Modern Research in Catalysis
Vol.2 No.2A(2013), Article ID:32387,9 pages DOI:10.4236/mrc.2013.22A003
Steam Reforming of Ethanol over CoMg/SBA-15 Catalysts
1Department of Chemical and Materials Engineering, Chung Cheng Institute of Technology, National Defense University, Taoyuan, Chinese Taipei
2Department of General Education, Army Academy, Taoyuan, Chinese Taipei
Email: *chenbinwang@gmail.com
Copyright © 2013 Josh Y. Z. Chiou et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 19, 2013; revised April 20, 2013; accepted May 17, 2013
Keywords: SBA-15; Steam Reforming of Ethanol; Cobalt; Magnesium
ABSTRACT
Hydrogen production through steam reforming of ethanol (SRE) over Mg modified Co-based catalysts supported on mesoporous SBA-15 was studied herein to evaluate the catalytic activity and the behavior of coke deposition. The CoyMgx/SBA-15 catalysts are obtained according to the steps of consecutive impregnation of Mg (x = 5 and 10 wt%) to be incorporated on SBA-15 and then follow the loading of Co (y = 10 and 20 wt%) using the incipient wetness impregnation method. The catalysts are characterized by using X-ray diffraction (XRD), temperature programmed reduction (TPR), transmission electron microscopy (TEM) and BET techniques. Also, the spent catalysts are further characterized by using XRD and TEM. The catalytic activity of the SRE is evaluated in a fixed-bed reactor under 22,000 h−1 GHSV and with an H2O/EtOH molar ratio of 13. All the CoyMgx/SBA-15 catalysts present a mesoporous structure, even after the SRE reaction. The optimum catalyst of Co20Mg5/SBA-15-H650 comes from the high loading of Co and high reduction temperature pretreatment, which show a high catalytic activity and stability at 550˚C with a hydrogen yield (YH2) up to 5.78 and CO selectivity around 3.10%.
1. Introduction
Hydrogen generation from biomass-derived alcohols has been the activity of choice recently. Ethanol is more attractive because it is non-toxic, has higher hydrogen content, is renewable energy and has an easy-to-handle nature when compared to methanol [1,2]. The main catalytic reaction using ethanol to produce hydrogen by steam reforming is shown in Equation (1), where only hydrogen and non-renewable CO2 are produced, providing 6 moles of H2 per mole of ethanol stoichiometrically [3].
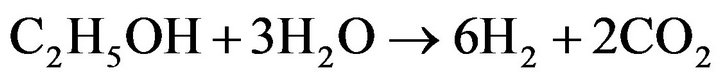 (1)
(1)
Several metallic active phases have been used as catalysts for the steam reforming of ethanol (SRE) to produce hydrogen. Since Co-based catalysts, mainly metal exhibiting appreciable activity for C-C bond broken and water-gas shift (WGS) reactions, generate a low temperature and few by-products, they are efficient when used in SRE. The early stage of SRE research has focused on Co-based catalysts. Haga et al. [4] found that the properties of cobalt catalysts were greatly influenced by the supports, where the hydrogen production decreased in the order of: Co/Al2O3 > Co/ZrO2 > Co/MgO > Co/SiO2 > Co/C. The Co/Al2O3 catalyst showed high hydrogen selectivity for SRE by suppressing CO methanation and ethanol decomposition. Supported cobalt catalysts showed a significant improvement in catalytic performance on the SRE compared with corresponding supports reported by Llorca et al. [5], a variety of oxides involving acidic/ basic and redox properties. Batista et al. [6] studied the high efficiency SRE over Co/Al2O3 and Co/SiO2 catalysts with little Co content (8%) in which the Co/SiO2 catalyst showed better CO removal. Llorca et al. [7] reported CO-free hydrogen produced from SRE over the Co/ZnO catalyst at low temperatures, where the highly stable catalyst was prepared by using Co2(CO)8 as a precursor.
The technique of doping extra components, such as alkali (Li, Na and K) [8], alkaline earth (Mg and Ca) [9, 10] and lanthanide (La and Ce) [10] to modify the originnal property and improve the performance of a catalyst is interesting. Pigos et al. [8] reported that the addition of Na and K significantly improved the formate decomposition rate on a WGS reaction over Pt/ZrO2 catalysts. Wang et al. [9] reported that the addition of Na improved the catalytic performance of a PtRu/ZrO2 catalyst on the oxidative steam reforming of ethanol, where the Na not only enhanced the WGS reaction at a low temperature, but also reduced coke deposition. Cheng et al. [10] also reported the promotional effect of doping alkaline earth oxides or lanthanide oxides on a Ni/Al2O3 catalyst for CO2 reforming of CH4.
Besides the selection of an active metal or promoter for the supported catalysts, the choice of a support with a high surface area to disperse the metal phase over their surface is a main consideration to enhance catalytic performance. Support material, such as γ-Al2O3, SiO2, ZSM-5 [11], MCM-41 [12] and SBA-15 [13], have been widely used in recent years as catalyst supports for catalytic reactions occurring at high temperatures, based on the support material’s larger pores, thicker walls and higher thermal stability. Of considerable interest in this regard are mesoporous materials as a support that will provide an improvement on hydrogen production via steam reforming reaction [14-19]. The promoter effect of alkaline earth metals (Mg and Ca) over Cu-Ni/SBA-15 [16] and Cu-Ni/SiO2 [18] catalysts has been studied; both of them improved the dispersion of the metallic phase and strengthened the metal-support interaction. High hydrogen selectivity was obtained with Mg and reduced deposited carbon with the incorporation of Ca. A promoter made up of a CexZr1−xO2 layer pre-coated on SBA-15 changes the redox properties and enhances the catalytic activity on steam reforming of methane over a Ni-based catalyst, as reported by Wang et al. [19].
