Modern Chemotherapy
Vol.2 No.2(2013), Article ID:30489,17 pages DOI:10.4236/mc.2013.22005
Drug therapy: Solamargine and other solasodine rhamnosyl glycosides as anticancer agents
![]()
Australasian Institute of Medical Research, Brisbane, Australia; bill.cham@gmail.com
Copyright © 2013 Bill E. Cham. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 24 February 2013; revised 26 March 2013; accepted 5 April 2013
Keywords: Cancer; Skin Cancer; Solamargine; Solasonine; BEC; Solasodine Rhamnosyl Glycosides; CuradermBEC5; Apoptosis; Antineoplastic; Targeted Therapy
ABSTRACT
In the last century, the discovery of cytotoxic agents was revolutionary for anticancer therapy. These therapies have resulted in better understanding of cancer in general. However, the development of agents that combine efficacy, safety and convenience remains a great challenge. The narrow, if not adverse, therapeutic index of most drugs, the damage not only to cancer cells, but also to normal and healthy tissue and the occurrence of resistance have limited anticancer efficacy. This review presents the development of promising novel cytotoxic solasodine rhamnosyl glycoside drugs that offer not only gains in specificity and efficacy, but also in safety, tolerability, non-resistance and convenience in the treatment of patients with cancer.
1. INTRODUCTION
In the past 100 years our understanding of the biology of cancer has come a long way. We now have a reasonable working knowledge of how tumors initially form, grow and spread. Importantly, substantial information about features distinguishing tumor from normal cells is being accumulated, resulting in major new insights into cancer biology.
However, translating this information into the development of new treatments, or even refining the use of the ones already in existence, has not been forthcoming. Limited biological information available at the time of the development of current cytotoxic chemotherapy regimens remain the mainstay for most cancers.
Cancer remains a major cause of mortality worldwide. Despite great progresses that have been in understanding the molecular basis of cancer, the progress in cancer detection and treatment, mortality is high and still there is no cure.
Targeted therapy is a new generation modality for cancer treatment. However, currently there are no really effective targeted therapies. New drugs have to be discovered and methods have to be developed to enable maximum application of these new drugs. One of the major limitations of currently used targeted therapies is the potential for cells to develop resistance to them.
There is a need for targeted therapies that induce cancer cells to undergo apoptosis or helping the immune system to destroy cancer cells. In addition, the targeted therapy should be immune to cellular resistance.
A class of glycoalkaloids, known as solasodine rhamnosyl glycosides, specifically induces apoptosis in cancer cells and cancer cells do not develop resistance to this class of glycoalkaloids. In addition, these glycoalkaloids also exert a positive immune response towards cancer cells. In chronological order, this review examines the scientific events that lead to the clinical application of these antineoplastic glycoalkaloids.
2. RESULTS
Solasodine rhamnosyl glycosides are secondary metabolites of plants. They are found mainly in Solanaceae and Liliaceae. The molecules have a mono or oligosaccharide chain attached at the C3 position of the nitrogenous steroid alkaloid backbone. The sugar chains are usually trisaccharides. Investigations have indicated that glycoalkaloids have evolved in nature to protect plants against bacteria, fungi, insects and animals. They are toxic to a wide range of organisms and are generally considered to be defensive allelo-chemicals. The biological activity of glycoalkaloids is largely due to their membrane-disruptive effects. The family Solanaceae plants S. linnaeanum (devil’s apple), S. nigrum (black nightshade) and S. melongena (eggplant), amongst others, possess anticancer properties. These plants contain the glycoalkaloids solasonine (SS) and solamargine (SM), which exert pharmacological effects. SS and SM singularly, or in combination, are effective antineoplastic biological therapeutic agents.
SM has the molecular formula C45H73NO15 with the mass of 868.04 Da. Its systematic name is (22R, 25R)- spiro-5-ene-3ß-yl-a-L-rhamnopyranosyl-(1à2glu)-0-a-L-rhamnopyranosyl-(1à4glu)-ß-D-glucopyranose.
SS has the molecular formula C45H73NO16 with the mass of 884.04 Da. Its systematic name is (22R, 25R)- spiro-5-ene-3ß-yl-a-L-rhamnopyranosyl-(1à2gal)-0-ß-D-glucopyranosyl-(1à3gal)-ß-D-galactopyranose.
Figure 1 shows the chemical structures of SM and SS. The aglycone alkaloid of SM and SS is solasodine [1].
In 1987 it was reported that a standardized mixture (BEC) of SM (33%), SS (33%) and di-and monoglycosides of solasodine (34%) extracted from S. sodomaeum, now reclassified as S. linnaeanum, possessed antineoplastic properties. BEC was effective in vivo against murine Sarcoma 180 (S180), whereas the aglycone solasodine at equimolar concentrations was ineffective [2]. In such studies, BEC was injected in single and multiple doses up to 4 days after inducing cancer activity in mice. Single doses of varying concentrations of BEC on the absolute survival of mice with S180 determined that ED50 of BEC was 9 mg/kg. Single dose toxicity studies of BEC in normal mice showed that the LD50 of BEC was 29 mg/kg, resulting in the therapeutic index LD50/ED50 of 3.3. It was further shown that the effectiveness of BEC
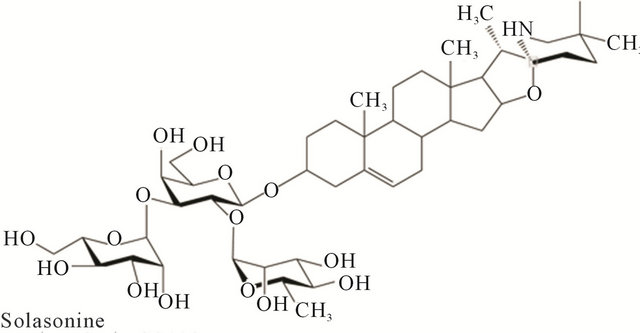
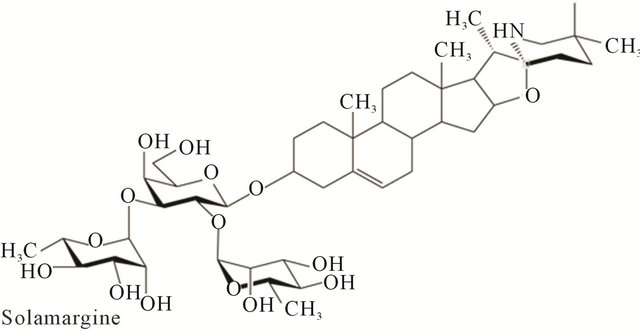
Figure 1. Chemical structures of solasonine and solamargine.
on the lethal S180 in mice was dependent on the number of BEC administrations. Multiple doses at low concentrations (8 mg/kg) resulted in much greater efficacy.
In 1990 [3] studies showed that the uptake of tritiated thymidine (cell survival) by cancer cells was preferentially inhibited by BEC. In contrast, BEC and/or its component SM at equivalent concentrations, had a limited effect on the uptake of tritiated thymidine in lymphocytes. The inhibition of tritiated thymidine uptake in cancer cells by SM, SS and mono-and diglycosides of solasodine were dependent upon their cellular uptake by endogenous endocytic lectins (EELs) present on the cancer cells. The aglycone solasodine at a wide concentration range did not have any observable effects on cancer cells. In contrast, in a dose dependent manner, but at doses much lower than used with solasodine, BEC caused the cells to shrink, the cytoplasm condensed and became dark staining, the nuclei became pyknotic, the chromatin clumped, and finally the nuclei disintegrated (Figure 2). These observations are the hallmark of apoptosis. It was concluded that the inhibition of thymidine uptake by SM was the result of cell lysis, which in turn was dependent on the uptake of SM by EELs on cancer cells but not normal cells.
From dose-response curves of ovarian cancer cells, HeLa cells, lymphoblastoid cells and fibroblasts the ED50 and LD50 of SM, vinblastine, cisplatin and chlorambucil were calculated. The LD50 is defined as the dose of the antineoplastic agent to kill 50% of normal noncancerous cells whereas, the ED50 is defined as the dose of the antineoplastic agent to kill 50% of cancer cells. The therapeutic index (TI) is LD50/ED50. Therefore, a TI value of 1.0 indicates that the antineoplastic agent at a
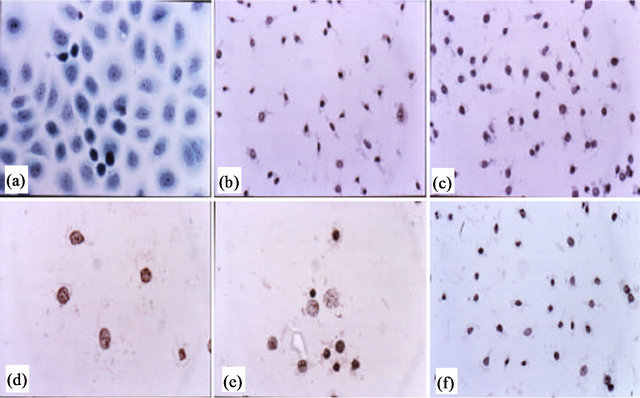
Figure 2. Untreated ovarian cancer cells, the cells are all viable (a); BEC causes the cytoplasm of the cancer cells to undergo dissolution, the nuclei contract and become dark staining (b); nuclei then enlarge (c); the chromatin (contents of nucleus) clumps (d) and finally the nuclei disintegrate (e); Only cellular debris is left after the interaction of the cancer cells with BEC (f). This cell death is characteristic of apoptosis, which is also known as programmed cell death.
given concentration is killing as many cancer cells as normal cells. TI values greater than 1.0 indicate that at a given concentration of the antineoplastic agent more cancer cells are being killed than normal cells. Using tritiated thymidine uptake by cancer cells and normal cells it was shown that the specificity of SM was much higher than cisplatin and vinblastine for killing ovarian cancer cells relative to fibroblasts (Table 1). In addition, it was shown that the absolute concentrations of SM required to kill cancer cells were 6 - 40 times less than that of other cytotoxics [3].
In another article in 1990 [4] it was reported that the efficacy of BEC against murine S180 in vivo could be inhibited by the monosaccharide rhamnose. Figure 3 illustrates that the survival of mice with S180 treated with 4 doses of 8 mg BEC/kg was dependent on given doses of rhamnose. Mice inoculated with S180 cells alone died in 2 - 3 weeks. When 4 doses of BEC of 8 mg/kg were given on consecutive days, complete inhibition of S180 activity was achieved and all the animals survived. The number of survivals was decreased with increasing concentrations of rhamnose. Five milligrams rhamnose/kg decreased the survival to 75%, whereas 10 mg rhamnose/kg decreased the survival to 50% ad 15 mg rhamnose/kg decreased the survival to 42%. Similar
Table 1. Dose (micromol/L) of anticancer drug to kill ovarian cancer and normal cells.
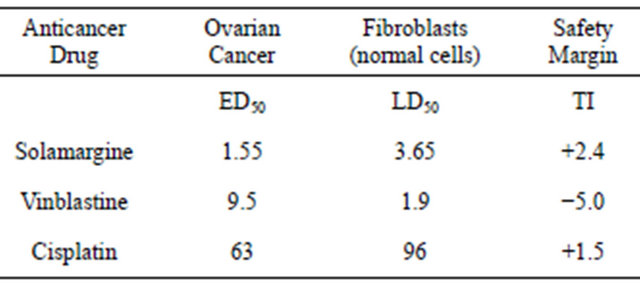
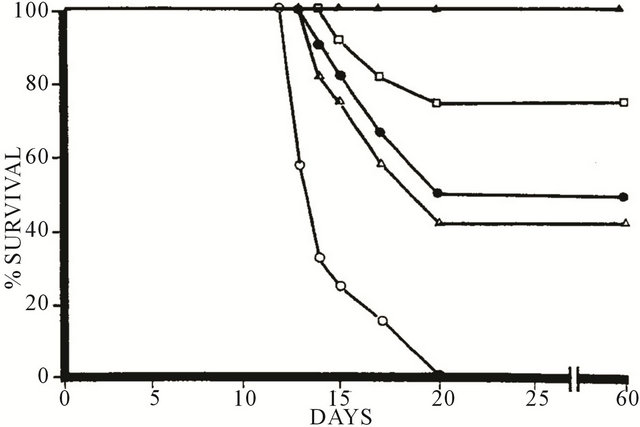
Figure 3. Effect of solasodine glycosides (BEC) and rhamnose on mouse survival with S180. -○-, Untreated S180; -Δ-, 4 doses BEC 8 mg/kg + rhamnose 15 mg/kg; -●-, 4 doses BEC 8 mg/kg + rhamnose 10 mg/kg; -□-, 4 doses BEC 8 mg/kg + rhamnose 5 mg/kg; -▲-, 4 doses BEC 8 mg/kg.
concentrations of rhamnose had no effect on S180 activity in the absence of BEC.
It was also shown that mice, inoculated with S180 cells, which were at their terminal stages could tolerate and become symptom-free of cancer by single dose administration of BEC at concentrations of BEC three times the LD100 for normal mice. It was concluded that the binding of solasodine glycosides on tumor cells was mediated through the rhamnose moiety, which forms part of SS, SM and diglycosides of solasodine in BEC and that cancer cells preferentially interacted and metabolized BEC. Evidence was also provided that BEC selectively destroyed tumor cells relative to normal cells.
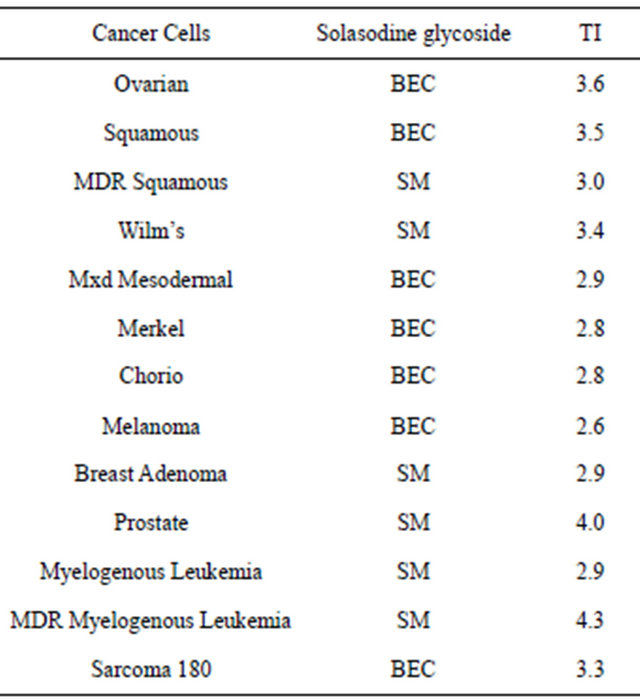
Subsequent to these early observations [1-4] many studies have confirmed and elaborated on these findings. For example in 1993 it was shown that BEC and in particular SM showed high antitumor specificity and efficacy when normal cells and a wide variety of primary tumor and cell cultured tumors were studied [5]. Table 2 shows the TIs of BEC and SM on some cancers.
The TI in cancer cells was in the same order as with whole animal studies (Table 1). It is interesting to note from data in Table 2 that SM effectively triggers cell death in multiple drug resistant (MDR) tumor cells. Clinically, MDR is one of the major causes for the failure of conventional chemotherapeutic treatment of cancer. MDR severely limits the effectiveness of chemotherapy in a variety of common malignancies. MDR is often associated with the expression of P-glycoprotein (P-gp), which functions as a drug efflux pump to unilaterally transport intracellular drugs out of the cells, resulting in drug resistance to tumor cells.
