, “Especies de Nematodos Fito-parasíticos en Venezuela,” Interciencia, Vol. 27, No. 7, 2004, pp. 354- 364.
- M. P. Sánchez de, “Las Endomicorrizas: Expresión Bioedáfica de Importancia en el Trópico,” Universidad Nacional de Colombia, Facultad de Ciencias Agropecuarias, Palmira, 2007, 352 Pages.
- M. Pozo and C. Azcón-Aguilar, “Unraveling MycorrhizaInduced Resistance,” Plant Biology, Vol. 10, 2007, pp. 393-398.
- A. Schüßler, D. Schwarzott and C. Walker, “A New Fungal Phylum, the Glomeromycota, Phylogeny and Evolution,” The British Mycological Society, Vol. 105, No. 12, 2001, pp. 1413-1421. doi:10.1017/S0953756201005196
- S. Smith and D. Read, “Mycorrhizal Symbiosis,” 3rd Edition, Academic Press, San Diego, 2008, 605 Pages.
- M. Toro, I. Bazo and M. López, “Micorrizas Arbusculares y Bacterias Promotoras de Crecimiento Vegetal, Biofertilizantes Nativos de Sistemas Agrícolas Bajo Manejo Conservacionista,” Agronomía Tropical, Vol. 58, No. 3, 2008, pp. 215-221.
- J. Whipps, “Microbial Interactions and Biocontrol in the Rizosphere,” Journal of Experimental Botany, No. 52, 2000, pp. 487-511.
- A. Barros, “Micorrizas Vesiculo-arbusculares em Cafeiros da Região Sul do Estado de Minas Gerais,” Escola Superior de Agricultura de Lavras, Brasil, 1987, 98 Pages.
- E. Casanova, “Introducción a la Ciencia del Suelo,” 2nd Edition, Universidad Central de Venezuela (Agronomía), Consejo de Desarrollo Científico y Humanístico, Caracas, 2005, 482 Pages.
- O. Abarca, “Conflictos de Intensidad de Uso de la Tierra en las Estaciones Experimentales de La Universidad Central De Venezuela. Análisis Espacial con Sistemas de Información Geográfica,” Agronomía Tropical, Vol. 55, No. 2, 2005, pp. 10-32.
- E. Sieverding, “Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems,” Deustsche Gesellsschaft fur Tecnische Zusammenarbeit (GTZ) GmbH, 2001, 371 Pages.
- A. Taylor and J. Sasser, “Biología, Identificación y Control de los Nematodos de Nódulo de Raíz (especies de Meloidogyne),” Proyecto Internacional de Meloidogyne. North Carolina State University Press, 1987, 111 Pages.
- I. Rodrigues and R. Crozzoli, “Efectos de Nematodos Agallador Meloidogyne exigua Sobre el Crecimiento de Plantas de Café en Vivero,” Nematología Mediterránea, Vol. 23, No. 2, 1995, pp. 325-328.
- J. Morton and S. Betivenga, “Levels of Diversity in Endomycorrhizal Fungi (Glomales, Zygomicetes) and Their Role in Defining Taxonomic and Non-Taxonomic Groups,” Plant and Soil, Vol. 159, No. 1, 1994, pp. 47-59.
- M. Jaizme-Vega, “Inoculación de Hongos Micorrícicos en Vid Durante la Fase de Vivero,” Aportaciones al Conocimiento del Vino Canario, Vol. 2, 2010, pp. 89-103.
- J. Becerra, J. Castaño and B. Villegas, “Efecto de la Micorrización sobre el Manejo de Nematodos en Plántulas de Plátano Híbrido ‘FHIA-20AAAB’,” Agronomía, Vol. 18, No. 1, 2010, pp. 7-18.
- C. Calvet, J. Pinochet and A. Hernández-Dorrego, “Field Microplot Performance of the Peach Almond Hybrid GF- 677 after Inoculation with Arbuscular Mycorrhizal Fungi in a Replant Soil Infested with Root-Know Nematodes,” Mycorrhizae, Vol. 10, No. 1, 2001, pp. 295-300. doi:10.1007/PL00009998
- L. Patterson and L. Melville, “Biofertilizantes, Bioprotectores y Biorestauradores Micorricicos para la Producción Agroecológica en las Fincas de los Productores de Café,” Fundación para el Desarrollo tecnológico Agropecuario y Forestal de Nicaragua (FUNICA, Managua, 2010, 87 Pages.
- C. Villenave, J. Leye and R. Duponnois, “Nematofauna Associated with Exotic and Native Leguminouspant Species in West Africa: Effect of Glomus Intradices Arbuscular Mycorhizal Symbiosis,” Biology and Fertility of Soils, Vol. 38, No. 3, 2003, pp. 161-169. doi:10.1007/s00374-003-0632-3
- A. Medina and R. Azcón, “Effectiveness of the Application of Arbuscular Mycorrhiza Fungi and Organic Amendments to Improve Soil Quality and Plant Performance under Stress Conditions,” Journal of Soil Science and Plant Nutrients, Vol. 10, No. 3, 2010, pp. 354- 372.
- P. Schreiner and J. Pinkerton, “Ring Nematodes (Mesocriconema xenoplax) Alter Root Colonization and Function of Arbuscular Mycorrhizal Fungi in Grape Roots in a Low P Soil,” Soil and Biochemistry, Vol. 3, No. 1, 2002, pp. 10-22.
- M. Luc, R. Sikora and J. Bridge, “Plant Parasitic Nematodes in Subtropical and Tropical Agriculture,” 2nd Edition, CABI Publishing, Masachussets, 2005, 918 Pages. doi:10.1079/9780851997278.0000

