Modern Research in Catalysis
Vol.1 No.3(2012), Article ID:23589,6 pages DOI:10.4236/mrc.2012.13007
Effect of the Preparation Method on Co/Al2O3 Catalyst Applied to Ethanol Steam Reforming Reaction Production of Hydrogen
Laboratory of Catalysis-Chemical Engineering Department, Federal University of Sao Carlos, São Carlos, Brazil
Email: silmararodriguesgarcia@gmail.com
Received August 29, 2012; revised September 30, 2012; accepted October 8, 2012
Keywords: Hydrogen; Ethanol; Reform; Cobalt; Alumina
ABSTRACT
Alumina supported cobalt catalysts were prepared, characterized and applied in ethanol steam reforming for hydrogen production. The support and the supported catalysts were prepared, respectively, by the solvothermal and precipitation, impregnation and deposition-precipitation methods. The cobalt was added by impregnation and deposition-precipitation in the Al2O3 supports using a Co(NO3)2·6H2O solution. The solids were characterized, Temperature-Programmed Reduction with H2 (RTP-H2), X-Ray Diffraction (DRX), BET Nitrogen Adsorption and Temperature Programmed Oxidation (TPO). The results indicated that the preparation method and the treatment conditions of samples were appropriate for obtaining the wanted compounds. Co3O4 phase was verified for all catalysts through analyses of DRX and RTP-H2 results. Catalytic tests were performed by varying the temperature from 450˚C to 600˚C, with water: ethanol molar ratio of 3:1. The ethanol conversion was superior of 99%, with greater hydrogen yield at 600˚C. The lower carbon deposition was observed in catalysts prepared with solvothermal/deposition-precipitation methods at 450˚C.
1. Introduction
H2 will have an important role in the future world economy scenario as a clean, renewable, and efficient fuel. The ethanol steam reforming has been rising as a promising alternative route in H2 production, which can be produced via biomass fermentation [1]. Moreover, such process seems to be promising for CO2 emission control, once the CO2 generated in the reforming process is consumed by the biomass, closing a cycle, thus, not contributing to the greenhouse effect [2]. H2 may be applied in fuel cells, a device that uses H2 and O2 to generate electricity and has water vapor as the only reaction product [3]. The development of industrial catalytic processes, demands research and development of new catalysts that are more active, and, especially more selective and stable [4].The overall ethanol steam reforming reaction (1) yields 6 moles of hydrogen per mole of ethanol.
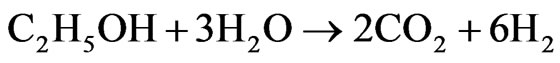 (1)
(1)
ΔH˚f(25˚C) = +171 kJ/mol, ΔG˚f(25˚C) = +65 kJ/mol The main problem encountered in the steam reforming process is in the catalysts deactivation by carbon deposition. Thermodynamic studies indicate the need of working with high water/ethanol ratio [5], which is an unfavorable condition for carbon formation. Another alternative may be the use of promoters for catalyst, which appear to be efficient for reduction of carbon formation in hydrocarbons steam reforming [6]. Therefore, research on catalysts that rapidly transform CO into CO2 (2), the so-called “Shift” reaction (or water gas shift reaction-WGS), and also must be selective for the overall ethanol steam reforming reaction (1) with H2 production [7] has been intensified in order to efficiently meet the constant challenges and the determination of appropriate conditions for the reaction [8].
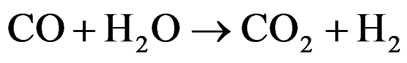 (2)
(2)
ΔH˚f(25˚C) = −41 kJ/mol, ΔG˚f(25˚C) = −28 kJ/mol Among the catalysts that present activity for the ethanol steam reforming reaction, those based on cobalt stand out for their high hydrogen production [9]. By analyzing some studies on cobalt catalysts supported on alumina, silica, magnesia, and carbon, it can be verified that the Co/Al catalyst presented the best results and hydrogen selectivity of approximately 67% [10]. Thus, attention has been focused on the development of active and stable catalysts that are selective to ethanol steam reforming reactions. So far, the main problems to be faced are metal sintering and carbon formation.
Therefore, the objective of this paper was to study the influence of the preparation methods of catalysts on activity and yield in order to produce hydrogen, as well as to evaluate coke deposition on these catalysts to contribute to the development of catalysts for the ethanol steam reforming reaction
2. Experimental Procedure
2.1. The Support Prepared with the Solvothermal Method
Was obtained with 1-butanol aqueous solutions and isopropoxyaluminum. Then, the solution was transferred to a 50 mL autoclave. The mixture was subjected to 210˚C in an oven with heating ramp of 10˚C/min for 5 hours. After cooling to room temperature, the solution was vacuum filtered with successive washings with acetone. The solid was dried at 60˚C for 24 hours and, then, left at room temperature for 24 hours for solvent removal. The sample was calcined at a temperature of 600˚C at a heating rate of 10˚C/min under a synthetic air flow rate of 80 mL/min until the desired temperature was reached, remaining at it for 2 hours and at a ramp of 10˚C/min.