BEC and its individual components SM and SS have been shown to be very effective in inducing apoptosis in the following cancer cells lines:
• Ehrlich Carcinoma
• Leukemia (K562)
• Colon Cancer (HT-29, HCT-15)
• Liver Cancer (HepG2, PLC/PRF/5, SMMC-7721)
• Lung Cancer (A549)
• Gastric Carcinoma (AGS)
• Pancreatic Carcinoma (MIA, PaCa-2)
• Renal Adenocarcinoma (786-0)
• Uterine Adenocarcinoma (HeLa 229)
• Ovarian Carcinoma (JAM)
• Mesothelioma (NO36)
• Glioblastoma, Astrocytoma (U87-MG)
• Prostate Carcinoma (DV-145, LNCap, PC-3)
• Melanoma (A2058)
• Breast Cancer (T47D, MDA-MB-231)
• Osteosarcoma (U20S)
• Squamous Cell Carcinoma (A431, SCC4, SCC9, SCC25)
Similar concentrations of BEC, SM and SS used in these malignant cell culture studies did not cause apoptosis in normal cells such as bone marrow cells, fibroblasts, normal hepatocyte cells HL7702 and H9C2.
The antineoplastic mode of action of BEC and SM described in the earlier studies [1-5] has now been confirmed by many investigators. For example, in 1996, it was reported that the presence of the terminal rhamnosyl residue of solasodine glycosides was required to exhibit strong cytotoxic activity [6]. Also, in 1996 it was shown that SM possessed potent cytotoxicity to human hepatocytes (Hep3B). A sub-G1 cell stage was drastically increased after incubation with SM and cell death was by apoptosis. In addition, the gene expression of TNFR1 was up-regulated by SM and the overexpression of TNFR1 contributed to the mechanism of the cytotoxicity of SM [7].
In 1998 it was shown that the rhamnose moiety of SM played a crucial role in triggering cell death by apoptosis. SM possessed potent cytotoxicity to human hepatoma cells and induced “sub-G1” apoptotic features. The 2 rhamnose moiety of SM played a crucial role in triggering cell death by apoptosis. It was implied that the car bohydrate moieties of steroidal alkaloids altered the binding specificity to steroid-associated receptors [8]. The trisaccharide of SM, in which two rhamnose units are connected to a glucose moiety, binds more efficiently to specific cell receptor sites than the corresponding trisaccharide of SS in which one rhamnose and one glucose units are bridged by a galactose monosaccharide (Figure 1) [1].
Table 2. The therapeutic indices denoting the safety margin and specificity of BEC and/or SM on various cancer cell lines. The normal cell lines were bone marrow cells and human retinal pigment epithelial cells.
In 1999 it was reported that SM promoted apoptosis of hepatic cancer cells and lung cancer cells with consequential observations of nuclear condensation, DNA fragmentation and Sub-G1 peak appearances, confirming the earlier observations (Figure 2). It was found that apoptosis of cancer cells caused by SM was achieved by activating the TNFRs and Fas of cancer cells resulting in apoptosis caused by the cellular hydrolytic enzymes Caspase-8 and Caspase-3. By combining SM with cisplatin, the effective killing of cisplatin resistant cancer cells can be enhanced, particularly lung cancer cells [9]. SM was shown to be much more effective than taxol, cisplatin or gemcitabine in killing lung cancer cells (Figure 4).
In 2000 it was shown that the anticancer action of SM was irreversible and that Hep3B cancer cells in the G(2)/M phases were susceptible to SM-mediated apoptosis. Up-regulation of TNFR-1 and -II on Hep3B cells were detected after SM treatment, and the SM mediated cytotoxicity could be neutralized with either TNFR-I or II specific antibodies. Therefore, these results revealed that the actions of TNFR-I and II on Hep3B cells may be independent and both are involved in the mechanism of SM-mediated apoptosis [10].
In 2002 it was reported that SS was very effective, in a dose dependent manner, against Ehrlich carcinoma cells and K562 leukemia cells. Acetylation of SS rendered the product inactive as an antineoplastic agent. The aglycone solasodine had minimal antiproliferative effect against the cancer cells. It was concluded that the role of the sugar moiety was very important for anticancer effects [11].
In 2004 studies with a variety of glycoalkaloids determined that SM and SS but not their aglycone solasodine inhibited the growth of human colon (HT29) and liver (HepG2) cancer cells, highlighting the importance of the carbohydrate side chain regarding the antineoplastic
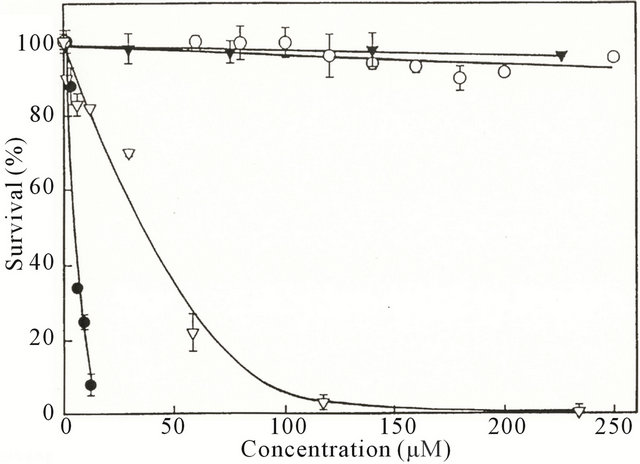
Figure 4. The effect of solamargine. -●-, taxol; -▽-, cisplatin; -○-, or gemcitabine; -▼-, on the survival of lung cancer cells.
properties of the glycoalkaloids [12,13].
Again in 2004 it was shown that SM in a dose-dependent manner displayed superior cytotoxicity in four human lung cancer cell lines. SM induced apoptosis in these cells and increased sub-G1 fractions were observed. It is known that suppression of TNFRs during the progress of human lung carcinogenesis occurs. SM treatment increased the binding activities of TNF-alpha and TNF-beta to the lung cancers, and the intrinsic TNFsresistant cancer cells became susceptible to TNF-alpha and -beta. SM also caused release of cytochrome c, downregulation of anti-apoptotic Bcl-2 and Bcl-xL, increase of Caspase-3 activity, and DNA fragmentation. It was concluded that SM could modulate the expressions of TNFs and Bcl-2, and might be a potential anticancer agent for TNFs and Bcl-2 related resistance of human lung cancer cells [14].
In 2005 a Rhamnose Binding Protein (RBP) was identified and isolated as a cellular receptor of the lectin group. It was previously shown that BEC and SM interacted specifically with an endogenous endocytic lectin (EEL) [3]. As with the previously described rhamnose specific EEL, it was shown that RBP was more abundant on neoplastic cells than non-cancer cells. RBP has a molecular weight of 65 - 70 kDa, a dissociation constant of 1.5 × 10–6 when bound to the rhamnose moiety of SM and the RBP is insoluble in aqueous solutions [12,15]. With lectins, the recognition of carbohydrates, in this case rhamnose is highly specific and thus comparable to the antigen-specificity of antibodies or the substratespecificity of enzymes. Using different cell populations of specific cancers it was determined that a single type of receptor is involved in virtually all cell lines. There are two types of binding of BEC on cancer cells, which were related to receptor affinity and numbers of receptors per cell (Figure 5) [15].
With A2058 melanoma in vitro cell culture studies it was again shown that rhamnose protected the cancer cells
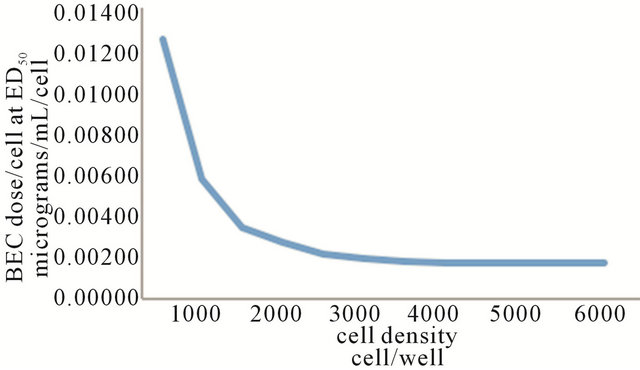
Figure 5. BEC dose/cell at ED50 vs cell density of five different cell lines. Two distinct regions are discernible. Cell densities of 1500 cells and below show that receptor affinity is a major determinant of cytotoxicity. Cell density above 1500 cells denotes a lower number of receptors per cell or slower cytotoxicity.
from BEC activity. Figure 6 shows that increasing concentrations of BEC resulted in decreasing melanoma A2058 cell survival, with an LD50 of 12 µg/mL of BEC and LD100 of approximately 20 µg/mL of BEC. When 5 mM of rhamnose is co-administered with the BEC to the melanoma cancer cells, virtually all the melanoma cells survived [15]. Thus, rhamnose exerted a protective effect against the efficacy of the anticancer BEC compounds. These observations complement the in vivo studies with S180 mice and confirm that the earlier described EEL [3] is indeed the RBP.
In 2006 studies were done on the structure-activity relationship of glycoalkaloids. The inhibition of SM and SS and their hydrolysis products beta 1, beta 2 and gamma SM, and beta 2 and gamma SS and their aglycone solasodine on HCT tumor cells was most active for SM and SS than their hydrolysis products. It was surmised that the carbohydrate side chains of the glycoalkaloids were paramount in influencing biological activity. Not only the number but also the type of carbohydrate, as well as the order of attachment affected the anticancer activity of the glycoalkaloids [16].
In 2006, Ono et al. [17] isolated 8 steroidal glycosides from the underground parts of S. sodomaeum. The antiproliferative activity against human promyelocytic leukemia (HL-60) cells revealed that the steroidal glycoside SM showed stronger activity than cisplatin.
In 2007 it was shown that in addition of inducing apoptosis, SM also sensitized breast cancer cells to cisplatin. SM exhibited a more pronounced cytotoxic effect than cisplatin, methotrexate, 5-fluorouracil, epirubicin and cyclophosphamide against breast cancer cells. SM up-regulated the expressions of external death receptors, such as tumor necrosis factor receptor 1 (TNFR-1), Fas receptor, TNFR-1-associated death domain (TRADD) and Fas-associated death domain (FADD). SM also enhanced the intrinsic ratio of Bax to Bcl-2 by up-regulating Bax and down-regulating Bcl-2 and Bcl-xl expressions. As a consequence, mitochondrial cytochrome c was released and Caspase-8, -9 and -3 were activated in cells, indicating that SM triggered extrinsic and intrinsic apoptotic pathways in breast cancer cells. It is known that resistance to cisplatin in cancer cells is correlated
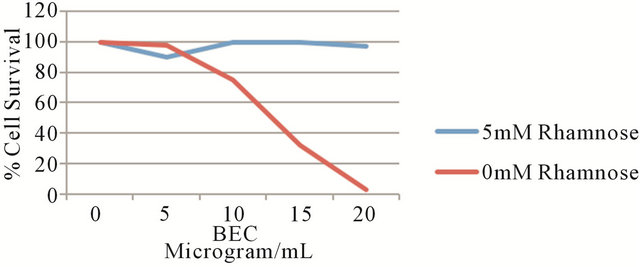
Figure 6. Effect of solasodine glycosides (BEC) and rhamnose on in vitro A2058 melanoma cells.
with Bcl-2 and Bcl-xL overexpression. Because SM down-regulates Bcl-2 and Bcl-xL, the combined treatment of SM and cisplatin significantly reduced Bcl-2 and Bcl-xL expressions and enhanced Bax, cytochrome c, Caspase-9 and -3 expressions in breast cancer cells. Thus, it was suggested that the combined use of low concentrations of SM and cisplatin may be effective in cisplatinresistant breast cancer [18].
Similarly, in 2007 it was shown that SM enhanced cytotoxicity of epirubicin in nonsmall-cell lung cancers (NSCLC). NSCLC is generally resistant to chemotherapy. Human epidermal growth factor receptor 2 (HER2) overexpression is associated with resistance to drugs. SM up-regulated Fas expression and down-regulated the expression of HER2 resulting in promoting chemotherapyinduced apoptosis in NSCLC A549 and H441 cells. SM also down-regulated topoisomerase II alpha (TOP2A). It was concluded that combination therapy of low concentrations of SM with low-toxic topoisomerase II inhibitor epirubicin resulted in the synergistic cytotoxic effect in NSCLC [19].
SM synergistically enhanced the effect of trastazumab in inhibiting cell proliferation. SM markedly co-regulated HER2/neu and topoisomerase II alpha expression and enhanced epirubicin induced cytotoxicity to breast cancer cells [20].
In 2010 Sun et al. [21] reported that SM displayed a superior toxicity to many human tumor cells. Studies with human K562 leukemia cells revealed that SM induced an early lysosomal rupture within 2 h. Subsequently, mitochondrial damage was observed. Downexpression of Bcl-2, up-regulation of Bax, Caspase-3 and Caspase-9, resulting in damages to the lysosomes and mitochondria indicated that the cytotoxicity of SM was involved in a lysosomal-mitochondrial death pathway induced by SM.
In 2011 the effects of SM on cell membrane integrity of human K562 leukemia and squamous cell carcinoma KB cells were studied. After interaction of SM with these cancer cells, oncosis occurred which was characterized by marked changes in cell shape and volume. Blebs appeared on the cell membranes followed by swelling of the mitochondria, the contents of the nuclei clumped and the cells died. It was proposed that apoptosis and oncosis shared certain mechanisms and alterations within the cells before they died by bursting. Low concentrations of SM killed cancer cells by apoptosis and at higher doses SM killed cancer cell by oncosis, and both types of cell death were induced by intermediate concentrations of SM [22].
Also in 2011, it was shown that SM substantially reduced cell viability and induced apoptosis in osteosarcoma U20S cells. SM increased the mRNA and protein expression of p53 and Bax (a pro-apoptotic protein downstream to p53). The expression of Bcl-2 (an antiapoptotic protein) was also reduced. SM induced mitochondrial translocation of p53, loss of mitochondrial potential, cytochrome c release and activation of Caspase-9 and -3. Inhibitors of the transcription of p53 or mitochondrial translocation partially reversed SM-induced apoptosis. It was concluded that SM activated the mitochondria-mediated apoptotic pathway in U20S cells via both p53 transcription-dependent and-independent mechanisms [23].
In 2011 an in situ visualization of the RBP receptor on cancer cell lines surfaces was shown by using biotinylated rhamnose and chacotriose (the triglycoside of SM) the RBP receptor showed different expression patterns on various cell lines. BEC was very effective in killing cancer cells [24]. These results confirm previous observations [15] signifying the potential of RBP to identify neoplastic cells and the possible design of new antineoplastic agents that will specifically interact and eliminate cancer cells.
Recently it was reported that in addition to apoptosis and, perhaps as a consequence thereof, BEC also has an effect of stimulating lasting immunity against cancer as shown with a mouse model and the terminal cancer S180. BEC could play an important role in clinical management of diseases such as malignancy and also be used as a preventative therapy [25]. These observations may have wider applications in addition to cancer. It has also been reported that BEC is effective in treating herpes simplex, herpes zoster, and genital herpes in humans [26]. BEC exhibited rapid activity against these viruses, and follow-up of the treated patients for one year showed no recurrences, whereas in control groups, recurrences of herpes infection ranged from one to six months indicating some immunity in addition to acute activity of BEC [26,27]. It was suggested that BEC may exert activity and lasting immunological effects with similar modes of action on a number of different diseased states including cancer [25].
In 2012 it was shown that SM significantly inhibited the growth of human hepatoma SMMC-7721 and HepG2 cells and induced cell apoptosis. SM caused cell cycle arrest at the G2/M phase. Moreover, SM up-regulated the expression of Caspase-3 [28].
In conclusion, the subcellular and cellular studies confirm the original observations that BEC and its individual glycoalkaloid components exert two main properties. Firstly, BEC recognizes specific receptors, which are present on cancer cells. These receptors known as specific EELs have been further characterized as RBPs. After binding of BEC to the RBP receptor, the complex is internalized in the cancer cell by receptor-mediated endocytosis through “coated pit endocytosis”. Secondly, after internalization of BEC, extrinsic and intrinsic apoptotic pathways in the cancer cells are triggered by up-regulating the expressions of external death receptors, such as TNFR-1, Fas receptors, TNFR-1 associated death domain and Fas-associated death domain. BEC enhances the intrinsic ratio of Bax to Bcl-2 by up-regulating Bax and down-regulating Bcl-2 and Bcl-xL expressions. These effects result in activation of Caspase-8, -9 and -3 in cancer cells with consequent apoptosis by a lysosomal-mitochondrial death pathway of the affected cancer cell.