It is well known that Co-based catalysts suffer from deactivation by carbon deposition at high reaction temperatures [20]. This is obviously an important point to consider in SRE reactions related to Co-based catalysts. The SBA-15 supported Co catalysts with high surface area and modified by an Mg promoter were prepared in this work. The catalytic performance and coking behavior of hydrogen production via SRE over mesoporous structure catalysts were also considered.
2. Experimental
2.1. Catalyst Preparation
SBA-15 was prepared according to the method described in the literature [13]. Briefly, a triblock copolymer P123 (8 g, Strem) was dissolved in 250 mL HCl (1.9 M). The solution was stirred at 40˚C for 2 h, and 16 g of tetraethyl orthosilicate (TEOS) were then slowly added to the mixture and stirred vigorously at 40˚C for 22 h. The solution was transferred into a Teflon bottle and aged at 100˚C for 24 h. The solid product was filtered, washed with deionized water and then dried at room temperature for 24 h, followed by calcination in air at 500˚C for 6 h with a heating rate of 7˚C /min.
Catalysts promoted with alkaline are much more sensitive to the preparation order for catalytic performance, and the promoting effect is more significant when the support is impregnated with the promoter oxides before the incorporation of the active phase [10]. For this reason, Mg-modified Co/SBA-15 catalysts are prepared by consecutive impregnation with Mg and then Co. Mgx/SBA- 15 samples were prepared from the aqueous solution of Mg(NO3)2·6H2O (Mg loading, x = 5 and 10 wt%, Showa) incorporating SBA-15 by the impregnation method. CoyMgx/SBA-15 samples were prepared by the incipient wetness impregnation method using Mgx/SBA-15 with aqueous Co(NO3)2·6H2O (Co loading, y = 10 and 20 wt%, Showa). All samples were dried at 100˚C overnight and then calcined at 300˚C for 3 h.
2.2. Catalyst Characterization
The metal loading of catalysts was determined by the atomic-emission technique (ICP-AES) using a Perkin Elmer Optima 3000 DV. The BET surface area and pore size distribution were measured by N2 adsorption at a liquid nitrogen temperature using a Micromeritics ASAP 2010 analyzer. X-ray diffraction (XRD) measurement was performed using a Siemens D5000 diffractometer with Cu Kα1 radiation (λ = 1.5406 Å) at 40 kV and 30 mA. The microstructure and particle size of the samples were observed by using transmission electron microscopy (TEM) with a JEOL JEM-2010 microscope equipped with a field emission electron source and operated at 200 kV. Reduction behavior of CoyMgx/SBA-15 catalysts was studied by temperature-programmed reduction (TPR). About 50 mg of the sample were heated in a flow of 10% H2/N2 gas at a flow rate of 10 ml∙min−1. During TPR, the temperature was increased by 7˚C∙min−1 from room temperature to 900˚C.
2.3. Activity Tests
Catalytic activity of CoyMgx/SBA-15 catalysts in an SRE reaction was determined at atmospheric pressure in a fixed-bed flow reactor. 100 mg of the catalyst were placed in a 4 mm i.d. quartz tubular reactor and held by glass-wool plugs. The temperature of the reactor was controlled by heating tape and measured by a thermocouple (1.2 mm i.d.) at the center of the reactor bed. The feed of the reactants was comprised of a gaseous mixture of ethanol (EtOH), H2O and Ar (purity 99.9995%, supplied by a mass flow controller). The composition of the reactant mixture (H2O/EtOH/Ar = 37/3/60 vol%) was controlled by the Ar flow stream (22 mL/min) through a saturator (maintained at 120˚C) containing EtOH and H2O. The gas hourly space velocity (GHSV) was maintained at 22,000 h−1 and the H2O/EtOH molar ratio was 13 (H2O:EtOH = 80:20 by volume). Prior to reactivity measurement, the catalyst was reduced in 10% H2 in N2 for 2 h at 400˚C. The SRE activity was tested stepwise, increasing the temperature from 350˚C to 550˚C. The reaction was carried out online by gas chromatography (GC) with columns of Porapak Q (for CO2, H2O, C2H4, CH3CHO, CH3OCH3 and EtOH) and using a Molecular Sieve 5 Å (for H2, CH4 and CO) for separation. It was also quantitatively analyzed by two sets of thermal conductivity detectors (TCD) on line. Response factors for all products were obtained, and the system was calibrated with appropriate standards before each catalytic test. Activity evaluation of all samples depended on the conversion of ethanol (XEtOH), the distribution of products (mol %) and the yield of hydrogen (YH2, mol H2/mol EtOH) according to the following equations.
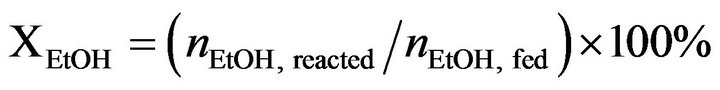 (2)
(2)
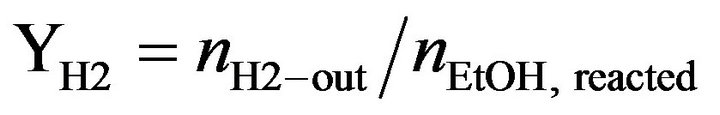 (3)
(3)
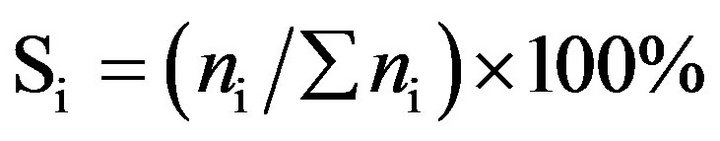 (4)
(4)
where ni was a mole of products and included H2.