2.2. The Support Prepared with the Precipitation Method
In a 0.6M solution of aluminum nitrate was added polyethylene glycol. Ammonium carbonate, 0.6 M, was slowly added to the solution under stirring at 70˚C. The pH of the mixture increased to 7. The mixture was aged at 70˚C for 6 h. The solution was vacuum filtered in order to separate the precipitation from the solution with successive washings with warm distilled water. The solid was dried at room temperature for 24 h. Next, it was kept in an oven at 80˚C for 6 h. The sample was first calcined in nitrogen at 330˚C at a heating rate of 3˚C/min under a flow rate of 80 mL/min for 3 h, and, then, it was calcined in synthetic air at 550˚C at a heating rate of 3˚C/min under a flow rate of 80 mL/min for 2 h and heating rate of 10˚C/min.
2.3. Impregnation and Deposition-Precipitation
The Co/Al catalyst was prepared by support impregnation from a cobalt nitrate aqueous solution in route evaporator at 80˚C and under stirring. Subsequently, the solid was dried at 60˚C for 24 hours and calcined at 600˚C for 2 hours under synthetic air flow rate of 80 mL/min and heating ramp of 10˚C/min. Another method used in the catalyst preparation was the deposition-precipitation method from aqueous solutions of 1:3 Co(NO3)2‧6H2O: urea in route evaporator at 80˚C under stirring. The precursor was dried in an oven at low temperature, 60˚C, for 24 h to remove excess solvent. Then, it was calcined at 600˚C for 2 hours under synthetic air flow rate of 80 mL/min a heating ramp of 10˚C/min.
3. Characterization of Materials
The following characterization techniques were used: XRay Diffraction (DRX), BET Specific Area, Temperature-Programmed Reduction with H2 (RTP-H2), Temperature Programmed Oxidation (TPO) and Energy-Dispersive X-Ray Spectroscopy (EDX), which allowed the qualification and quantification of the physical and chemical properties of catalysts.
Catalysts were characterized by: X-Ray Diffraction (DRX) in a Rigaku Multiflex diffractometer with CuKα
radiation, 10˚ to 80˚ (2θ) scanning range; measurements of Temperature-Programmed Reduction (RTP) in a Micromeritcs Chemissorb with 30 mL/min flow rate of 5% H2/N2 mixture and ramp of 10˚C/min up to 1000˚C; BET specific area measurements performed by physical absorption of nitrogen in a Quantachrome NOVA 1200 P; Temperature Programmed Oxidation (TPO) to quantify carbon formation in the catalyst. An SDT 2960 SIMULTANEOUS DSC-TGA TA-INSTRUMENTS was used in oxidizing environment and heating rate of 10˚C/min up to 1000˚C.
4. Catalytic Trials
Trials were performed with feeding of a liquid mixture of water and ethanol, at a molar 3:1 water: ethanol ratio, and nitrogen was used as carrier gas, once feeding rate is considered too low. The gas flow rate was controlled by a mass flow controller (MKS Instruments, model 247 with 4 channels), and the liquid flow rate was obtained with the help of a metering pump. The catalytic trials were performed by varying the temperature from 450˚C and 600˚C.
The reactions were performed in a quartz reactor during steam reforming. The reactor was set as follows: quartz wool was placed in the center of the reactor, and 150 mg of each catalyst sample was placed on the wool. The reaction trials were performed at pressures close to the ambient one. The reaction products were analyzed through gas chromatography in a Varian 3800 chromatograph, after water separation through condensation. Gas adsorption was performed after condensation on a molecular sieve filter in order to remove the remaining moisture.
5. Results and Discussion
In this study, the catalysts were named as: Al S, Co/Al S - I, Co/Al S - DP, Al P, Co/Al P - I, Co/Al P - DP.
5.1. Characterization of Materials
5.1.1. Specific Surface Area (B. E. T)
The results of the analysis performed to determine the specific areas are presented in Table 1. They show that the γ-alumina support prepared through the precipitation
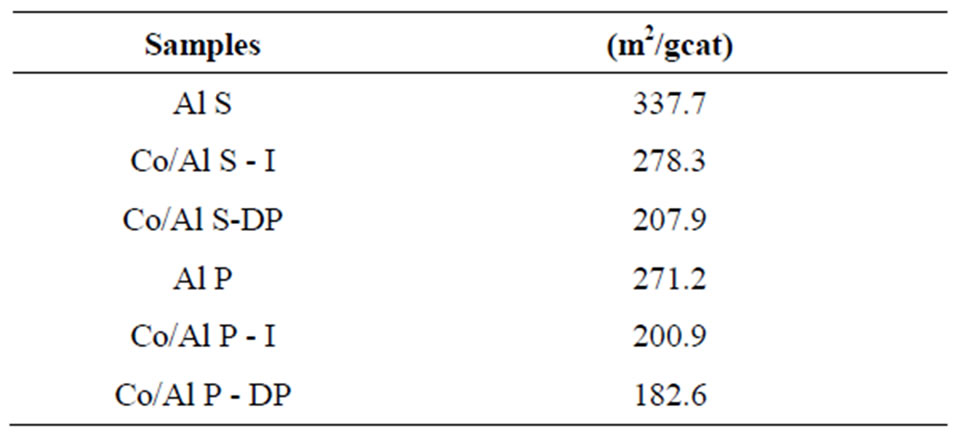
Table 1. The BET surface area.
method presents smaller surface area when compared to the one prepared through the solvothermal method. The analysis of the results for surface area shows that, once the oxides were impregnated on the support, a decrease in the catalyst surface area occurred. This decrease is due to the Co introduction method, such as for the sample with Co/Al S - I in relation to Co/Al S - DP, which causes a decrease in area of approximately 45%; it is also due to alumina pore coating by cobalt oxide and to the small specific area of these cobalt oxides, which leads to a decrease in the surface area of the catalyst/support set.