The BEC antineoplastic properties also result in additional benefits when compared with conventional chemotherapeutic drugs. Drug resistance is a real problem for cancer patients. MDR is a major drawback of cancer chemotherapy and can result in patients becoming immune to the effects of many different drugs at once. Resistance can often result in patient death as a result of lack of effective treatment available. SM inhibits the overexpression of P-gp, which has been well established as the cause of the MDR phenotype in many in vitro selected drug resistant cell lines. It was shown that SM possessed potent killing capacity in a range of MDR tumor cell lines; it showed strong broad-spectrum cytotoxicity against MDR cells. SM cytotoxicity against MDR tumor cells is nearly equal to or even more potent than against the corresponding parental non-MDR tumor cell lines [29]. These observations confirm earlier data that were presented in Table 1.
Conventional cytotoxic chemotherapies affect fast growing cells by killing such cells when they are proliferating. When such cells are not proliferating the conventional anti-mitotic chemotherapeutic agents have very limited effects upon these cells. Consequently the time course for treating cancer cells with conventional drugs is long and repetitive treatments are required. Toxicity, lack of specificity and lack of efficacy with current cytotoxic chemotherapies have limited the progress in cancer therapy. The lack of specificity of conventional anticancer drugs is a great limitation of their use. They enter both cancer and normal cells mainly through diffusion. Due to their DNA reactivity, these drugs can cause a second tumor, which may be different than the one originally treated several years after “curative” treatment. BEC and SM exhibit much higher cytotoxic effects when compared with a number of currently used antineoplastic agents such as Vinblastine [3,9], Camptothecin [13], Vincristine [3,9], Methotrexate [18], Cisplatin [3,9,17, 18], 5-Fluorouracil [18], Gemcitabine [9], Epirubicin [18], Taxol [9], Cyclophosphamide [18] and Doxorubicine [13]. The TIs of BEC and SM are superior to these drugs. The absolute concentrations of these drugs to obtain similar efficacy as BEC and SM are in the order of 6 - 40 times higher.
Unlike established cytotoxic chemotherapy, BEC and its individual SM and SS, are not anti-mitotic in their actions. BEC directly induces apoptosis without first affecting the DNA replication of the cell. Furthermore, and very importantly, BEC kills cancer cells whether they are proliferating or not! Observations showing that BEC exerts acute activity against cancer and additionally, lasting immunological effects, add a new dimension to the promise that BEC may be potent antineoplastic biological therapeutic agents [25,30-33]. BEC therapy lacks the mutagenic and carcinogenic potential of currently used chemotherapy drugs [12,15]. A recent report confirmed that SM did not exert any mutagenic effect, but SM also significantly reduced the frequency of chromosomal aberrations induced by the chemotherapeutic agent doxorubicin in both V79 cells and micronuclei in Swiss mice [34]. So, in addition of not having mutagenic effects, SM displays antimutagenic properties.
2.1. Clinical Studies—Animals
2.1.1. Intravenous Chemotherapy with BEC
wang#title3_4:spMice
Significant inhibition of H22 liver cancer and Ehrlich ascites tumor in mice were observed when SM was administered intravenously [35].
SR-T100 extracted from S. incanum containing SM as the main active ingredient when injected i.v. induced apoptosis and was effective against microinvasive squamous cell carcinoma in hairless mice [36].
2.1.2. Intralesion Chemotherapy with BEC
wang#title3_4:spMice
In this communication it was earlier stated that BEC was very effective in mice with the terminal tumor S180. Moreover, it was shown that intraperitoneal administration of BEC cleared the cancer in early stages of cancer development and also at the terminal stages of cancer infectivity. After elimination of S180 by BEC treatment, mice experienced normal life spans, indicating that BEC cured the cancer in mice.
It was also shown that the rhamnose moieties of the solasodine glycosides were essential for the expression of antineoplastic activity. Additionally, BEC exerted lasting immunological effects against S180 [25].
Horses
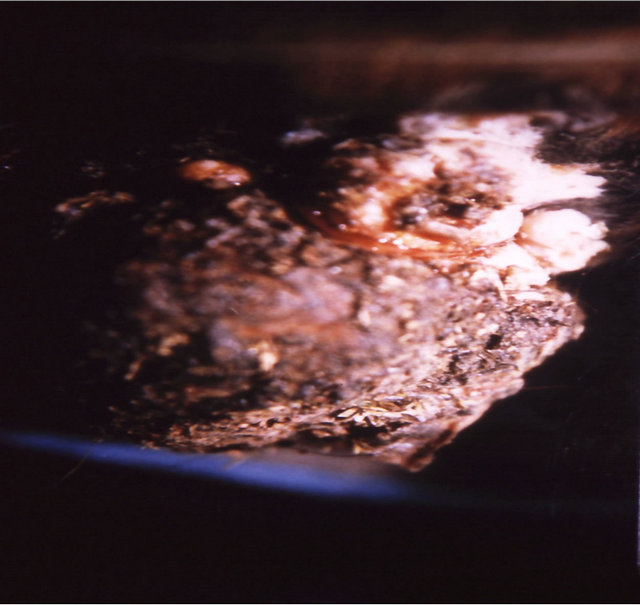
Intralesion administration of BEC into large chondrosarcomas, melanomas and squamous cell carcinoma in horses resulted in rapid clearances of these tumors with no recurrences for at least 5 years after treatment. Only 2 injections, 48 h apart, were necessary to obtain complete clearances of large tumors. The doses injected intralesionally were approximately 100 mg BEC for 1 kg tumor weight.
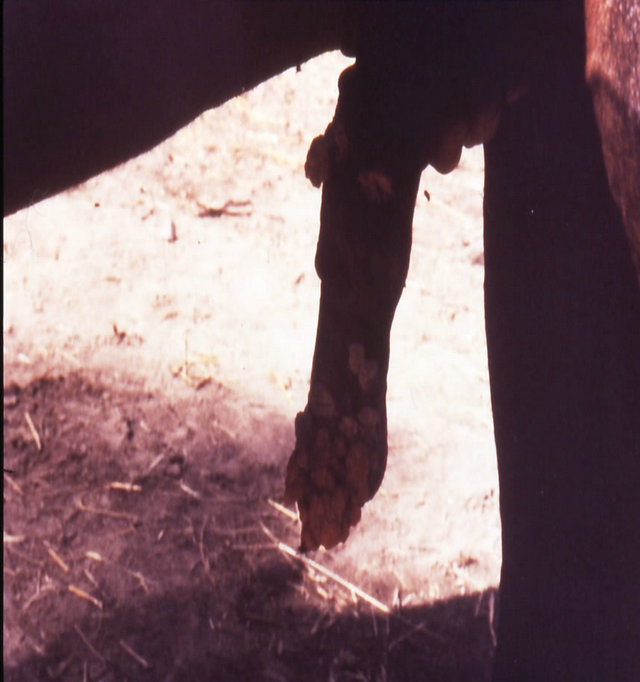
Figure 7 illustrates BEC intralesion therapy on a chondrosarcoma on a horse.
Figure 8 illustrates BEC intralesion therapy on squamous cell carcinoma on a horse.
Figure 9 shows that large melanomas in horses were also eliminated by intralesion injection of BEC.
2.2. Clinical Studies—Humans
2.2.1. Intravenous Studies with BEC or Its Individual Components
The pharmacodynamics of BEC is by specific RBP receptors present on cancer cells but to a lesser extent on normal cells. The mode of action is by apoptosis.
Pharmacokinetic data revealed that in humans the plasma biological half-life of SM was 8.4 ± 2 h and for SS this was 5.57 ± 1.27 h. The clearance was 3.0 ± 0.7 L/h for SM and 5.6 ± 1.6 L/h for SS. Plasma protein binding for both SM and SS was consistent in human, rat and dog. SM protein binding ranged from 76.7% - 96.3%, for SS it was 76.4% - 97.3% [37]. It was also shown that SM and SS were essentially stable when incubated with human, rat and dog cryopreserved hepatocytes indicating that SM and SS were metabolically stable. A 1:1 mixture of SM and SS applied intravenously in a Phase I study produced limiting hepatotoxicity at doses above 1.0 mg/kg/day over 2 h or 1.5 mg/kg/day over 4 h. It was recommended that 1.0 mg/kg/day over 2 h was suitable for Phase II studies. In the Phase I study some activity was seen against resistant solid tumors [12,37,38].
A reasonable, but not optimal effect of BEC i.v. injection on cancer cells has been reported [38]. Over 40 patients with very late stage cancer diseases have been treated by i.v. injections with BEC. Glioblastoma multiform, Colon rectal cancer, Bladder cancer, Liver cancer, Metastasized melanoma and Respiratory Cancers have been treated with i.v. administration of BEC.
There were no serious adverse reactions or death attributable to the treatment. Observed overall benefits with i.v. administration of BEC were extended life span, tumor size reduction, tumor marker reduction, reduction in tumor growth rates, improved quality of life, reduced use of analgesia, reduction of swelling, pain reduction and improved appetite.
The treated patients were in very late stage disease with
 (a)
(a)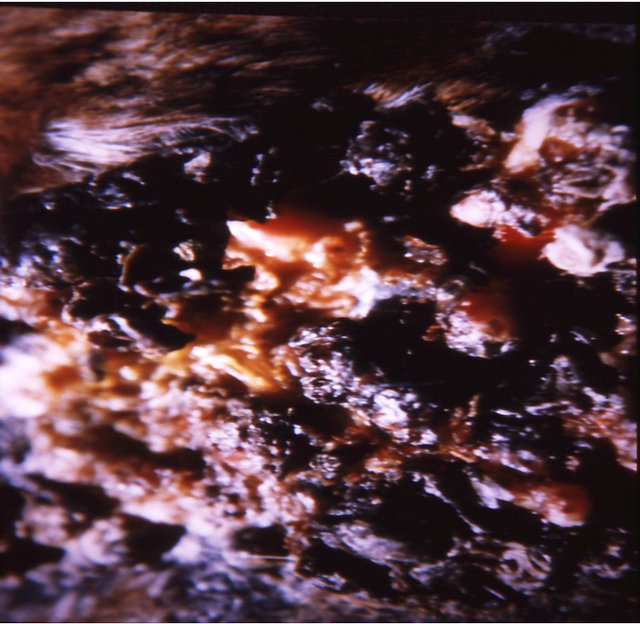 (b)
(b)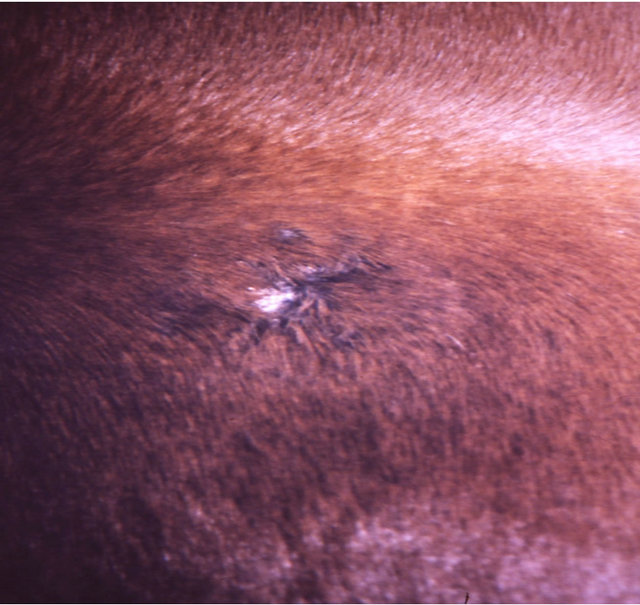 (c)
(c)
Figure 7. A sarcoid of approximately 500 grams on the chest of a horse before injection (a); after two injections of BEC, showing the rapid degradation of the cancer (b); and the site where the cancer was after completion of BEC therapy (c).
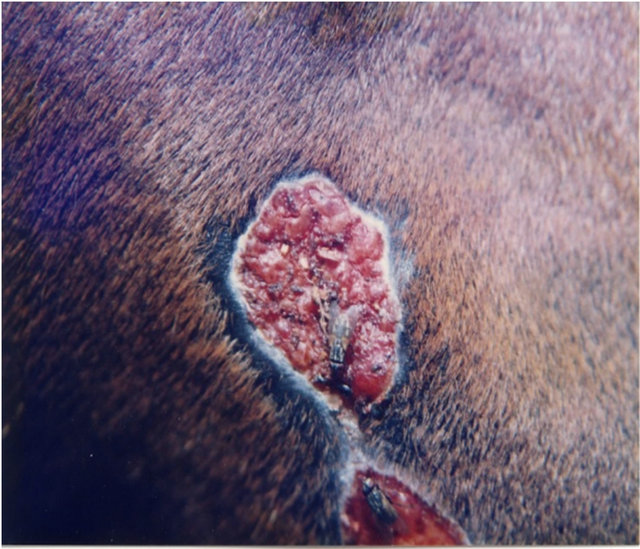

(a) (b)
|
|
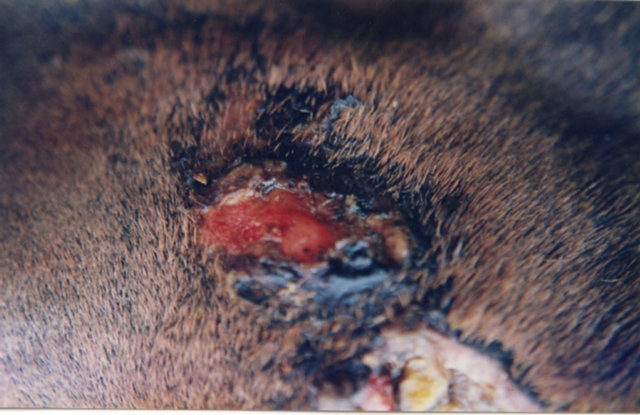 (a)
(a)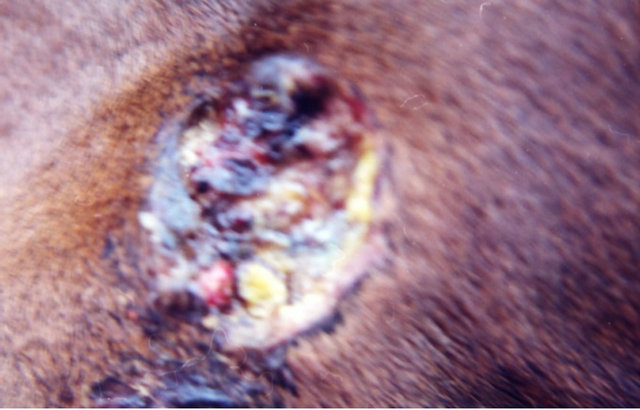 (b)
(b)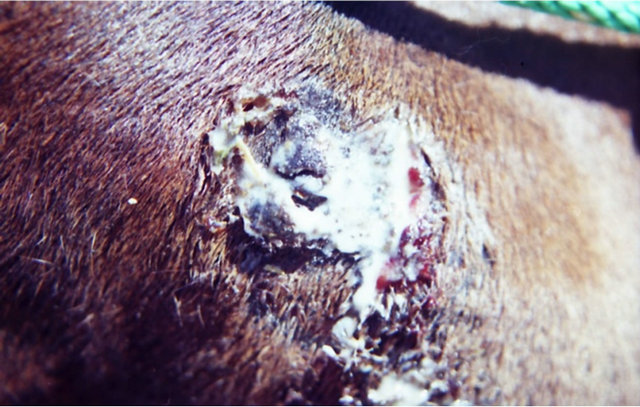 (c)
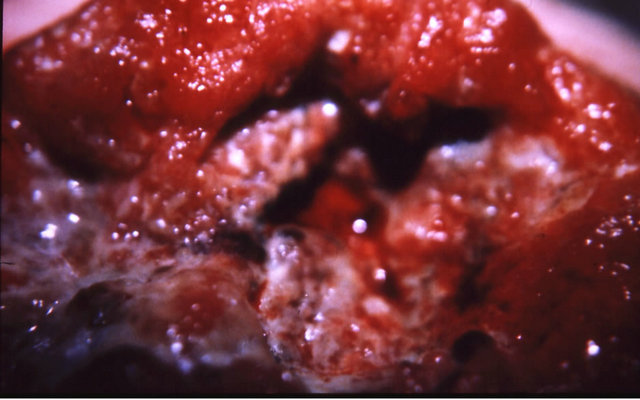
(c)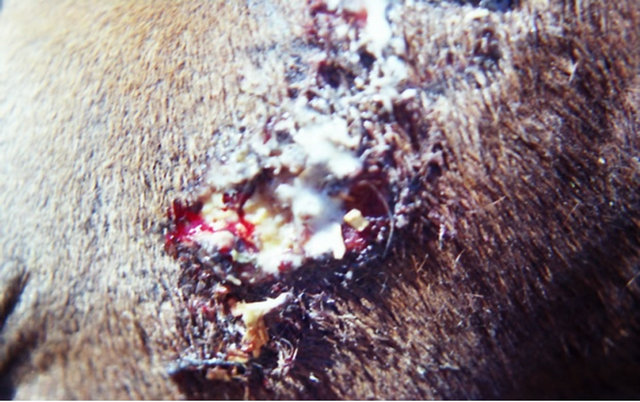 (d)
(d)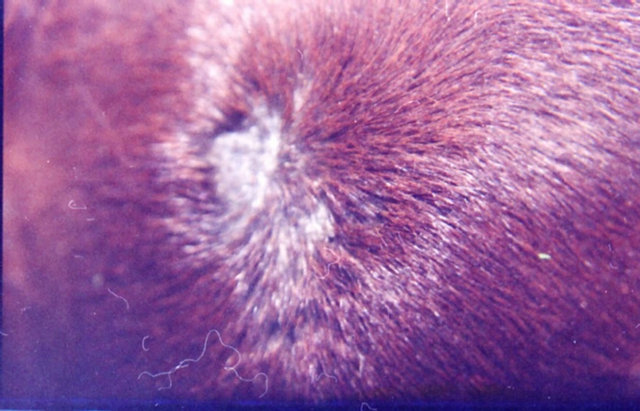 (e)
(e)
Figure 9. A melanoma on the chest of a horse before intralesion injection of BEC (a); After the first injection the lesion started to breakdown (b) and after the second and last intralesion administration of BEC, massive necrosis of the tumour was observed ((c) and (d)); No further treatment was done after the second intralesion injection. Twelve weeks after intralesion injection of BEC, the melanoma was completely eliminated (e). There was no recurrence for at least 5 years after treatment.