3. Results and Discussion
3.1. Characterization of Supports and Catalysts
The XRD patterns at small angles of SBA-15, Mgx/ SBA-15 and CoyMgx/SBA-15 (x = 5 and 10; y = 10 and 20) samples are shown in Figure 1. The SBA-15 support (Figure 1(a)) shows a pattern with three well-resolved peaks observed at 2θ values of 0.92˚, 1.54˚ and 1.77˚ that correspond to the diffraction of (100), (110) and (200) planes, respectively, indicating their ordered 2D hexagonal structure with space group p6mm [13]. The d-spacing of this structure, calculated from nλ = 2dsinθ is 9.6 nm, which is also in the mesoporous range. Both Mgx/ SBA-15 samples (x = 5 and 10) are presented in Figures 1(b) and (c), respectively. The intensity of the diffraction peaks of the hexagonal mesostructure decreases gradually with the increase of x from 5 to 10. Moreover, a similar trend can be observed with the decrease in d-spacing where the d-spacing for x = 5 and 10 are 9.3 and 9.0 nm, respectively. The intensity of diffraction peaks for the CoyMgx/SBA-15 (y = 10 and 20) catalysts (Figures 1(d)-(h)) decreases with the increase of x and y, and weakens more than the Mgx/SBA-15 samples. Furthermore, the material composed of a high surface area, larger pores and thicker walls seems to disintegrate with increasing metal loading, raising doubt about the structural integrity.
The N2 adsorption-desorption analysis of the CoyMgx/ SBA-15 catalysts is shown in Figure 2. All of the samples exhibit a Type IV isotherm with a clear H1-type

Figure 1. Small angle XRD patterns of the samples: (a) SBA-15 (b) Mg5/SBA-15 (c) Mg10/SBA-15 (d) Co10Mg5/ SBA-15 (e) Co20Mg5/SBA-15 (f) Co20Mg5/SBA-15-H650 (g) Co10Mg10/SBA-15 (h) Co20Mg10/SBA-15.
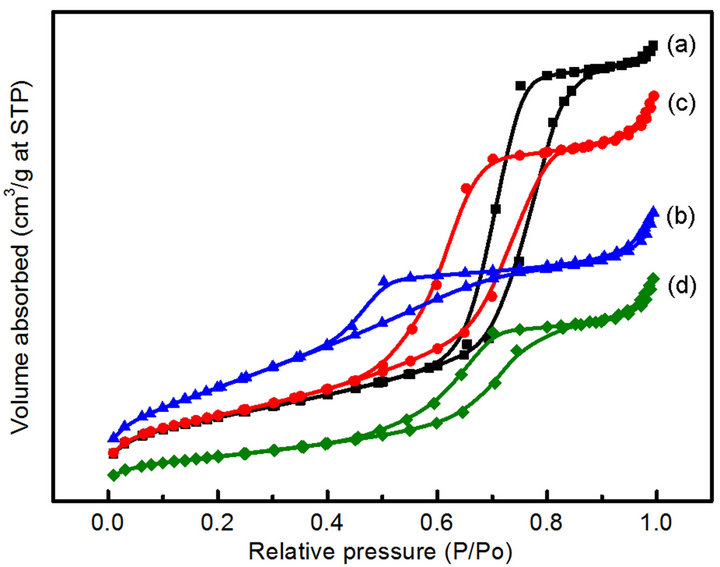
Figure 2. N2 adsorption/desorption isotherms of the samples: (a) Co10Mg5/SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/ SBA-15 (d) Co20Mg10/SBA-15.
hysteresis loop, with metal loading or not (SBA-15 and Mgx/SBA-15 samples are not shown), which is typical for mesoporous materials. Even though the XRD analysis showed the destruction of the hexagonal structure with impregnation of cobalt, the SBA-15 supported catalysts still maintained the mesoporous structure. Table 1 summarizes the physical characterization of CoyMgx/SBA-15 catalysts, which includes the metal loading, surface area and phase composition. The surface area decreases with the increase of the (x + y) value, where the surface areas are 359, 313, 234 and 130 m2/g, respectively for the values of 15, 20, 25 and 30. The decrease of surface area indicates that the mesoporous structure may be blocked by large amounts of Mg and Co loading.
The wide-angle XRD patterns of the CoyMgx/SBA-15 catalysts are shown in Figure 3. The broad and wide
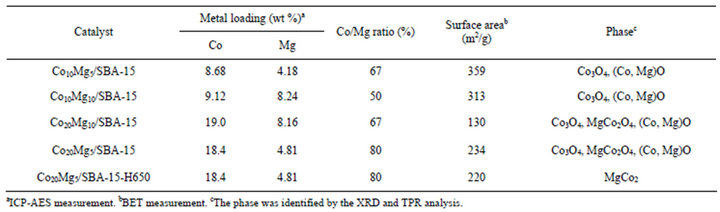
Table 1. Physical characterization of the CoyMgx/SBA-15 catalysts.
peak at 2θ around 15˚ - 30˚ is characteristic of amorphous silica. The peak related to MgO (2θ ≈ 42˚) is unobservable in the XRD patterns for CoyMgx/SBA-15 catalysts, which indicate the Mg shows highly dispersed on SBA-15 or becomes the nickel-magnesia solid solution oxides (Co, Mg)O [21,22]. Both the Co10Mg5/ SBA-15 and Co10Mg10/SBA-15 catalysts (Figure 3(a) and (b)) show the characteristic diffraction peaks corresponding to the (220), (311), (511) and (440) planes at 31.3˚, 36.8˚, 59.0˚ and 64.8˚, respectively. These are related to the cubic phase of Co3O4 (JCPDS No: 76-1802). The spinel structure of magnesium cobaltite MgCo2O4 [23,24] (JCPDS No: 81-0671) shows the corresponding planes of (111), (220), (311), (400), (511) and (440) at 18.9˚, 31.1˚, 36.6˚, 44.5˚, 58.9˚ and 64.7˚, respectively. These are obtained on the high Co loading catalysts of Co20Mg5/SBA-15 and Co20Mg10/SBA-15 (Figures 3(c) and (d)). Otherwise, the higher Co loading would show the stronger diffraction signal. In here, both the Co3O4 and MgCo2O4 phases are not able to give clear assignment, because their diffraction peaks are overlapped. Choudhary et al. [25] reported that the MgCo2O4 phase was only observed in the case of catalysts with high Co loadings, such as over 20%, which was supported by our results when y = 20. Therefore, the CoyMgx/SBA-15 catalysts may contain two phases of Co3O4 and MgCo2O4, and further investigation will be discussed on TPR analysis.