5.1.2. X-Ray Diffraction (DRX)
Figures 1 and 2 show the analysis results of X-ray diffraction obtained for the samples whose supports were prepared with the solvothermal and precipitation methods. Identification of crystalline phases was performed by comparison to data from literature and from JCPDS (2001). The diffraction peaks at 2θ = 37.01˚, 46.91˚ and 67.97˚ might be attributed to gamma-alumina, and the ones at 2θ = 25˚, 31˚, 37˚ and 61˚ to the presence of Co3O4.
5.1.3. Temperature-Programmed Reduction
In Figures 3-6, the peaks formed below 400˚C, correspond to the direct reduction of the Co3O4 phase to Co0, while the peaks formed in the temperature range from 500˚C to 700˚C, might be attributed to the reduction of highly disperse Co3O4. Some authors suggest that these peaks are results of reduction in two steps, from relatively large particles of Co3O4 to Co0, via CoO. As for the peaks formed above 700˚C, they correspond to the reduction of a mixed phase, CoO-Al2O3 (non-stoichiometric aluminate) [11]. It can be observed that the impregnated samples present a higher number of peaks, which indicates a higher distribution of phases and interactions with the support with little homogeneity, which differs from the behavior observed on the samples prepared via the deposition-precipitation method.
5.1.4. Reactions on Co/Al Catalyst
The results of reaction with the catalyst prepared in this study are presented in Figures 7-10. The influence of operating parameters on product formation in relation to converted ethanol (yield) was analyzed.
In Figure 7, the catalyst Co/Al S - I, at 450˚C was more stable, but still shows activity to ethylene formation, which is evidence of the competition between the ethanol dehydration reaction on acid alumina sites and the ethanol steam reforming reaction on metallic sites [12]. This catalyst presents higher CO2 yield at lower reaction temperature. After 3 hours of reaction at 450˚C, a drop in H2 is observed. During this period, there was an increase
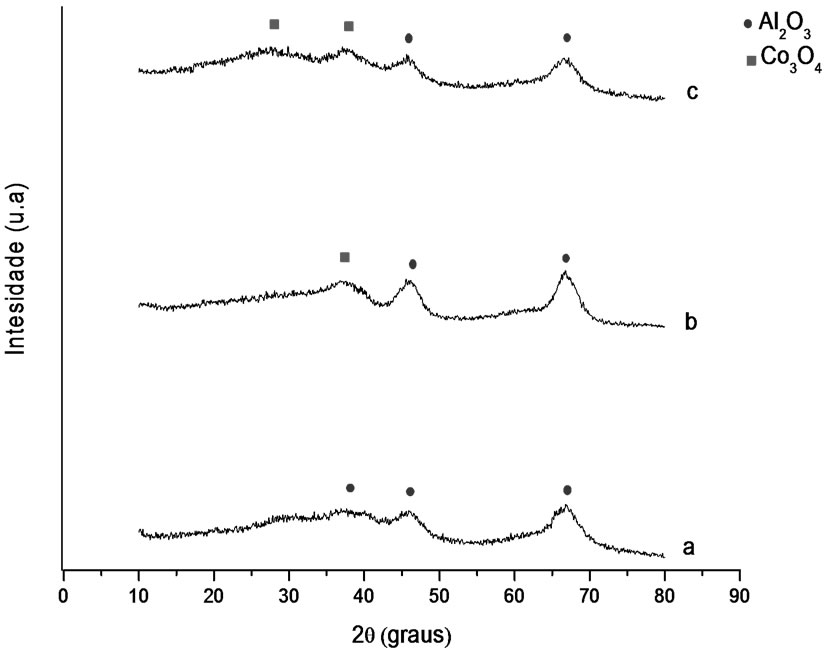
Figure 1. X-ray diffractograms. (a) γ-Al2O3 S, (b) Co/Al S-I, (c) Co/Al S-DP.
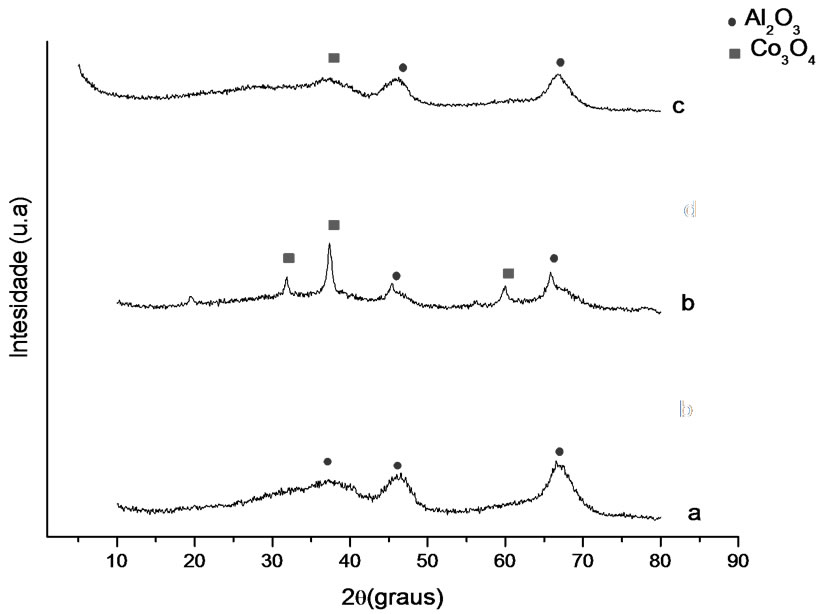
Figure 2. X-ray diffractograms. (a) γ-Al2O3 P, (b) Co/Al P-I, (c) Co/Al P-DP.