significant non-responsive tumor mass to other therapies [12]. These results are very encouraging, but must be regarded with much caution as these studies were uncontrolled and the patients were often on more than one therapy. Indeed, as shown earlier, a combination of BEC with cisplatin resulted in the effective killing of cisplatin-resistant cancer cells, particularly lung cancer cells [19] and breast cancer cells [20].
2.2.2. Intralesion Chemotherapy with BEC
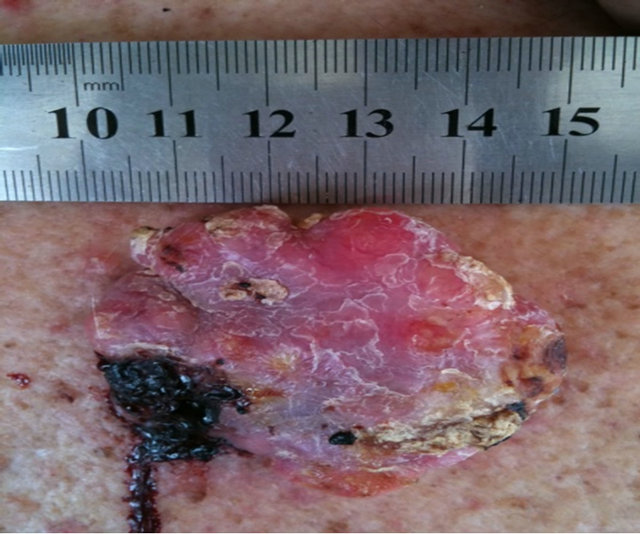
Figure 10 shows the extent of a large squamous cell carcinoma tumor before BEC therapy commenced. Multiple injections on 2 occasions with a 2 day interval caused the tumor to breakdown rapidly [39].
Intralesion injection of BEC into a large 5 cm × 5 cm × 1.5 cm basal cell carcinoma caused necrosis and completely eliminated the basal cell carcinoma (Figure 11) [40].
2.2.3. Oral Administration
Limited but informative studies have been done with the ingestion of BEC in tablet forms. BEC tablets taken orally have shown promising results in secondary endometrial cancer to the lung. After 3 months treatment the cancer had disappeared (multiple nodules present before treatment). Long term remission of late stage pancreatic cancer and total remission of small lung tumors in a patient with metastatic melanoma was observed whilst on oral therapy [12,37,38,41]. These results, although very promising, are far from being proof of effectiveness, because the studies were uncontrolled. There were no toxic manifestations with these studies.
2.2.4. Topical chemotherapy with BEC
Humans
Keratosis
In 1987 it was reported that 10% BEC in a topical cream formulation obtained regression in 23 of 23 keratotic lesions in patients [42]. In an open study in 1991 clinical and histological observations indicated that 56 keratoses were cleared with very low concentrations (0.005%) of BEC in a cream formulation Curaderm [43]. In both 1987 and 1991 studies, it was reported that no adverse effects in the liver, kidneys or hematopoietic system were observed. In 2011 it was reported that SRT100 extracted from S. incanum containing mainly SM, as the main active ingredient was effective against actinic keratoses. The treatment period for actinic keratoses was for 16 weeks and there were negligible discomforts [36]. In 2013 a single-blind, randomized, placebo-controlled, parallel group study revealed that CuradermBEC5 cream applied topically twice daily for 3 days was effective for the treatment of actinic keratoses [44]. Figure 12 illustrates an example of an actinic keratosis treated with CuradermBEC5.
Keratoacanthoma
Two patients with a total of 9 keratoacantomas treated with BEC in a topical cream formulation for 3 - 5 weeks
 (a)
(a)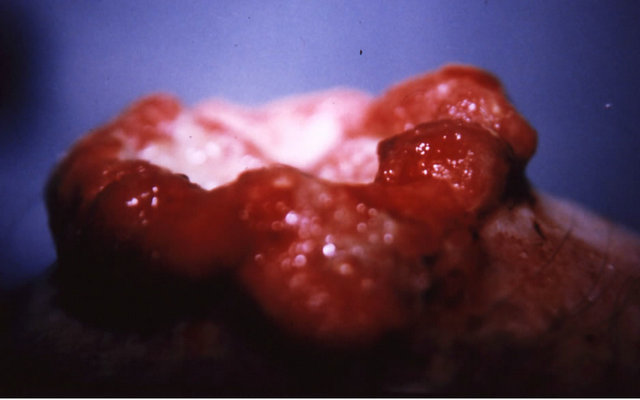 (b)
(b)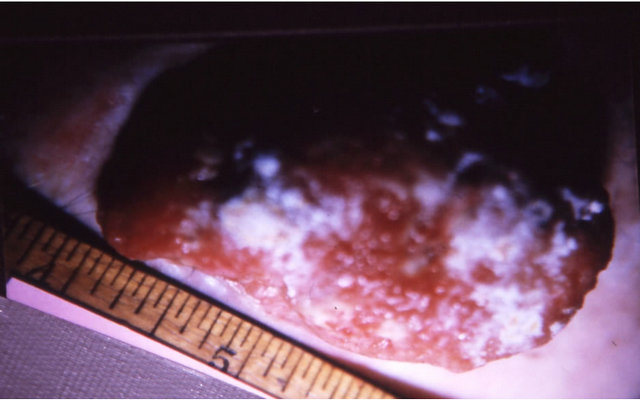 (c)
(c)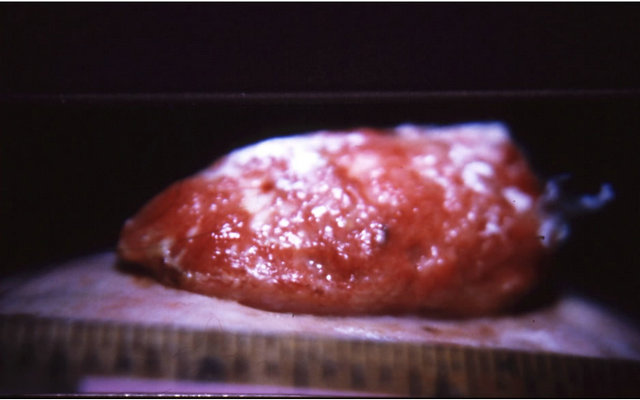 (d)
(d)
Figure 10. A large SCC on the head of a male subject (a) and (b). The patient was given two injections of BEC which resulted in the tumor collapsing and reducing in size (c) side view, (d) top view.
 (a)
(a)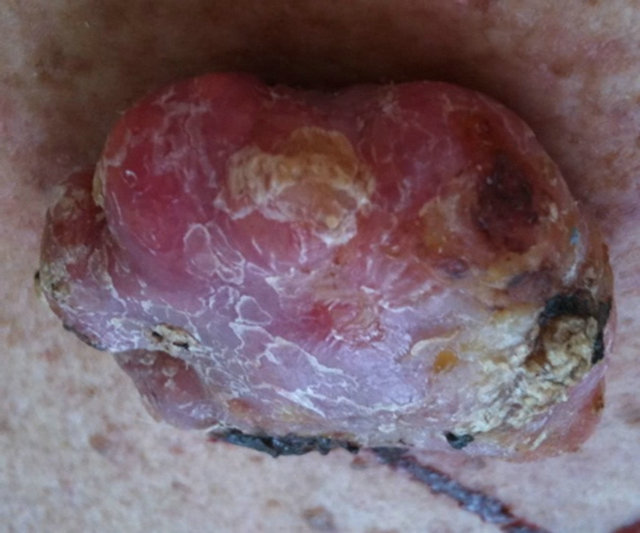 (b)
(b)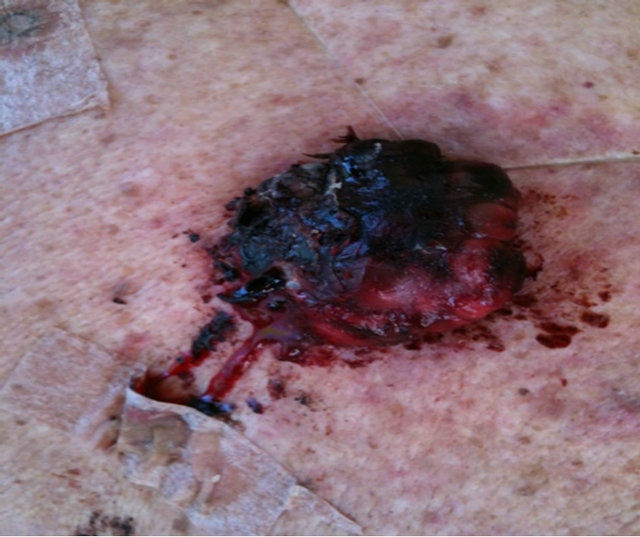 (c)
(c)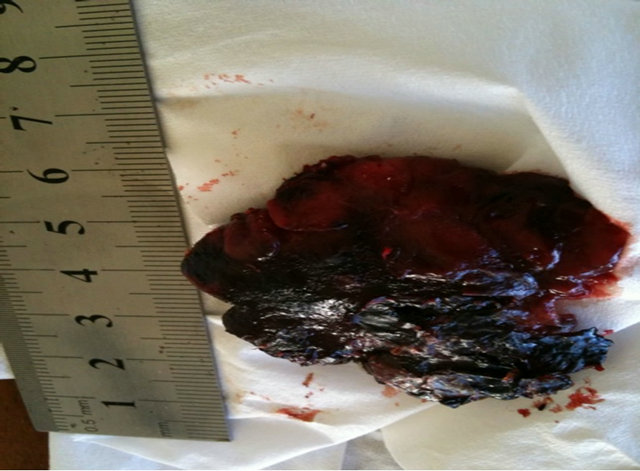 (d)
(d)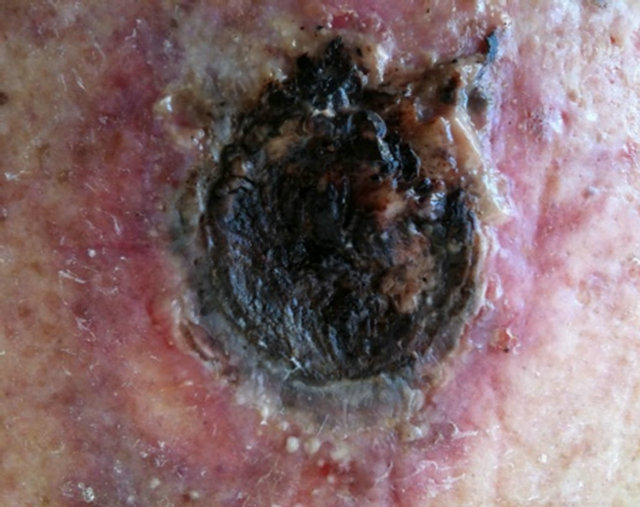 (e)
(e)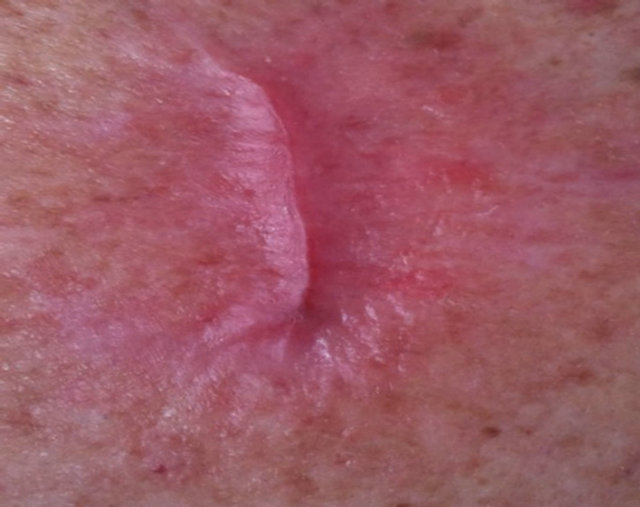 (f)
(f)
Figure 11. A large BCC, 5 cm × 5 cm (a), protruding 1.5 cm from the skin (b) before combination intralesion-topical applications. Two days after the first intralesion injection the lesion started to breakdown (c). Two days after the second and final intralesion injection the lesion released itself from the skin (d). There was an indentation in the skin where the lesion had separated itself (e). From here on the lesion was then treated for six weeks with topical application of CuradermBEC5 treatment only. Sixteen weeks after commencement of the combination treatment, there was no evidence of residual tumour (f). The observed scar tissue was probably from a previous surgical procedure.
 (a)
(a)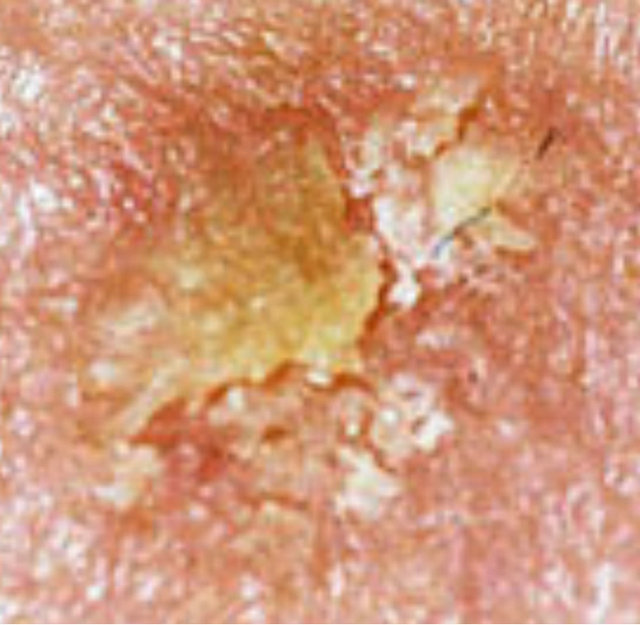 (b)
(b)
Figure 12. Actinic Keratosis before CuradermBEC5 therapy (a), and 56 days after 3 days of treatment (b).
resulted in regression of all lesions [42]. Figure 13 shows an example of a keratoacanthoma treated with CuradermBEC5.