Figure 4 shows the TPR profiles of the CoyMgx/SBA- 15 catalysts. There are two continuous reduction peaks around 180˚C to 350˚C and broad peak around 500˚C to 700˚C, respectively. While the lower temperature peaks may be related to the two-steps reduction of Co3O4 [26] and the higher temperature peak is assigned the reduction of MgCo2O4 [25]. Besides, a faint peak over 800˚C may be attributed to the reduction of cobalt-magnesia solid solution oxides (Co, Mg)O formed on the catalysts [27]. Further, the reduction signal of Co3O4 would be raised by increasing the Co loading. These results are confirmed to the XRD study, the Co3O4 and MgCo2O4 phases are coexisting in CoyMgx/SBA-15 catalysts. Particularly, the
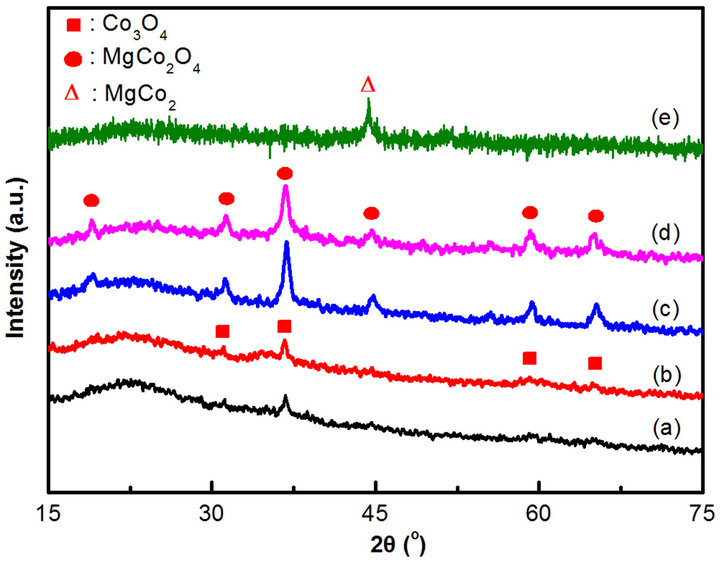
Figure 3. Wide angle XRD patterns of the samples: (a) Co10Mg5/SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/SBA-15 (d) Co20Mg10/SBA-15 (e) Co20Mg5/SBA-15-H650.
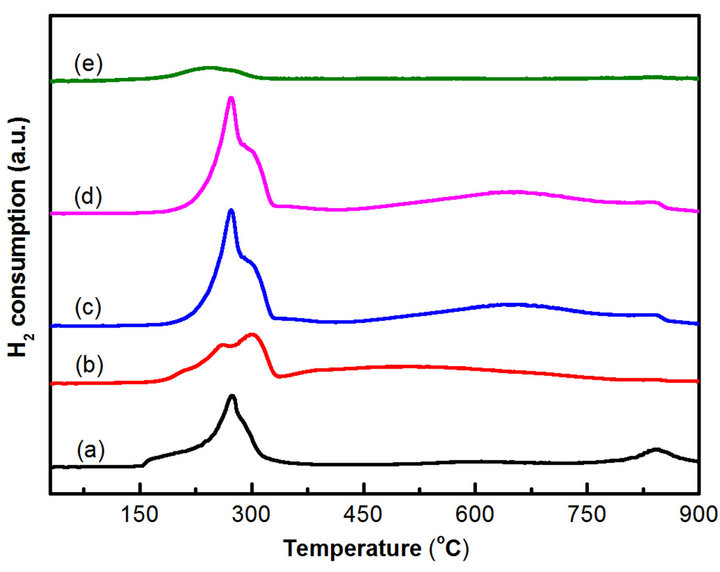
Figure 4. TPR profiles of the samples: (a) Co10Mg5/SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/SBA-15 (d) Co20Mg10/ SBA-15 (e) Co20Mg5/SBA-15-H650.
lower Mg loading will product the less amount of MgCo2O4 phase.
3.2. Catalytic Performance
Catalytic performance of ethanol conversion (XEtOH), products distribution and hydrogen yield (YH2) for the CoyMgx/SBA-15 catalysts are summarized in Table 2. The XEtOH reaches completion for Co10Mg5/SBA-15Co10Mg10/SBA-15 and Co20Mg10/SBA-15 catalysts as the reaction temperature (TR) approaches 475˚C; while the YH2 only approaches less than 2.0 at 550˚C. Otherwise, both the Co20Mg5/SBA-15 Co20Mg5/SBA-15-H650 catalysts show that the YH2 increases with TR and up to 5.02 and 5.78, respectively at 550˚C.