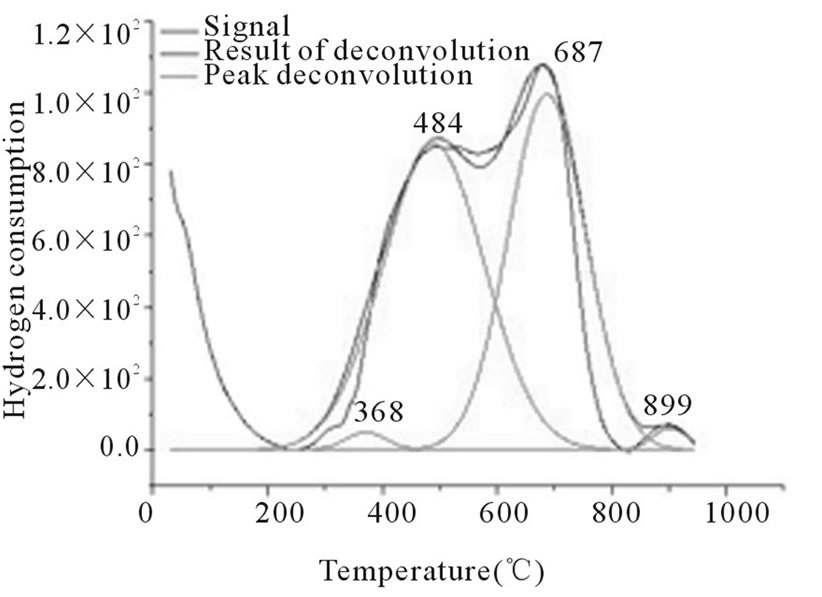
Figure 3. RTP Co/Al S-I
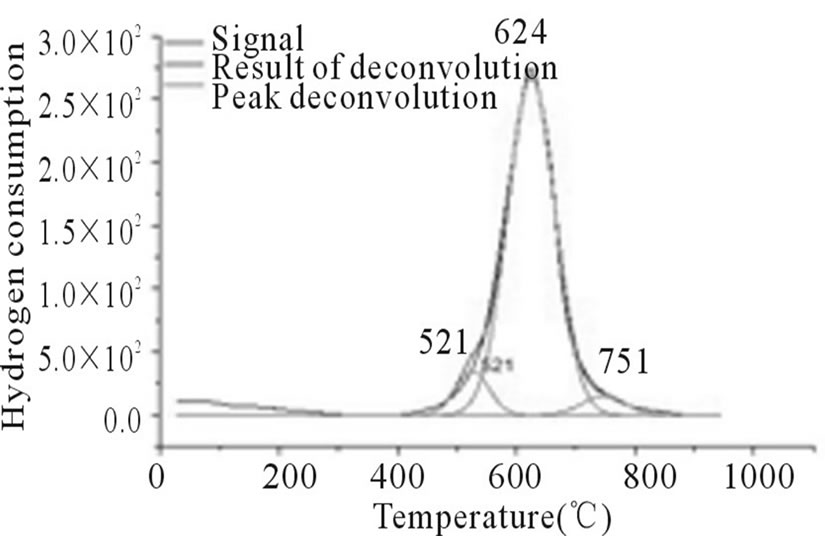
Figure 4. RTP Co/Al S-DP.
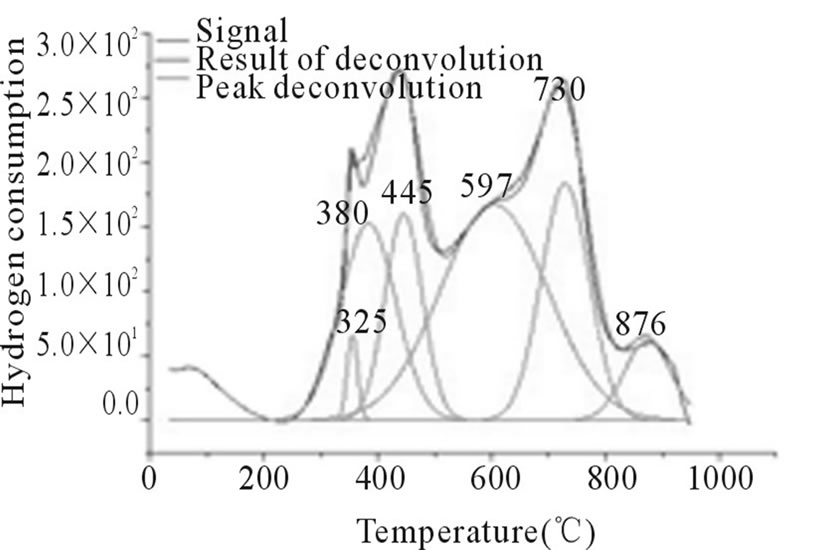
Figure 5. RTP Co/Al P-I.