 (a)
(a)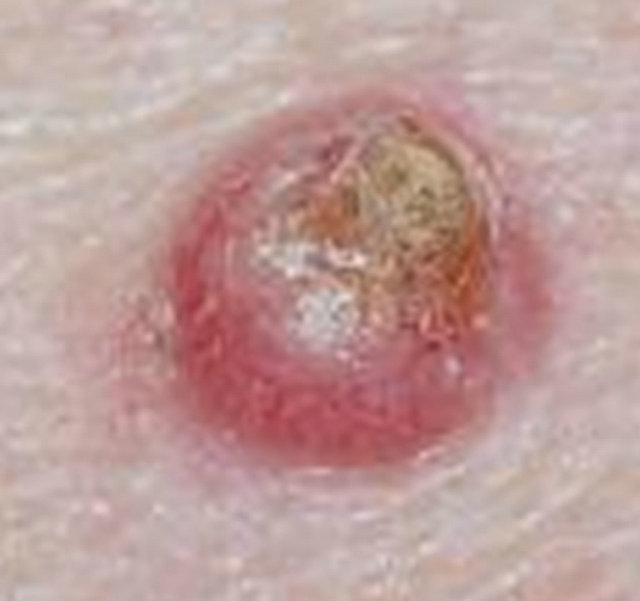 (b)
(b)
Figure 13. Keratoacanthoma before CuradermBEC5 therapy (a), and 60 days after 5 weeks of treatment (b).
Basal Cell Carcinoma
In 1987 it was first reported in an open controlled clinical trial that BEC in a topical cream formulation was effective in treating 20 of 24 basal cell carcinomas. There were no serious adverse effects within the treatment period and there were no recurrences after 5 years [42].
In a subsequent open study in 1991 with CuradermBEC5 39 of 39 cases with basal cell carcinomas were effectively removed. In this study the treatment period necessary to obtain complete removal of the lesions by CuradermBEC5 ranged from several weeks to 12 weeks [43]. Eight weeks of treatment with CuradermBEC5 resulted in 75% success rates and with the study it was shown that 12 weeks of treatment was required to obtain virtually 100% success rates.
This observation was confirmed in a double-blind, randomized, placebo-controlled, parallel group, multicentre study with Zycure (which essentially is CuradermBEC5), for the treatment of basal cell carcinoma. In this trial the patients were treated for 8 weeks. Sixty six percent of patients treated with CuradermBEC5 and 25% of patientson placebo appeared to be treated successfully. Followup for 1 year revealed histologically that 78% who were treated with CuradermBEC5 remained cancer-free, whereas, in the placebo group there was a 50% recurrence rate [45]. When calculated overall, CuradermBEC5 treatment of basal cell carcinoma was five-fold superior to the placebo. Regeneration of new epidermis at the application site during treatment of skin cancers with CuradermBEC5 supports the preclinical and clinical observations that BEC is preferential in its action towards transformed cells. This also explains the impressive cosmetic outcomes with CuradermBEC5 therapy as shown in Figure 14.
Further clinical studies showed that CuradermBEC5
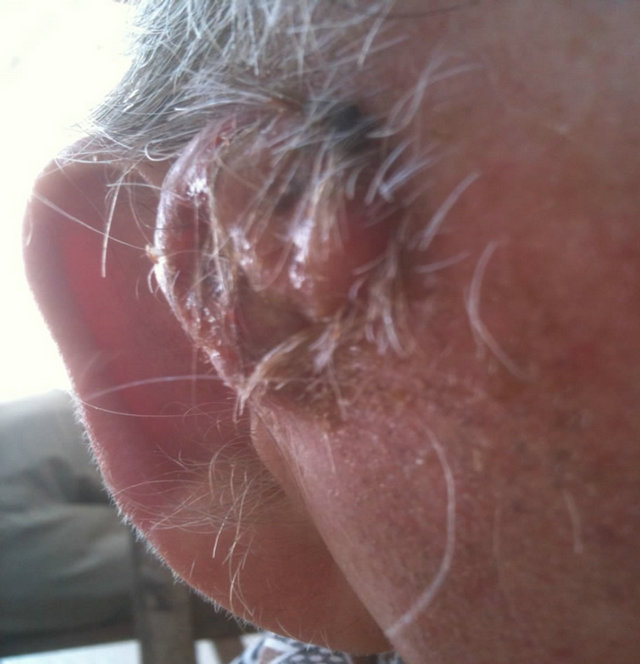
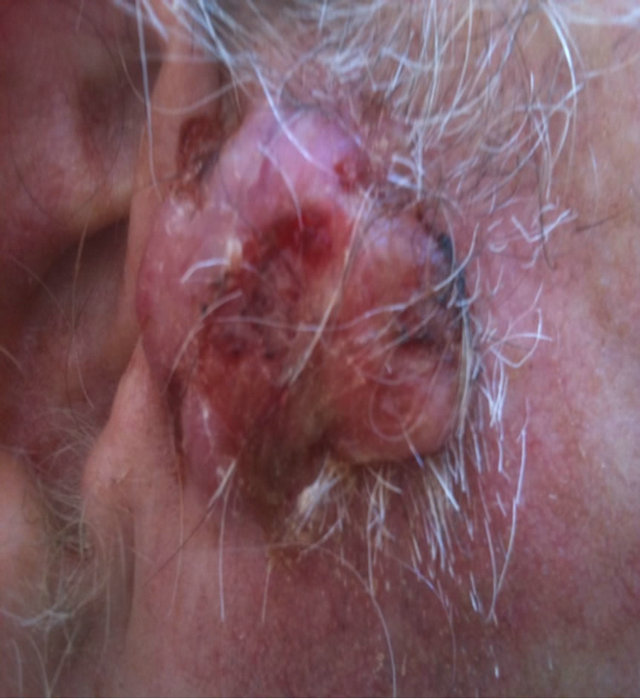
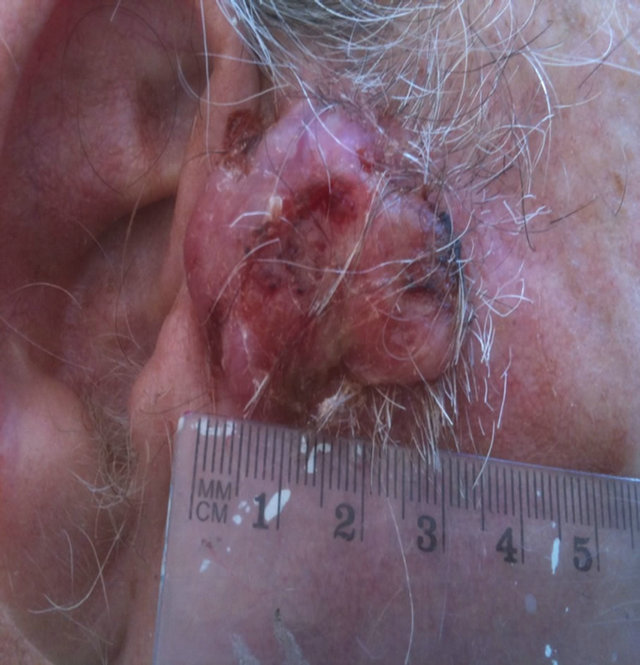
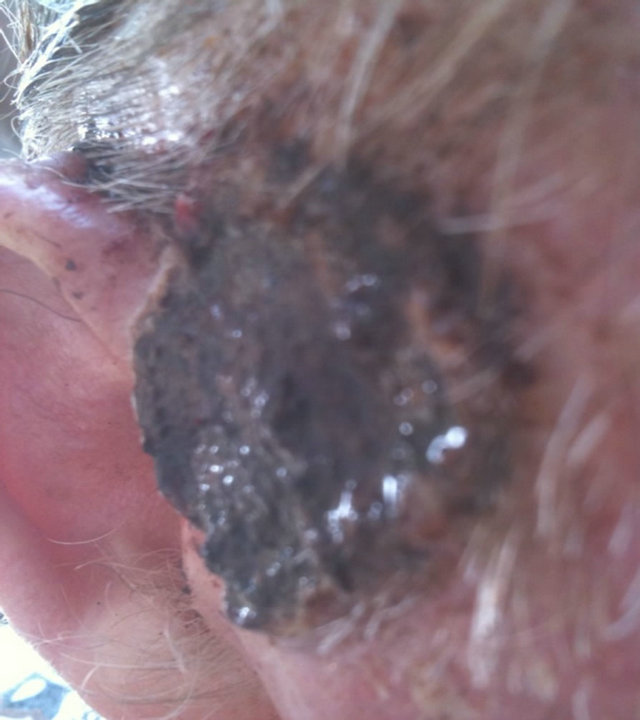
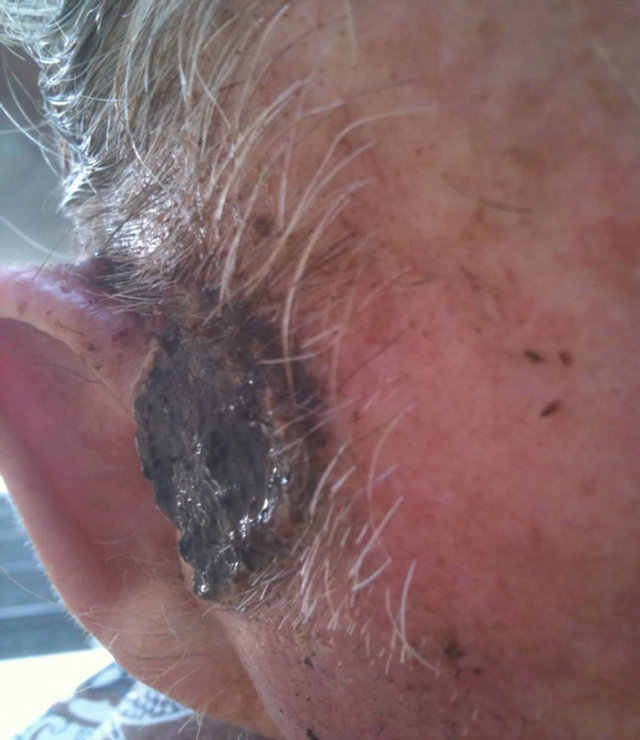
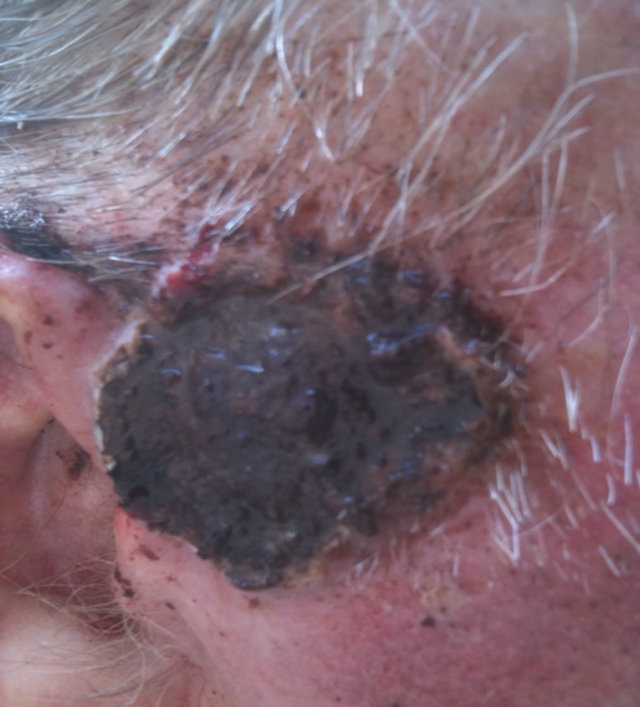

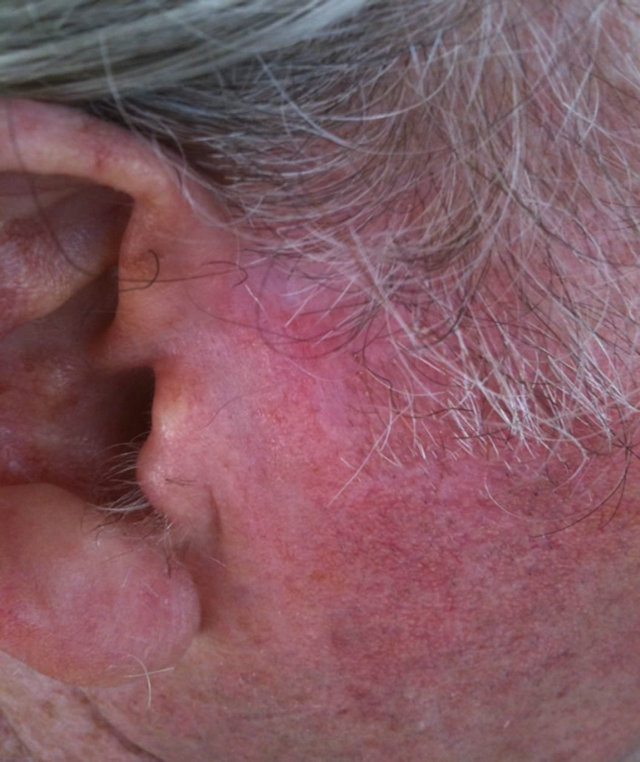
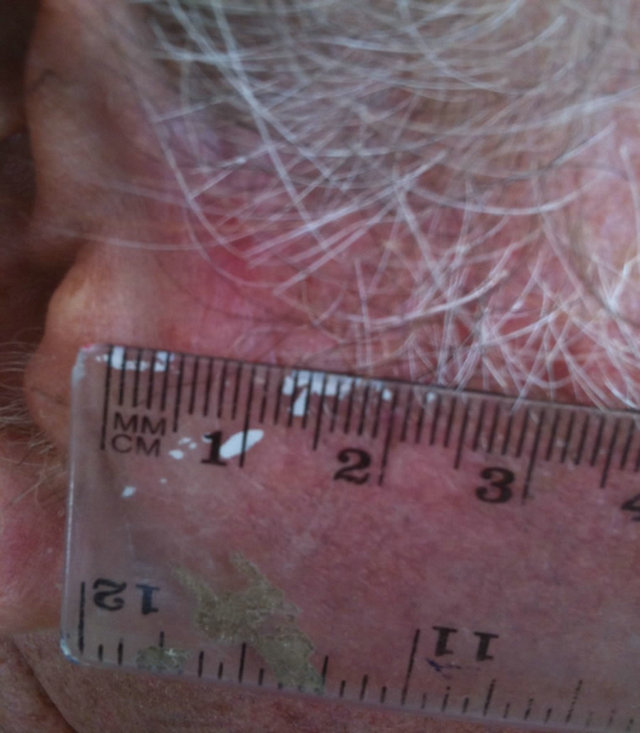
Figure 14. An extensive protruding (4 cm × 4 cm × 2 cm) BCC with central ulceration and raised curly borders on the right side of his face next to his ear is seen in this patient (top row). Treatment with Curaderm resulted in rapid breakdown of the tumour and after 2 weeks of treatment the lesion was reduced to about a half of the original size. Minor bleeding had occurred during this treatment period (middle row). After 14 weeks of treatment the lesion was clinically eliminated. Normal skin cells had replaced the tumour and the cosmetic end result was excellent, with no scar tissue formation. Even the hairs had regrown where the tumour was originally (bottom row).
eliminated basal cell carcinoma in areas that were difficult to treat by any modality [46]. Very large basal cell carcinomas were shown to be successfully treatable with CuradermBEC5 [40,47].
2.2.5. Cutaneous Squamous Cell Carcinoma
Cell culture [3] and whole animal work [36] showed that BEC recognized and interacted with RBP receptors in squamous cell carcinoma. After internalization BEC caused apoptosis in the cancer cells. These observations also applied to cutaneous squamous cell carcinoma. Specificity of BEC towards cutaneous squamous cell carcinoma resulted in clearing of squamous cell carcinoma. Normal skin cells were not affected by BEC. This resulted in impressive cosmetic outcomes with BEC therapy.