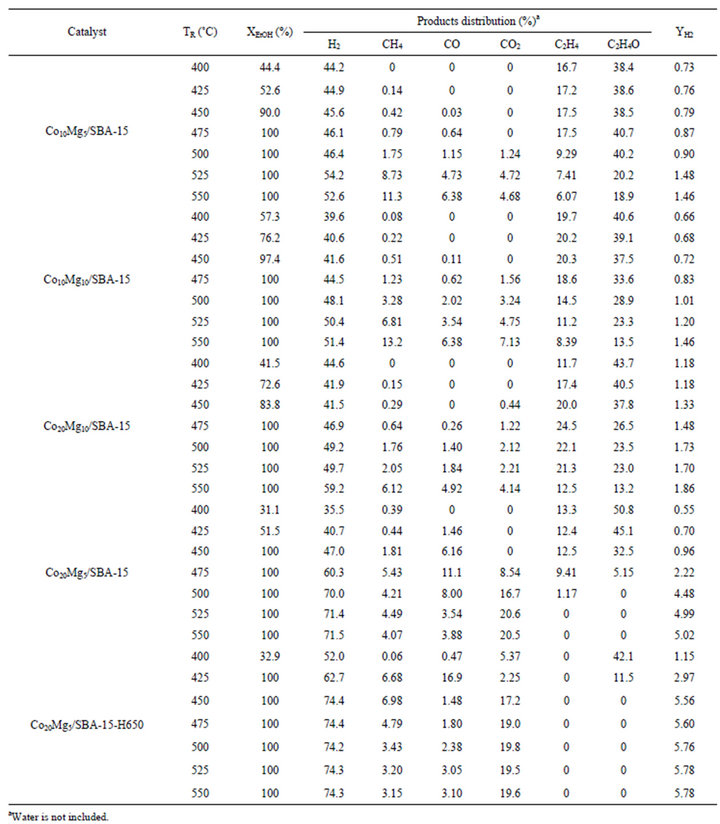
Table 2. Products distribution of SRE reaction over CoyMgx/SBA-15 catalysts.
Based on the phase diagram of the Mg-Co system [28], there is an equilibrium phase for MgCo2 when Co loading is over 67%. Two conditions are required to obtain MgCo2O4: a high Co loading over 20 wt% [25] and a Co/Mg ratio over 67% [28]. In regard to the pretreatment with reduction temperature effects for the sample with a Co/Mg ratio over 67%, a εCo structure is the major type for a reduction temperature below 422˚C. Otherwise, an αCo structure shows for a reduction temperature over 422˚C [28]. Compared to the pretreatment of temperature effects, a Co20Mg5/SBA-15 catalyst is reduced by H2 at 650˚C for 2 h (denoted as Co20Mg5/SBA-15-H650). The XRD characterization is shown in Figure 3(e) and the TPR analysis is shown in Figure 4(e). The XRD of Co20Mg5/SBA-15-H650 catalyst presences only a diffraction peak around 45˚C that can be identified and assigned to the (400) plane of the MgCo2 phase (JCPDS No. 29-0486). Since the Co20Mg5/SBA-15-H650 sample is storage in atmosphere, the oxidation of sample may be occurred. A TPR profile of Co20Mg5/SBA-15-H650 shows a weak peak below 350˚C which relates to the reduction of Co3O4.
In order to understand the variation in the Co/Mg ratio over 67% and pretreatment with the reduction temperature effect, both the Co20Mg5/SBA-15 and Co20Mg5/ SBA-15-H650 samples are further discussed. Temperature profiles of catalytic performance on the SRE reaction over the Co20Mg5/SBA-15 and Co20Mg5/SBA-15- H650 samples are described in Figures 5 and 6. There are significant differences in catalytic activity and products distribution due to the high temperature reduction. The Co20Mg5/SBA-15-H650 sample is better than the Co20Mg5/SBA-15 sample. The results indicate that the XEtOH approaches completion around 425˚C for Co20Mg5/ SBA-15-H650 samples while requiring 450˚C for Co20 Mg5/SBA-15 samples to complete the conversion. The YH2 increases up to 5.78 and SCO is 3.10% for the Co20 Mg5/SBA-15-H650 sample, while the YH2 approaches 5.02 and SCO is 3.88% for the Co20Mg5/SBA-15 sample at 550˚C. Dehydration especially from ethanol to ethylene is a dominant reaction for all samples that are not pretreated under high temperature reduction, where the selectivity of C2H4 is over 10%.
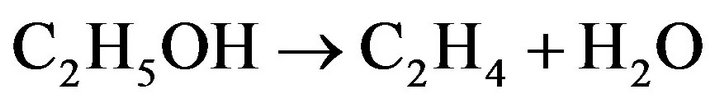 (5)
(5)
The main reaction is the dehydrogenation of ethanol to acetaldehyde at low temperature. As the temperature raised, a major reaction proceeded the decomposition of acetaldehyde into methane and CO for Co20Mg5/SBA-15 and Co20Mg5/SBA-15-H650 samples.
C2H5OH → CH3CHO + H2 (6)
CH3CHO → CH4 + CO (7)
Comparing the temperature effect on the decomposi-
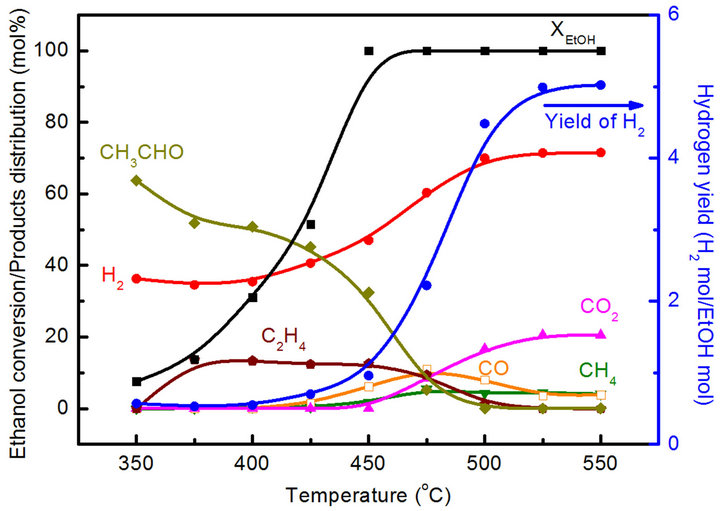
Figure 5. Catalytic performance of SRE reaction over Co20Mg5/SBA-15 catalyst.