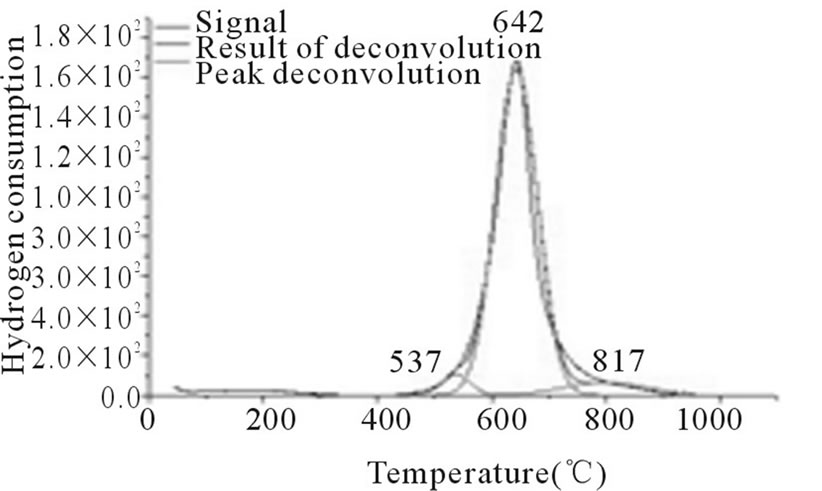
Figure 6. RTP Co/Al P-DP.
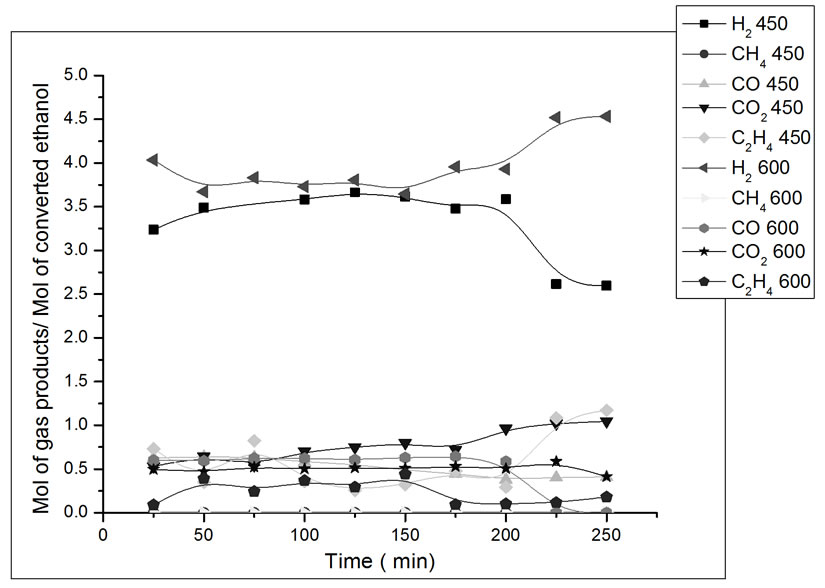
Figure 7. Yield in mol of gas products/mol of converted ethanol as a function of ethanol steam reforming reaction time. Catalyst Co/Al S-I, T = 450˚C and 600˚C. Ethanol: water ratio = 3:1.
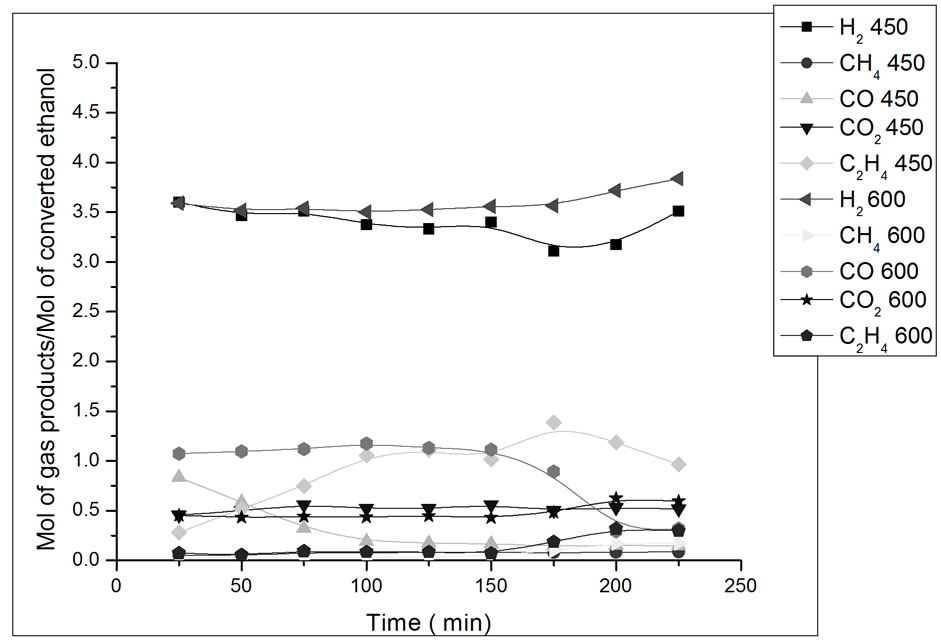
Figure 8. Yield in mol of gas products/mol of converted ethanol as a function of ethanol steam reforming reaction time. Catalyst Co/Al S-DP, T = 450˚C and 600˚C. Ethanol: water ratio = 3:1.