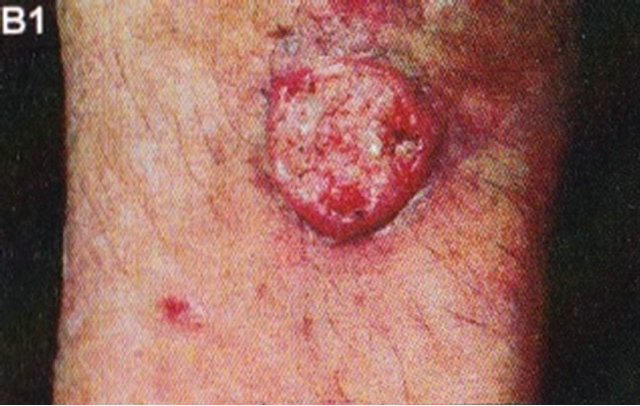
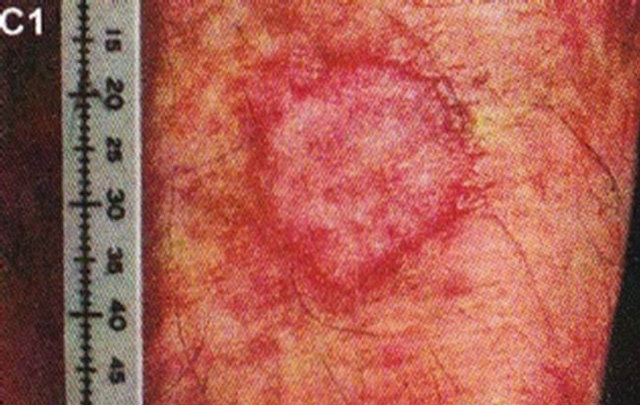
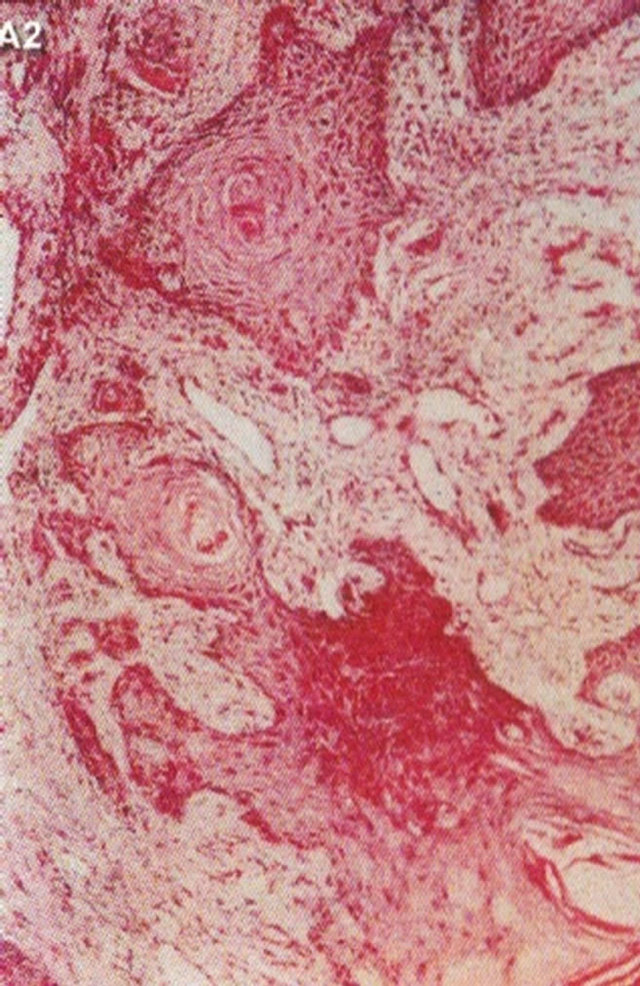
In 1987 it was shown that topical application of BEC in a cream formulation in 5 patients with 6 squamous cell carcinoma lesions resulted in 83% complete regression with no recurrences after 3 years. The BEC treatment period ranged from 4 - 12 weeks with a mean of 6.2 ± 2.7 weeks. Normal skin was not affected with topical BEC treatment. Plasma biochemical profiles, full blood count and differential and urinalysis indicated that these parameters were unaltered by BEC therapy. Mild pruritis and modest burning sensation surrounding the treated lesions occurred in a few cases [42]. Figure 15 illustrates clinical and histological progress of a squamous cell carcinoma on a leg of a patient [42]. Here it is seen that CuradermBEC5 causes apoptosis, which is in agreement with the cell culture studies.
In 1991 it was reported that CuradermBEC5 was effective in the treatment of squamous cell carcinoma. In an open study, clinical and histological observations indicated that all 29 squamous cell carcinomas treated with CuradermBEC5 had regressed. A placebo formulation had no effect on a smaller number of treated lesions. CuradermBEC5 had no adverse effects on the liver, kidneys or hematopoietic system [43].
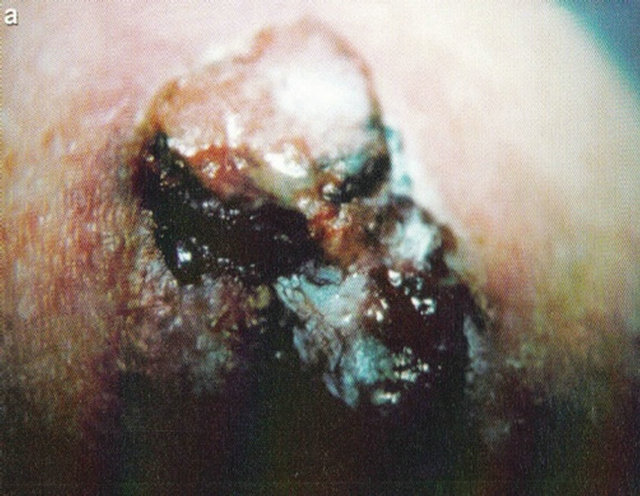
In 2007 an open clinical study with 11 patients who had squamous cell carcinoma determined that CuradermBEC5 therapy resulted in complete regression of all lesions as shown histologically by analyses of biopsies at the completion of treatment period and clinical assessment 5 years post treatment. The mean treatment periods were 9 weeks (range 5 - 16 weeks). The duration of CuradermBEC5 therapy varied depending on size of the particular lesions. The period of treatment was longer than previously reported [46] because the treated squamous cell carcinoma lesions were much larger than those previously reported. Figure 16 illustrates an example of that study.
In 2011, a case report of a large squamous cell carcinoma was presented. The lesion was confirmed histologically as a squamous cell carcinoma and was 4 cm in diameter. Before treatment with CuradermBEC5 the lesion sometimes oozed exudates. After 3 weeks of CuradermBEC5 treatment the lesion appeared larger. Another 3 weeks of treatment the lesion appeared much “cleaner” an was starting to fill in with normal tissue and this continued until after 14 weeks of treatment, the lesion had regressed and normal skin tissue had replaced the squamous cell carcinoma. There was no scar tissue at the completion of the treatment. The patient experienced itching and moderate transient stinging surrounding the treated lesion for the first week of CuradermBEC5 therapy. There were no recurrences 5 years after treatment. Figure 17 illustrates the treatment of this squamous cell carcinoma [47,48].
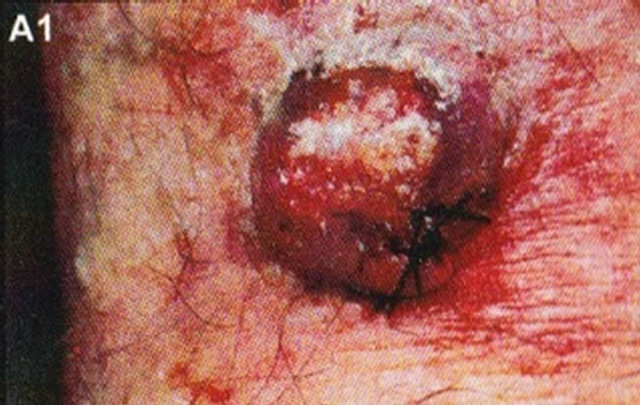
 (a)
(a)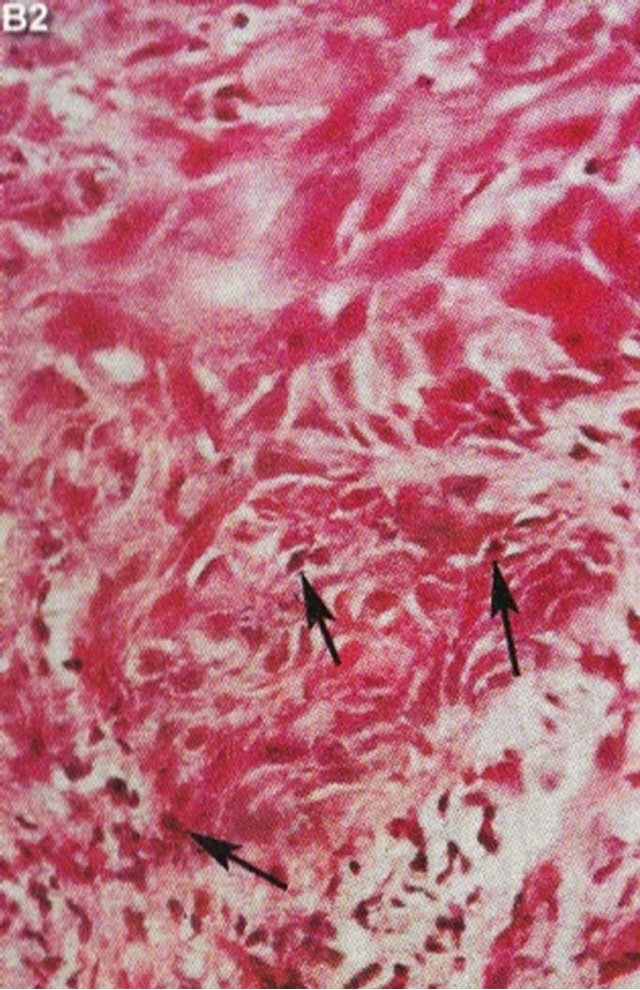 (b)
(b)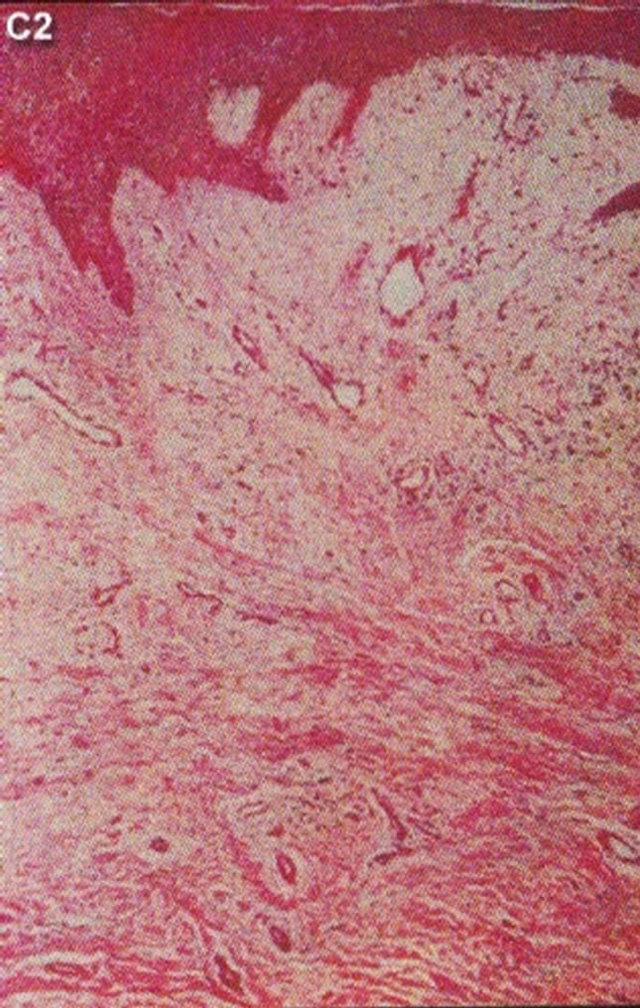 (c)
(c)
Figure 15. Clinical and histological diagnosis of an SCC on a leg of a patient before treatment (lane (a)), during therapy (lane (b)) and site of treated SCC after completion of therapy (lane (c)). 1) Clinical diagnosis; 2) Histological diagnosis. Arrows indicate cancer cells dying during Curaderm treatment (lane B2). The observation of this type of cell death caused by Curaderm is similar to those obtained in cell culture studies.
 (a)
(a)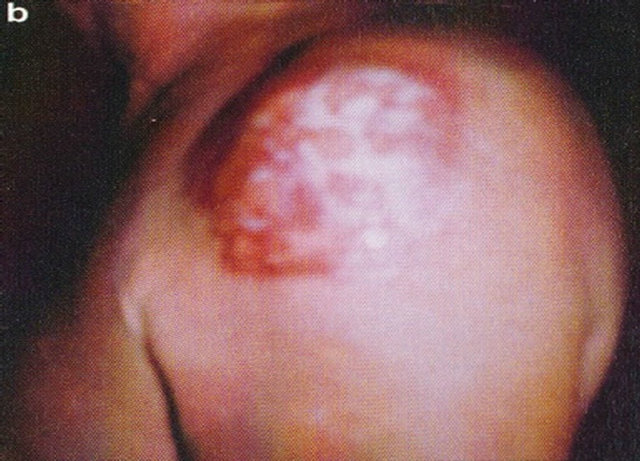 (b)
(b)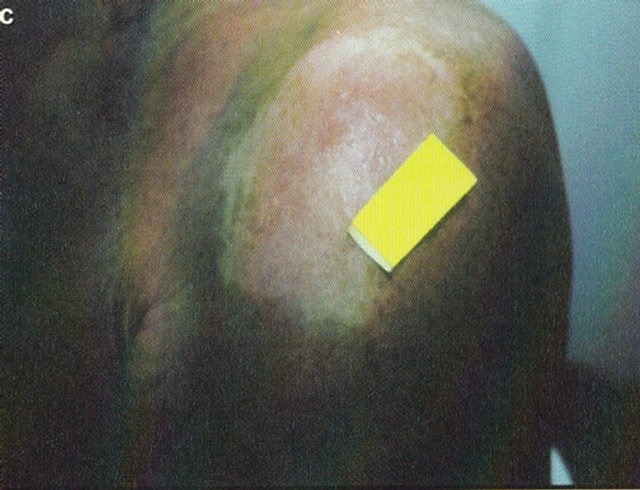 (c)
(c)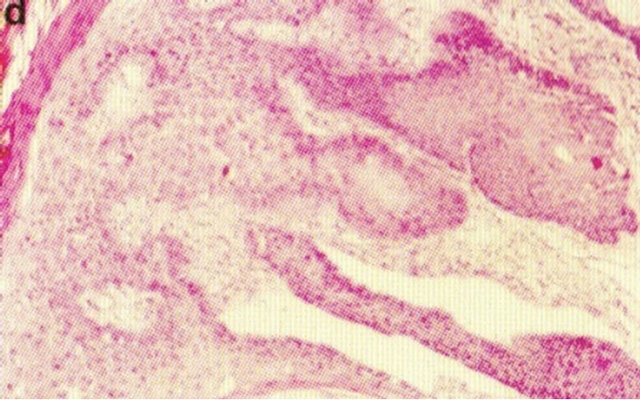 (d)
(d)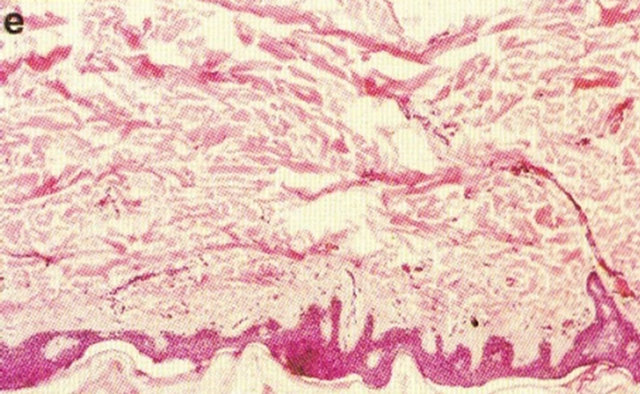 (e)
(e)
Figure 16. A large SCC (approximately 8 cm × 6 cm) on the shoulder of a patient before (a), during (b) and after (c) treatment with Curaderm. After 10 weeks the tumor was completely healed. The clinical diagnosis was confirmed histologically by punch biopsy (d). After completion of the therapy histopathology determined that no residual cancer was present (e). Clinical assessment 5 years post treatment revealed that there was no recurrence.