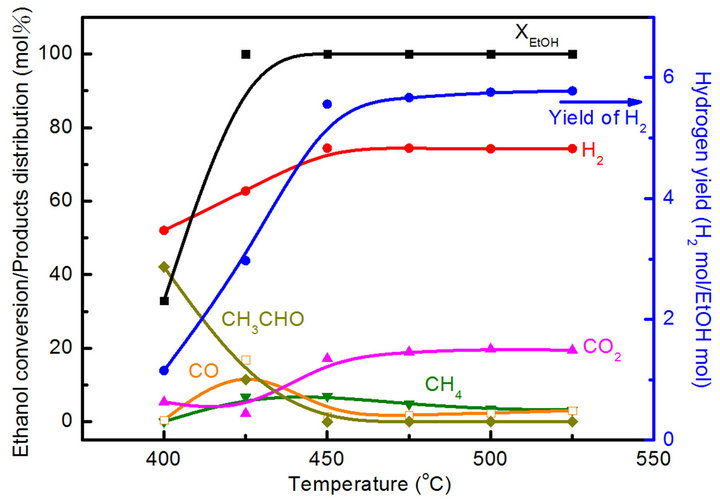
Figure 6. Catalytic performance of SRE reaction over Co20Mg5/SBA-15-H650 catalyst.
tion of acetaldehyde (DT), shows that the easy cracking of acetaldehyde promotes the increase of hydrogen yield. However, the promoting effect of a Co20Mg5/SBA-15- H650 sample is more pronounced than that of a Co20Mg5/ SBA-15 sample. The DT of a Co20Mg5/SBA-15-H650 sample is lower than 400˚C, while it is above 450˚C for a Co20Mg5/SBA-15 sample.
The distribution of CO is minor when the TR is above 425˚C for a Co20Mg5/SBA-15-H650 sample. This demonstates that the water-gas shift reaction (WGSR) is an important side-reaction in the SRE reaction producing H2 and CO2.
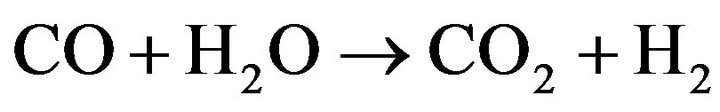 (8)
(8)
At 525˚C, the selectivity of CH4, CO and CO2 arrive at 3.20%, 3.05% and 19.5%, respectively, for a Co20Mg5/ SBA-15-H650 sample. The hydrogen selectivity is close to its stoichiometric value (75%), whereas an increase of up to 74% is obtained at over 450˚C. Unlike the Co20Mg5/SBA-15 and Co20Mg5/SBA-15-H650 samples, other CoyMgx/SBA-15 catalysts show poor catalytic performance in an SRE reaction. The low H2 yields (<1.9) and CO2 selectivity are produced by the ethanol dehydration to ethylene followed by steam reforming, where C2H4 is up to 20% at 450˚C. However, the formation of carbon through C2H4 is a possible route, which leads to catalyst deactivation.
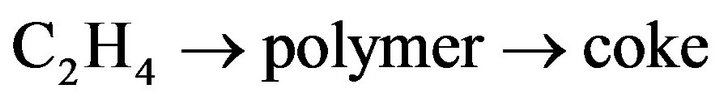 (9)
(9)
3.3. Characterization of Used Catalyst
XRD and TEM analysis are used to characterize the CoyMgx/SBA-15 catalysts after the SRE reaction. XRD patterns reveal MgO (JCDPS No. 4-829) and CoO (JCDPS No. 78-0431) diffraction patterns on Co10Mg5/ SBA-15, Co10Mg10/SBA-15 and Co20Mg10/SBA-15 samples (Figures 7(a), (b) and (e)). Only the Co20Mg5/ SBA-15 and Co20Mg5/SBA-15-H650 samples show metallic Co (JCDPS No. 89-4307) reflections of (111) and (200) planes (Figures 7(c) and (d)). These results are in good agreement, helping to convince researchers that the Co20Mg5/SBA-15 and Co20Mg5/SBA-15-H650 samples show the better catalytic activity than others in an SRE reaction, exhibiting an active site of metallic Co. The metallic Co usually generated via the reduction of Co3O4, which was easily sintered if the interaction with the support was absent [27]. However, the Co20Mg5/SBA-15 and Co20Mg5/SBA-15-H650 samples could form MgCo2O4 or MgCo2 phases and formatted well-dispersed Co clusters, which are more resistant to sintering due to a stronger interaction between MgO and the support [25]. Based on previous reports [27,29], coke formation would not be stimulated on well-dispersed Co clusters to deactivate the catalyst.
The TEM images (Figure 8) show that carbon deposited as large filaments and tubes emerged with the catalyst particles and/or as an amorphous coating carbon on
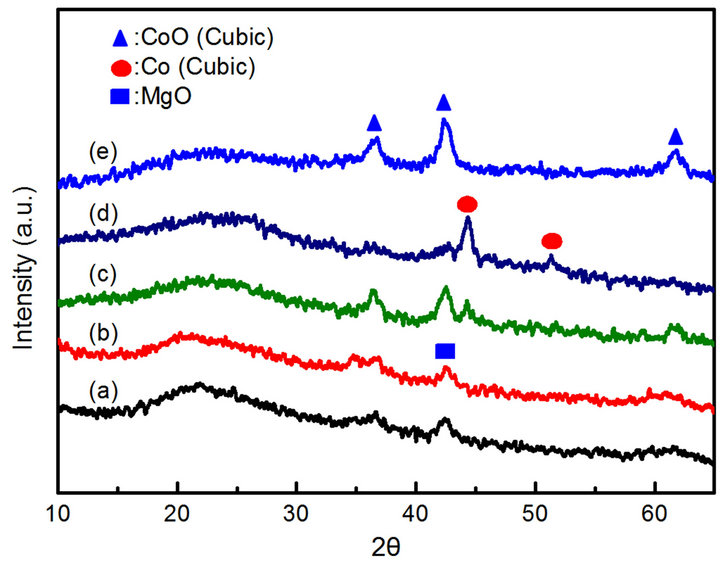
Figure 7. Wide angle XRD patterns of spent catalysts: (a) Co10Mg5/SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/SBA-15 (d) Co20Mg5/SBA-15-H650 (e) Co20Mg10/SBA-15.