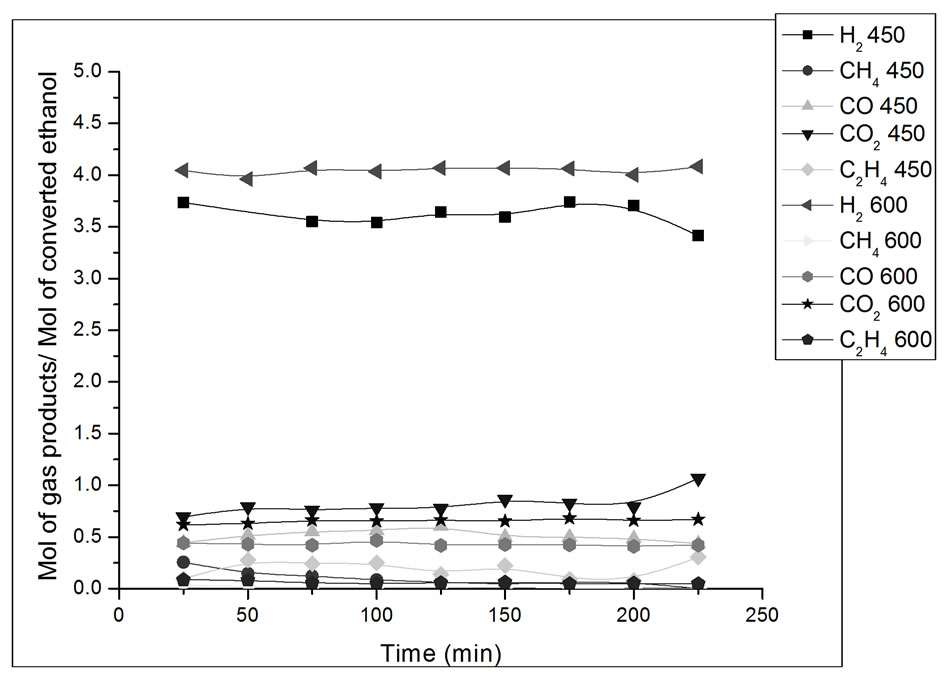
Figure 9. Yield in mol of gas products/mol of converted ethanol as a function of ethanol steam reforming reaction time. Catalyst Co/Al P-I, T = 450˚C and 600˚C. Ethanol: water ratio = 3:1.
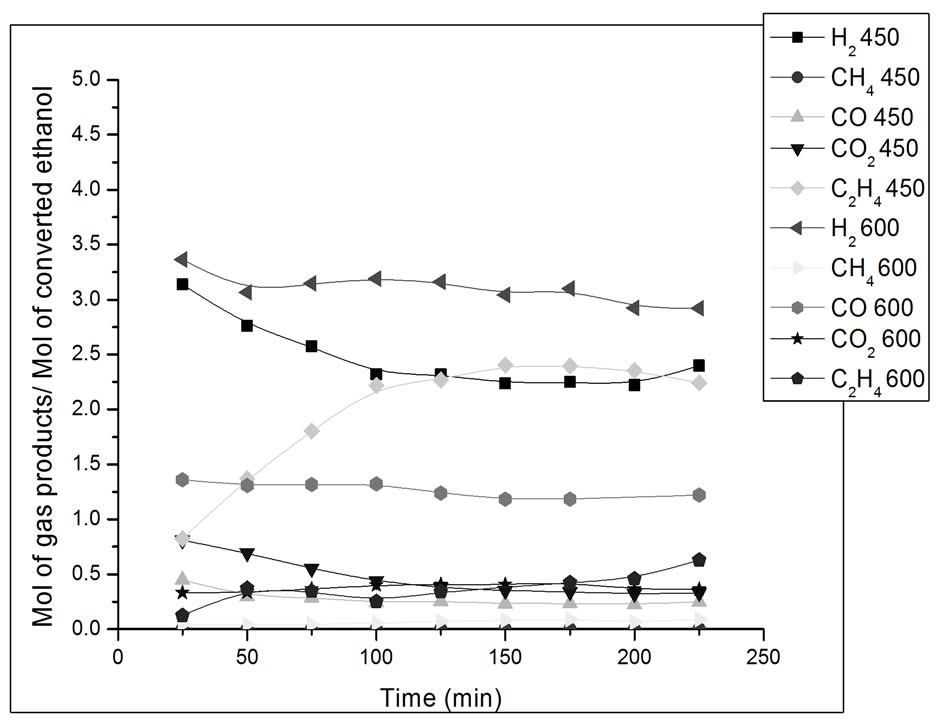
Figure 10. Yield in mol of gas products/mol of converted ethanol as a function of ethanol steam reforming reaction time. Catalyst Co/Al P-DP, T = 450˚C and 600˚C. Ethanol: water ratio = 3:1.
in the yield of ethylene, which is a product of the dehydration reaction. There was not methane formation at 450˚C nor at 600˚C. This catalyst promotes the combination of the steam reforming reaction and the gas-water displacement reaction (Reaction 2), which is shown by the higher production of H2 and CO2 at 450˚C [13].