 (a)
(a) (b)
(b)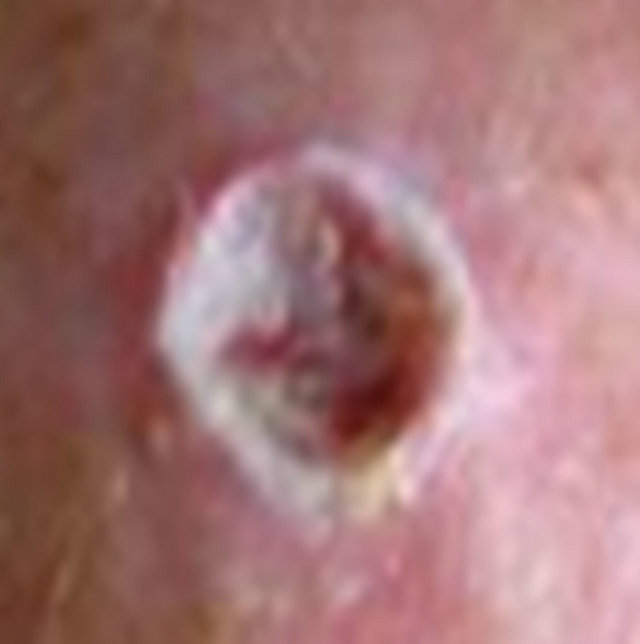 (c)
(c)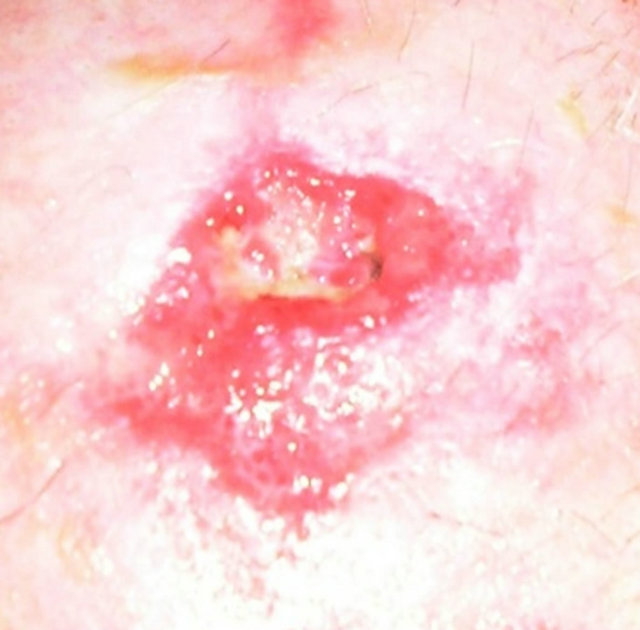 (d)
(d)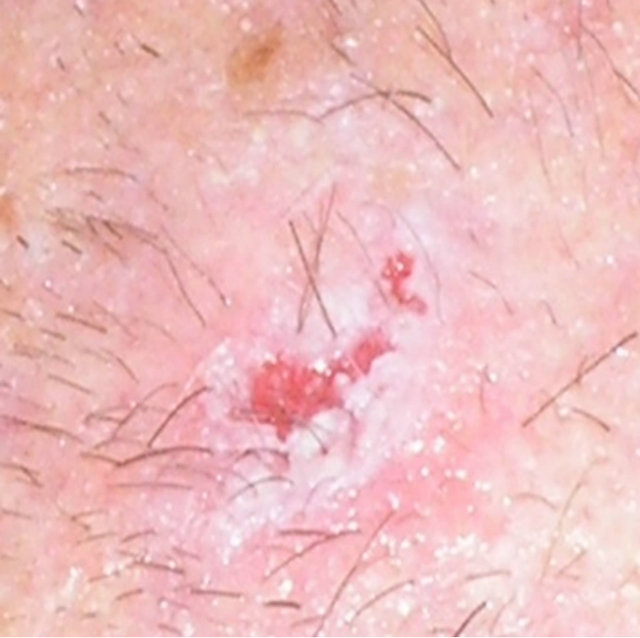 (e)
(e)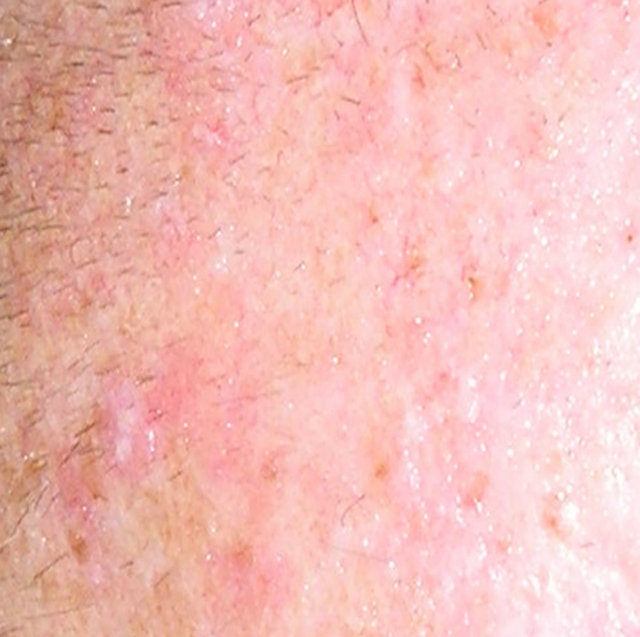 (f)
(f)
Figure 17. A large (4 cm diameter) sometimes oozing SCC on the head of patient 2 (a). Three weeks after Curaderm treatment the lesion appeared to have increased in size (b). Another 3 weeks treatment resulted in the lesion appearing much “cleaner” with some replacement of cancer tissue with normal tissue (c). After another 3 weeks treatment the lesion was much smaller and normal skin tissue had replaced the treated cancerous tissue whilst continually being treated with Curaderm (d). Two weeks later, the treated lesion was in the final stages of healing (e). After a total of 14 weeks treatment the entire lesion was clinically removed and there was no scar tissue at the completion of treatment (f).
2.2.6. Squamous Cell Carcinoma on the Penis
Intralesion administration of BEC completely cleared a severe squamous cell carcinoma on a horse’s penis (Figure 18) [39].
SM was effective against microinvasive squamous cell carcinoma in hairless mice [36]. In separate studies it has been shown that CuradermBEC5 eliminates intraepidermal squamous cell carcinoma (Bowen’s Disease) on the penis of humans [49,50].
2.2.7. Tissue-Sparing and Preservation of Functionality
Figures 19 and 20 show examples of tissue-sparing and preservation of functionality with topical application of CuradermBEC5. Treatment of these tumors with CuradermBEC5 is superior to other more destructive treatment options for Bowen’s disease of the penis. After treatment with CuradermBEC5 functionality was completely restored and it was difficult to locate where the lesions once were.
These observations are in agreement with the intralesion injections of an SCC on the penis of a horse as shown in Figure 18.
3. OVERALL CONCLUSIONS
The original publications in 1987 have gained momentum due to the resurgence in seeking naturally oc-
 (a)
(a)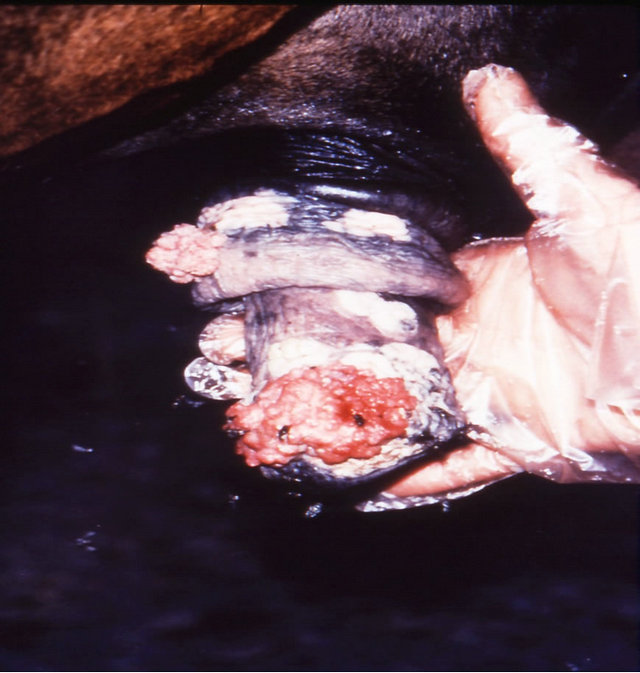 (b)
(b)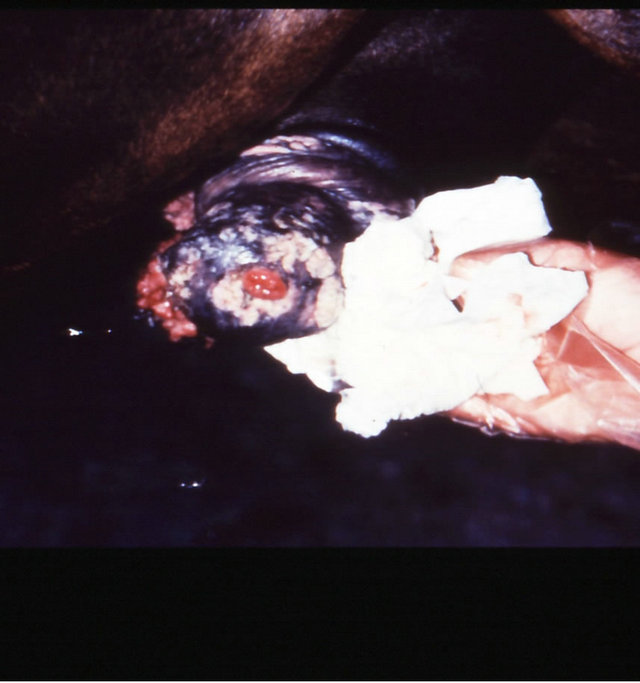 (c)
(c)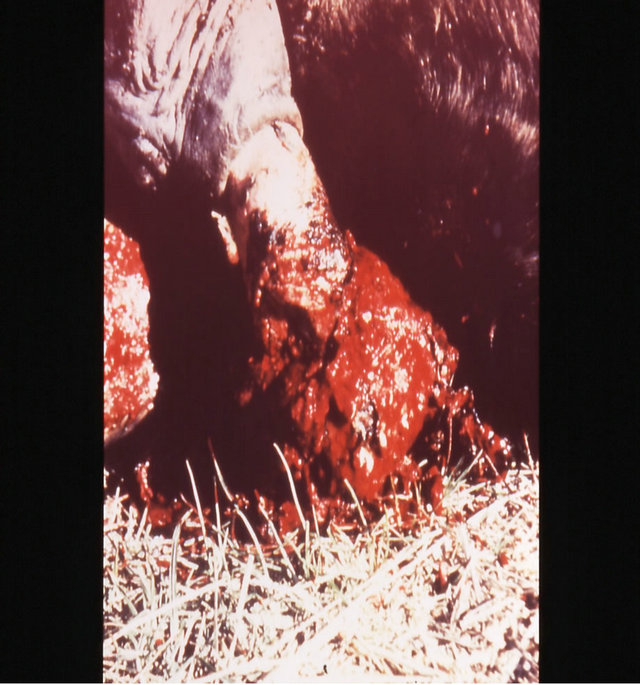 (d)
(d)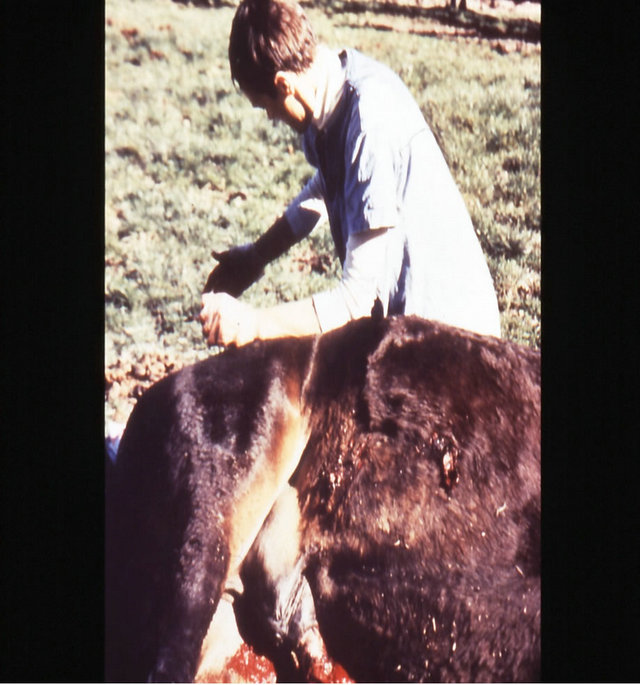 (e)
(e)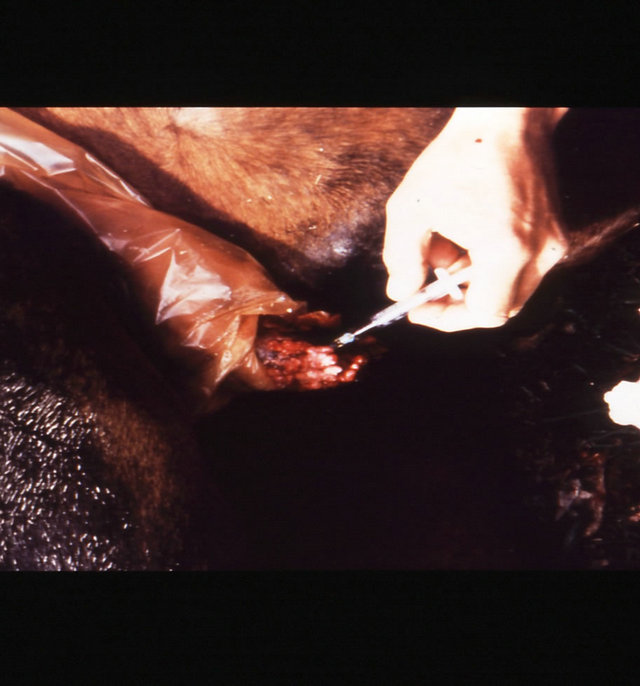 (f)
(f) (g)
(g)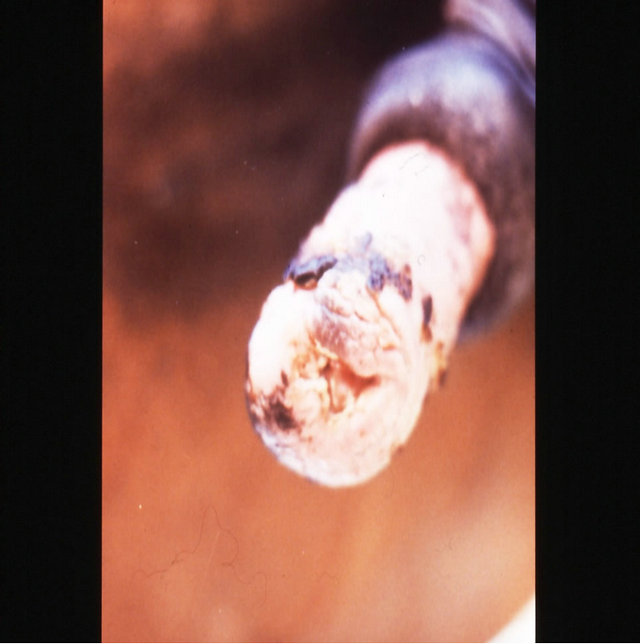 (h)
(h)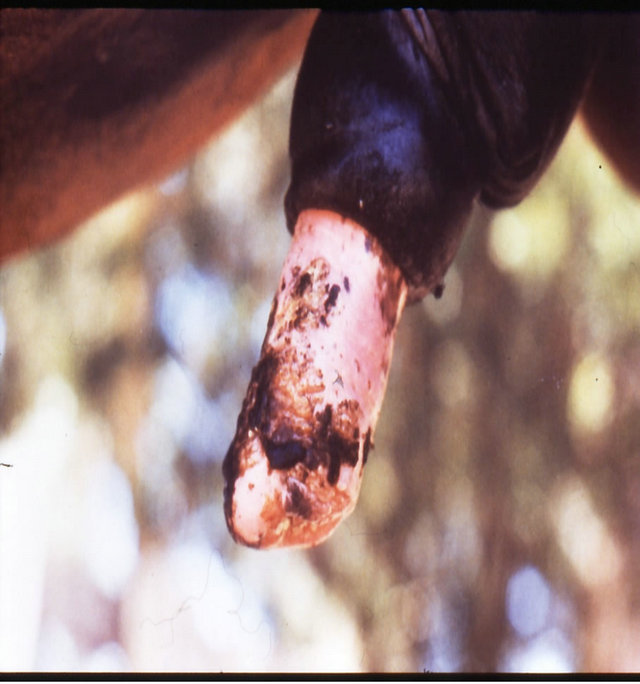 (i)
(i) (j)
(j)
Figure 18. Multiple large SCCs on the penis of a horse. This horse was given three courses of BEC injections before complete remissions of all the tumours were achieved. The extent of multiple lesions are seen in (a) and (b). (c) shows that the tumours were extended throughout the entire penis. The veterinarian injected each individual tumour mass with BEC (d). The horse needed general anaesthetic during BEC therapy. Massive haemorrhagic necrosis of the tumour masses occurred during the treatment course; (e) and (f). After the final treatment (third injection) the tumour separated entirely and fell off while the horse was waking up (g). Successfully treated penis showing no signs of any tumour (h) and (i) two years after the initial diagnosis and treatment of the cancer. The horse was in excellent condition after treatment (j).