Figure 8. TEM images of spent catalysts: (a) Co10Mg5/ SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/SBA-15 (d) Co20Mg5/SBA-15-H650 (e) Co20Mg10/SBA-15.
the catalyst particles. The filaments and tubes carbon is shown in the Co20Mg5/SBA-15 and Co20Mg5/SBA-15- H650 samples (Figures 8(c) and (d)), and amorphous carbon is presented in the Co10Mg5/SBA-15, Co10Mg10/ SBA-15 and Co20Mg10/SBA-15 catalysts (Figures 8(a), (b) and (e)). According to the deactivation with the deposited carbon, the coating carbon could shorten the lifetime of a catalyst rather than filaments carbon [30], which agreed with our results. Moreover, all the samples maintain a mesoporous structure of SBA-15 after the SRE reaction. A good thermal stability is presented.
4. Conclusion
Steam reforming of ethanol was studied over SBA-15 supported catalysts with a Mg promoter and a cobalt loading of 10 and 20 wt%. The Co/Mg ratio and pretreatment of catalysts play a major role on the catalytic performance regarding the structural properties. A high catalytic performance and hydrogen yield were obtained on the high loading of Co, where the Co/Mg ratio was 0.8. According to the phase diagram of an Mg-Co system, a reduction temperature of 650˚C would form MgCo2 as the main phase convinced by XRD, which leads to the active site to enhance the catalytic performance. The YH2 approaches 5.78 and the SCO is 3.10% for Co20Mg5/SBA- 15-H650 sample as the TR approaches 550˚C.
5. Acknowledgements
We are pleased to acknowledge the financial support for this study by the National Science Council of the Republic of China under contract numbers of NSC 99-2113- M-606-001-MY3 and NSC 101-2623-E-155- 001-ET.
REFERENCES
- R. D. Cortright, R. R. Davda and J. A. Dumesic, “Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water,” Nature, Vol. 418, No. 6901, 2002, pp. 964-967. doi:10.1038/nature01009
- D. K. Liguras, D. I. Kondarides and X. E. Verykios, “Production of Hydrogen For Fuel Cells by Steam Reforming of Ethanol over Supported Noble Metal Catalysts,” Applied Catalysis B: Environmental, Vol. 43, No. 4, 2003, pp. 345-354. doi:10.1016/S0926-3373(02)00327-2
- P. Ramirez de la Piscina and N. Homs, “Use of Biofuels to Produce Hydrogen (Reformation Processes),” Chemical Society Review, Vol. 37, No. 11, 2008, pp. 2459-2467. doi:10.1039/b712181b
- F. Haga, T. Nakajima, H. Miya and S. Mishima, “Catalytic Properties of Supported Cobalt Catalysts for Steam Reforming of Ethanol,” Catalysis Letters, Vol. 48, No. 3- 4, 1997, pp. 223-227. doi:10.1023/A:1019039407126
- J. Llorca, N. Homs, J. Sales and P. Ramirez de la Piscina, “Efficient Production of Hydrogen over Supported Cobalt Catalysts from Ethanol Steam Reforming,” Journal of Catalysis, Vol. 209, No. 2, 2002, pp. 306-317. doi:10.1006/jcat.2002.3643
- M. C. Batista, R. K. S. Santos, E. M. Assaf, J. M. Assaf and E. A. Ticianelli, “High Efficiency Steam Reforming of Ethanol by Cobalt-Based Catalysts,” Journal of Power Sources, Vol. 134, No. 1, 2004, pp. 27-32. doi:10.1016/j.jpowsour.2004.01.052
- J. Llorca, P. Ramirez de la Piscina, J.-A. Dalmon, J. Sales and N. Homs, “CO-Free Hydrogen from Steam-Reforming of Bioethanol over ZnO-Supported Cobalt Catalysts Effect of the Metallic Precursor,” Applied Catalysis B: Environmental, Vol. 43, No. 4, 2003, pp. 355-369. doi:10.1016/S0926-3373(02)00326-0
- J. M. Pigos, C. J. Brooks, G. Jacobs and B. H. Davis, “Low Temperature Water-gas Shift: The Effect of Alkali Doping on the C-H Bond of Formate over Pt/ZrO2 Catalysts,” Applied Catalysis A: General, Vol. 328, No. 1, 2007, pp. 14-26. doi:10.1016/j.apcata.2007.04.001
- C. H. Wang, K. F. Ho, J. Y. Z. Chiou, C. L. Lee, S. Y. Yang, C. T. Yeh and C. B. Wang, “Oxidative Steam Reforming of Ethanol over PtRu/ZrO2 Catalysts Modified with Sodium and Magnesium,” Catalysis Communications, Vol. 12, No. 10, 2011, pp. 854-858. doi:10.1016/j.catcom.2011.02.002
- Z. Cheng, Q. Wu, J. Li and Q. Zhu, “Effects of Promoters and Preparation Procedures on Reforming of Methane with Carbon Dioxide over Ni/Al2O3 Catalyst,” Catalalysis Today, Vol. 30, No. 1-3, 1996, pp. 147-155. doi:10.1016/0920-5861(95)00005-4
- D. H. Olson, G. T. Kokotailo, S. L. Lawton and W. M. Meler, “Crystal Structure and Structure-Related Properties of ZSM-5,” The Journal of Physical Chemistry, Vol. 85, No. 15, 1981, pp. 2238-2243. doi:10.1021/j150615a020
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, “Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-crystal Template Mechanism,” Nature, Vol. 359, No. 6397, 1992, pp. 710-712. doi:10.1038/359710a0