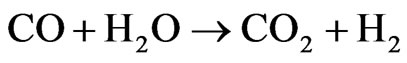 (2)
(2)
ΔH˚f(25˚C) = −41 kJ/mol, ΔG˚f(25˚C) = −28 kJ/mol
In Figure 8, we can observe that, in the beginning of the reaction with the catalyst Co/Al S-DP at 450˚C, there is a drop in CO yield, which remains stable throughout the rest of the remaining experiment time. At 600˚C, catalyst Co/Al S-DP, presents higher CO yield and a decrease in CO2 yield, which suggests the occurrence of the shift reverse reaction (Reaction 2) [14]. Catalyst promoted the steam reforming reaction along with the gas-water displacement reaction (Reaction 2) at 450ºC, which is evidenced by the higher CO2 yield in comparison to CO.
 (3)
(3)
ΔH˚f(25˚C)= 277.69 kJ/mol, ΔG˚f(25˚C) = −174.78 kJ/mol
In Figure 9, the catalyst Co/Al P-I presents better H2 yield at 600ºC than at 450˚C. It can also be observed that at 450˚C and 600˚C, the yield of CO2 was better than that of CO, which favors the shift reaction (Reaction 2).
In Figure 10, we can observe that the catalyst Co/Al P-DP presents higher ethylene and CO2 yield at 450˚C than at 600˚C. During the first 3 hours of reaction at 450˚C, there was a drop in H2 yield and an increase in the yield of ethylene, which is a product of the ethanol dehydration reaction on acid sites of alumina surface (Reaction 3) [15]. The formation of methane in low concentrations at 450˚C and 600˚C can also be seen. This catalyst promoted the steam reforming reaction along with the gas-water displacement reaction (Reaction 2) at 450ºC, which is evidenced by the higher CO2 yield in comparison to CO.
 (3)
(3)
ΔH˚f(25˚C)= 277.69 kJ/mol, ΔG˚f(25˚C) = −174.78 kJ/mol
5.1.5. Liquid Products Formed in the Ethanol Reforming Reactions
During the ethanol steam reforming reaction procedure, the formation of liquid products was observed, and they were condensed during the reaction and collected at the end for analysis and identification of the formed products. This residue presented unreacted ethanol, water and acetaldehyde.
Hydrogen was the main gaseous effluent obtained when analyzing the products yield as a function of time and at all temperatures and on all tested catalysts. Only traces of acetaldehyde were identified among liquid
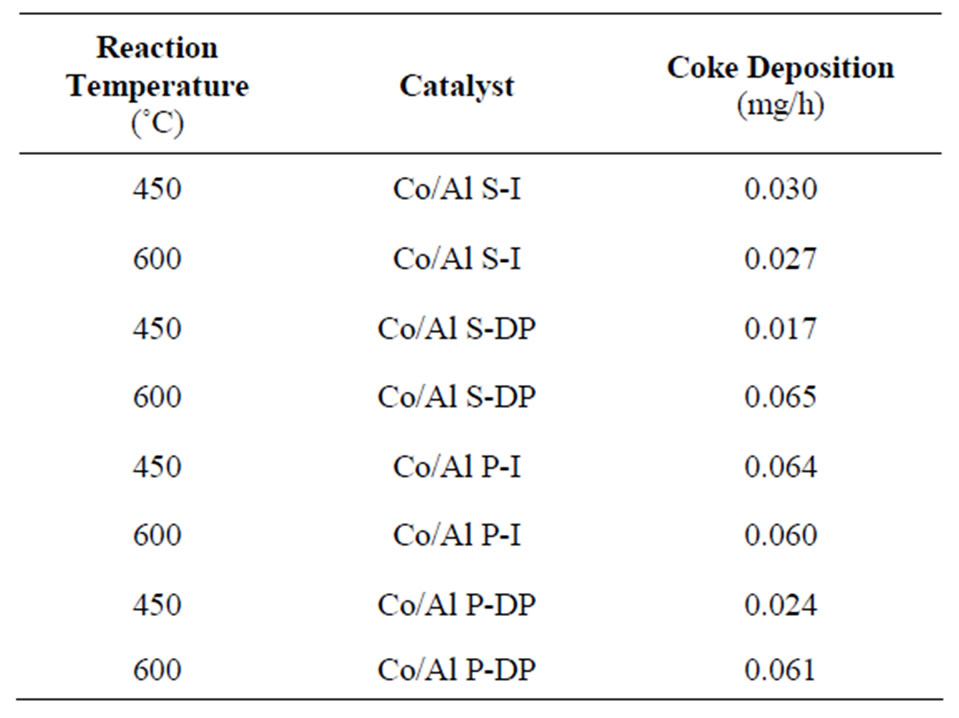
Table 2. Amount of formed carbon in mass.
products, which suggests low occurrence of ethanol dehydrogenation due to the high H2 yield presented by catalysts [16].
5.1.6. Analysis of Carbon Formation during Reactions
In Table 2, we can verify that all catalysts present low carbon formation during the ethanol steam reaction, and the catalyst Co/Al S - DP, at 450˚C, stands out, once it presents the lowest coke level at the end of the reaction. It can also be observed that catalysts used at 450˚C and prepared by deposition-precipitation systematically present lower amounts of deposited carbon.
6. Conclusion
Evaluation of the obtained results for catalysts prepared using the two preparation methods used in this study allows the conclusion that catalysts prepared by the deposition-precipitation method at 450˚C presented greater H2 and CO2 yield when compared to CO yield. Analysis of carbon formation showed that it was lower for the catalyst Co/Al S - DP at T = 450˚C.
7. Acknowledgements
The authors wish to thank CNPq for its financial support.
REFERENCES
- R. N. Das, A. Bandyopadhyay and S. Bose, “Nanocrystalline-Al2O3 Using Sucrose,” Journal of the American Ceramic Society, Vol. 84, No. 10, 2001, pp. 2421-2423. doi:10.1111/j.1151-2916.2001.tb01024.x
- B. Zhang, X. T. Tang, Y. Li, W. Cai, Y. Xu and W. Shenn, “Steam Reforming of Bio-Ethanol for the Production of Hydrogen over Ceria-Supported Co, Ir e N,” Catalysis Communications, Vol. 7, No. 6, 2006, pp. 367- 372.
- V. Fierro, O. Akdim, H. Provendier and C. Miroda-Tos, “Oxidative Reforming of Biomass Derived Ethanol for Hydrogen Production in Fuel Cell Applications,” Catalysis Today, Vol. 75, No. 1-4, 2002, pp. 141-144. doi:10.1016/S0920-5861(02)00056-1
- A. N. Fatsikostas and X. E. Verykios, “Production of Hydrogen for Fuel Cells by Reformation of Bio-Ethanol over Niand Ru-Based Catalysts,” Proceedings of the International Hydrogen Energy Congress and Exhibitions, 2005, pp. 13-15.
- N. Srisiriwat, A. Therdthianwong and S. Therd-Thianwong, “Sustainable Energy and Environment (SEE 2008),” The 2nd Joint International Conference, Thailand, 21-23 November 2006.
- T. Ioannides and S. Neophytides, “Efficiency of a Solid Polymer Fuel Cell Operating on Ethanol,” Journal of Power Sources, Vol. 91, No. 2, 2000, pp. 150-156. doi:10.1016/S0378-7753(00)00473-0
- E. C. Wanat, K. Venkataraman and L. D. Schimidt, “Steam Reforming and Water-Gas Shift of Ethanol on Rh and Rh-Ce Catalysts in a Catalytic Wall Reactor,” Applied Catalysis A: General, Vol. 276, No. 1-2, 2004, pp. 155-162. doi:10.1016/j.apcata.2004.08.001
- T. V. Reche, “Dissertação (Mestrado em Físico-Química),” Instituto de Química de São Paulo, São Carlos, 2004.
- P. S. Santos, P. K. Kiyohara and H. S. Santos, “Efeitos Causados Pela Mistura de Gasolina e. Álcool em,” Bol. Téc. Petrobrás, Vol. 41, No. 3-4, 1998, pp. 45-63.
- M. S. Batista, R. K. S. Santos, E. M. Assaf, J. M. Assaf, and S. Ticianelly “Characterization of the Activity and Stability of Supported Cobalt Catalysts for the Steam Reforming of Ethanol,” Journal of Power Sources, Vol. 124, No. 1, 2003, pp. 99-103. doi:10.1016/S0378-7753(03)00599-8
- P. Arnoldy and J. A. Moulinijin, “Temperature-Programmed Reduction of CoO/AI2O3 Catalysts,” Journal of Catalysis, Vol. 93, No. 1, 1985, pp. 38-54. doi:10.1016/0021-9517(85)90149-6
- R. K. S. Santos, M. S. Batista, E. M. Assaf and J. M. Assaf, “Effect of Metal Content in Catalysts Co/Al2O3 Applied to the Reaction of Steam Reforming of Ethanol New Chemical,” Química Nova, Vol. 28, No. 4, 2005, pp. 587-590. doi:10.1590/S0100-40422005000400006
- B. Varghese, Y. Zahang, L. Daí, V. B. C. Tan, C. T. Lim and C. Sow, “Structure-Mechanical Property of Individual Cobalt Oxide Nanowires,” Nano Letters, Vol. 8, No. 10, 2008, pp. 3226-3232. doi:10.1021/nl801555d
- A. Kaddouri and C. Mazzocchia, “A Study of the Influence of the Synthesis Conditions upon the Catalytic Properties of Co/SiO2 or Co/Al2O3 Catalysts Used for Ethanol Steam Reforming,”Catalysis Communications, Vol. 5, No. 6, 2004, pp. 339-345. doi:10.1016/j.catcom.2004.03.008
- J. Llorca, N. Homs, J. Sales and P. L. Piscina, “Efficient Production of Hydrogen over Supported Cobalt Catalysts from Ethanol Steam Reforming,” Journal of Catalysis, Vol. 209, No. 2, 2002, pp. 306-317. doi:10.1006/jcat.2002.3643
- S. Cavallaro, N. Mondello and S. Freni, “Hydrogen Produced from Etanol Reforming Molten Carbonate Fuel Cell,” Journal of Power Sources, Vol. 102, No. 1-2, 2001, pp. 198-204. doi:10.1016/S0378-7753(01)00800-X