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, “Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores,” Science, Vol. 279, No. 5350, 1998, pp. 548-552. doi:10.1126/science.279.5350.548
- A. J. Vizcaíno, A. Carrero and J. A. Calles, “Hydrogen Production by Ethanol Steam Reforming over Cu-Ni Supported Catalysts” International Journal of Hydrogen Energy, Vol. 32, No. 10-11, 2007, pp. 1450-1461. doi:10.1016/j.ijhydene.2006.10.024
- A. Carrero, J. A. Calles and A. J. Vizcaíno, “Hydrogen Production by Ethanol Steam Reforming over Cu-Ni/ SBA-15 Supported Catalysts Prepared by Direct Synthesis and Impregnation,” Applied Catalysis A: General, Vol. 327, No. 1, 2007, pp. 82-94. doi:10.1016/j.apcata.2007.04.030
- A. J. Vizcaíno, A. Carrero and J. A. Calles, “Ethanol Steam Reforming on Mgand Ca-modified Cu-Ni/SBA-15 Catalysts,” Catalysis Today, Vol. 146, No. 1-2, 2009, pp. 63- 70. doi:10.1016/j.cattod.2008.11.020
- J. A. Calles, A. Carrero and A. J. Vizcaíno, “Ce and La Modification of Mesoporous Cu-Ni/SBA-15 Catalysts for Hydrogen Production through Ethanol Steam Reforming,” Microporous and Mesoporous Materials, Vol. 119, No. 1-3, 2009, pp. 200-207. doi:10.1016/j.micromeso.2008.10.028
- A. Carrero, J. A. Calles and A. J. Vizcaino, “Effect of Mg and Ca Addition on Coke Deposition over Cu-Ni/SiO2 Catalysts for Ethanol Steam Reforming,” Chemical Engineering Journal, Vol. 163, No. 3, 2010, pp. 395-402. doi:10.1016/j.cej.2010.07.029
- K. Wang, X. Li, S. Ji, X. Shi and J. Tang, “Effect of CexZr1−xO2 Promoter on Ni-Based SBA-15 Catalyst for Steam Reforming of Methane,” Energy & Fuels, Vol. 23, No. 1, 2009, pp. 25-31. doi:10.1021/ef800553b
- H. Wang, Y. Liu, L. Wang and Y. Qin, “Study on the Carbon Deposition in Steam Reforming of Ethanol over Co/CeO2 Catalyst,” Chemical Engineering Journal, Vol. 145, No. 1, 2008, pp. 25-31. doi:10.1016/j.cej.2008.02.021
- B. Huang, X. Li, S. Ji, B. Lang, F. Habimana and C. Li, “Effect of MgO Promoter on Ni-based SBA-15 Catalysts for Combined Steam and Carbon Dioxide Reforming of Methane,” Journal of Natural Gas Chemistry, Vol. 17, No. 3, 2008, pp. 225-231. doi:10.1016/S1003-9953(08)60055-9
- W. Liu, S. Y. Lai, H. X. Dai, S. J. Wang, H. Z. Sun and C. T. Au, “MgO-Modified VOx/SBA-15 as Catalysts for the Oxidative Dehydrogenation of n-Butane,” Catalysis Today, Vol. 131, No. 1-4, 2008, pp. 450-456. doi:10.1016/j.cattod.2007.10.054
- M. A. Zamudio, S. Bensaid, D. Fino and N. Russo, “Influence of the MgCo2O4 Preparation Method on N2O Catalytic Decompositio,” Industrial & Engineering Chemistry Research, Vol. 50, No. 5, 2011, pp. 2622-2627. doi:10.1021/ie100658w
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, “Studies on Spinel Cobaltites, FeCo2O4 and MgCo2O4 as Anodes for Li-ion batteries,” Solid State Ionics, Vol. 179, No. 15-16, 2008, pp. 587-597. doi:10.1016/j.ssi.2008.04.007
- V. R. Choudhary, K. C. Mondal and T. V. Choudhary, “CO2 Reforming of Methane to Syngas over CoOx/MgO Supported on Low Surface Area Macroporous Catalyst Carrier: Influence of Co Loading and Process Conditions,” Industrial & Engineering Chemistry Research, Vol. 45, No. 13, 2006, pp. 4597-4602. doi:10.1021/ie060260a
- C. B. Wang, C. C. Lee, J. L. Bi, J. Siang, J. Y. Liu and C. T. Yeh, “Study on the Steam Reforming of Ethanol over Cobalt Oxides,” Catalysis Today, Vol. 146, No. 1-2, 2009, pp. 76-81. doi:10.1016/j.cattod.2008.12.010
- H. Y. Wang and E. Ruckenstein, “CO2 Reforming of CH4 over Co/MgO Solid Solution Catalysts—Effect of Calcination Temperature and Co loading,” Applied Catalysis A: General, Vol. 209, No. 1-2, 2001, pp. 207-215. doi:10.1016/S0926-860X(00)00753-5
- A. A. Nayeb-Hashemi and J. B. Clark, “The Co-Mg (Cobalt-Magnesium) System,” Bulletin of Alloy Phase Diagrams, Vol. 8, No. 4, 1987, pp. 352-354.
- E. Ruckenstein and H. Y. Wang, “Carbon Deposition and Catalytic Deactivation during CO2 Reforming of CH4 over Co/γ-Al2O3 Catalysts,” Journal of Catalysis, Vol. 205, No. 2, 2002, pp. 289-293. doi:10.1006/jcat.2001.3458
- I. Suelves, M. J. Lázaro, R. Moliner, B. M. Corbella and J. M. Palacios, “Hydrogen Production by Thermo Catalytic Decomposition of Methane on Ni-Based Catalysts: In- fluence of Operating Conditions on Catalyst Deactivation and Carbon Characteristics,” International Journal of Hydrogen Energy, Vol. 30, No. 15, 2005, pp. 1555-1567. doi:10.1016/j.ijhydene.2004.10.006
NOTES
*Corresponding author.