 (a)
(a)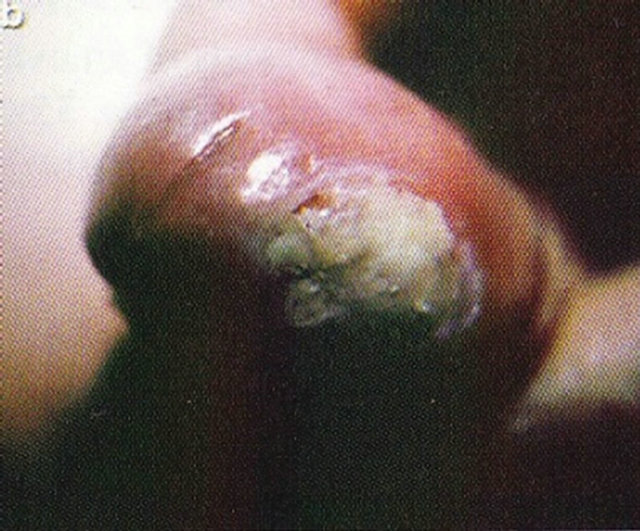 (b)
(b)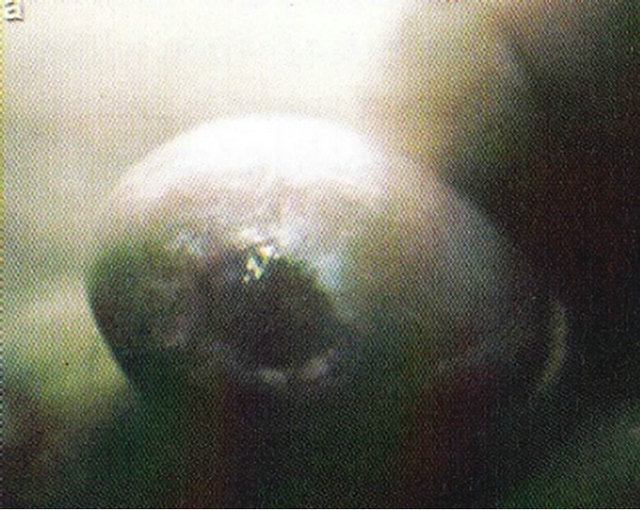 (c)
(c)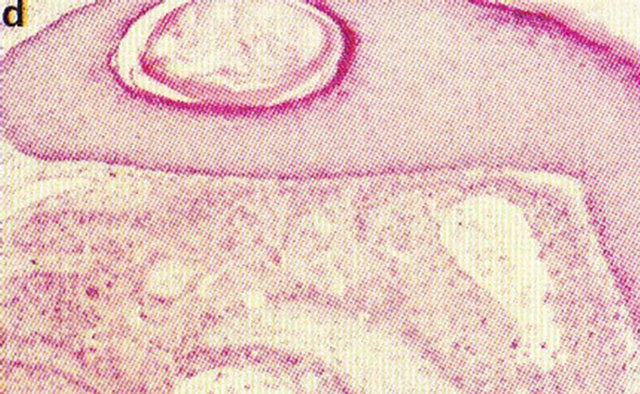 (d)
(d)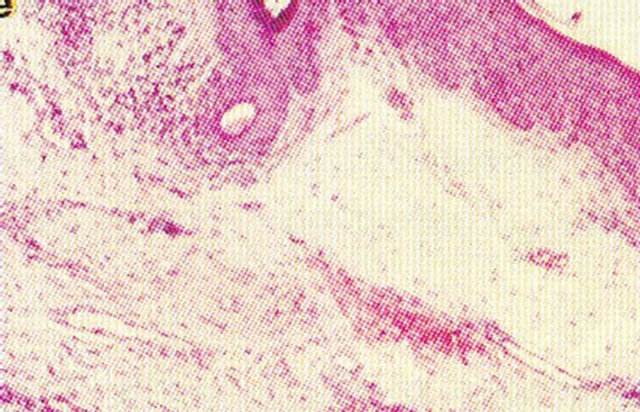 (e)
(e)
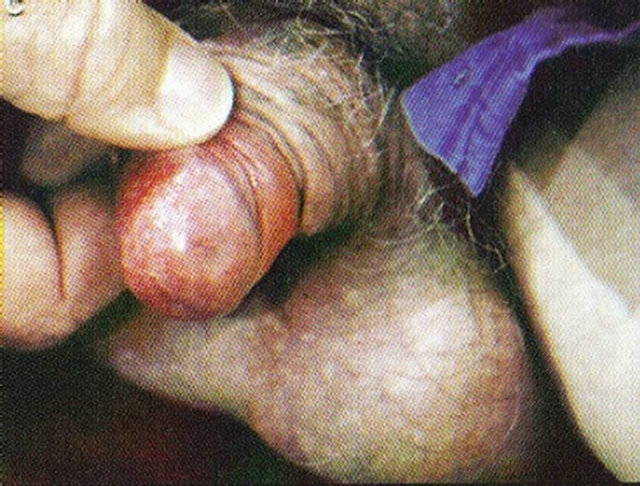
Figure 19. An intra-epithelial SCC on the penis of a patient before (a), during (b) and after Curaderm therapy (c). The prognosis of this patient before treatment with Curaderm was amputation. Treatment period was 6 weeks. The clinical diagnosis was confirmed histologically by biopsy (d). After completion of the therapy histopathology determined that no residual cancer was present (e). Clinical assessment 5 years post treatment revealed that there was no recurrence.
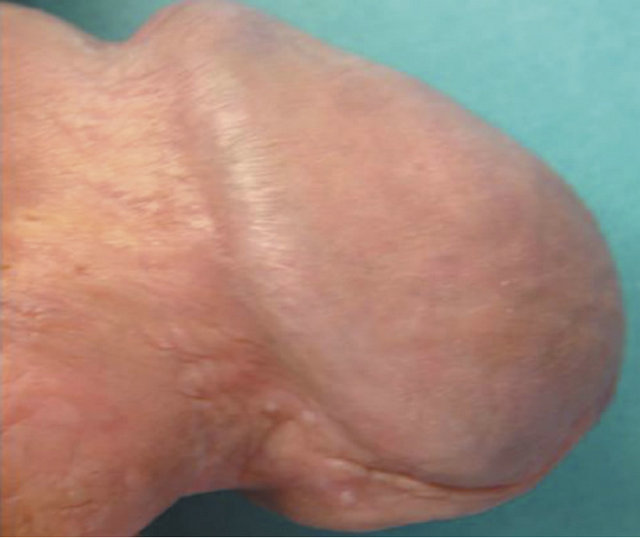 (a)
(a)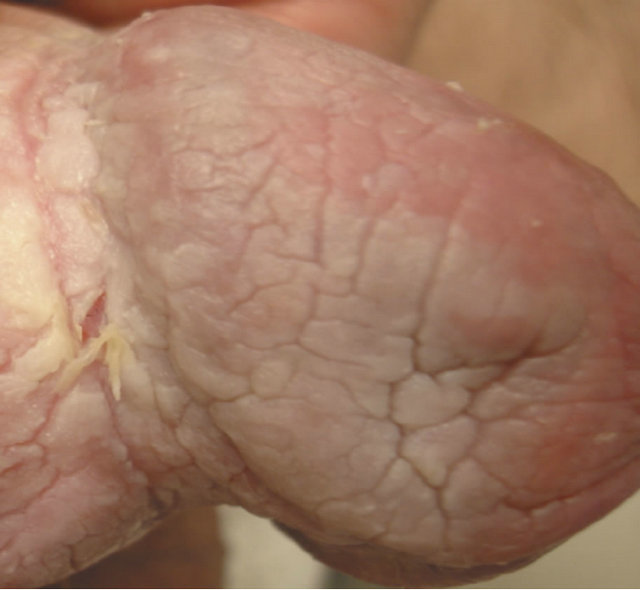 (b)
(b)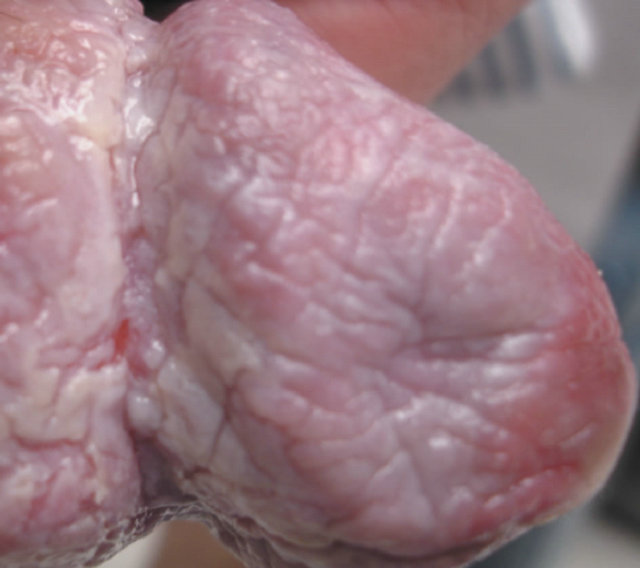 (c)
(c)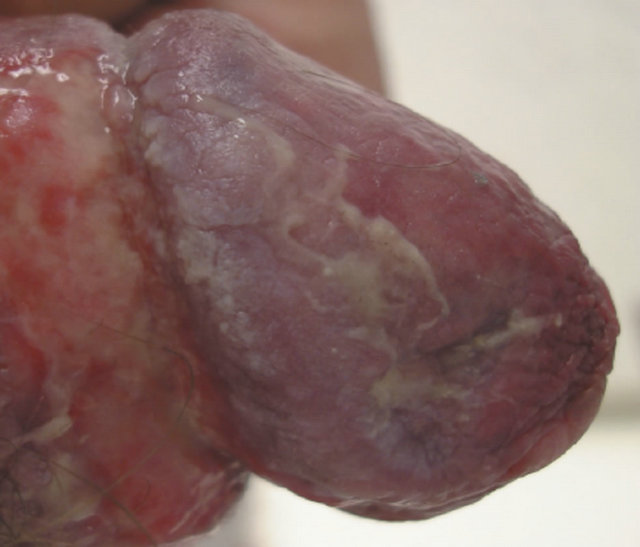 (d)
(d)
Figure 20. Bowen’s disease on the penis before treatment (a), two months after starting treatment with topical CuradermBEC5 (b), nine months after starting treatment (c), and two years follow-up after completion of treatment with CuradermBEC5. Liquid nitrogen was used to treat verrucous lesions.
curring antineoplastic agents in addition to targeted therapies. It is concluded that BEC and its individual glycoalkaloids SM and SS specifically interact with cancer cells by attaching themselves to EELs, which are RBP receptors. After internalization BEC causes apoptosis by inducting pro-apoptotic pathways and reducing anti-apoptotic pathways.
BEC overcomes MDR in cancer cells, and a combined use of SM and other anticancer drugs results in effective treatment of cancer cells that are resistant to currently used antineoplastics. Acute activity of BEC against cancer cells also leads to lasting immunological effects against cancer.
In the clinical setting, BEC shows some promise as an oral and intravenous treatment for cancer. These observations must be seen as very preliminary and much more work on these aspects are required.
Intralesion administration of BEC has shown that this type of application results in the clearing of large tumors. The prospects of BEC intralesion injections continue to be very encouraging.
Topical application of BEC is now available for the treatments of premalignant and malignant skin cancers with high cure rates. The resultant cosmetic outcomes after BEC therapy are very impressive.
REFERENCES
- Cham, B.E. and Wilson, L. (1987) HPLC of glycoalkaloids from Solanum sodomaeum L. Planta Medica, 53, 59-61. doi:10.1055/s-2006-962621
- Cham, B.E., Gilliver, M. and Wilson, L. (1987) Antitumour effects of glycoalkaloids isolated from Solanum sodomaeum L. Planta Medica, 53, 34-36. doi:10.1055/s-2006-962612
- Daunter, B. and Cham, B.E. (1990) Solasodine glycosides. In vitro preferential cytotoxicity for human cancer cells. Cancer Letters, 55, 209-220. doi:10.1016/0304-3835(90)90121-D
- Cham, B.E. and Daunter, B. (1990) Solasodine glycosides. Selective cytotoxicity for cancer cells and inhibition of cytotoxicity by rhamnose in mice with sarcoma 180. Cancer Letters, 55, 221-225. doi:10.1016/0304-3835(90)90122-E
- Cham, B.E. (1993) Efficacy and mode of action of solasodine glycosides (BEC) on cancer cells. Proceedings of 4th Oceania Symposium, Bioconcepts Publishing, 41- 51.
- Nakamura, T., Komori, C., Lee, Y., Hashinoto, F., Yahara, S., Nohara T. and Ejina, A. (1996) Cytotoxic activities of Solanum steroidal glycosides. Biological Biopharmaceutical Bulletin, 19, 564-566. doi:10.1248/bpb.19.564
- Hsu, S.H., Tsai, T.R., Lin, C.N., Yen, M.H. amd Kuo, K.W. (1996) Solamargine purified from Solanum incanum Chinese herb triggers gene expression of human TNFR1 which may lead to apoptosis. Biochemical and Biophysical Research Communication, 229, 1-5. doi:10.1006/bbrc.1996.1748
- Chang, L.C., Tsai, T.R., Wang, J.J., Lin, C.N. and Kuo, K.W. (1998) The rhamnose moiety of solamargine plays a crucial role in triggering cell death by apoptosis. Biochemical and Biophysical Research Communication, 242, 21-25. doi:10.1006/bbrc.1997.7903
- Kuo, K.W. and Lin, C.N. (1999) Pharmacological composition for treating cancer cells. US Patent No. 6214803.
- Kuo, K.W., Hsu, S.H., Li, Y.P., Lin, W.L., Liu, L.F., Chang, L.C., Lin, C.C., Lin, C.N. and Sheu, H.M. (2000) Anticancer activity evaluation of the Solanum glycoalkaloid solamargine. Triggering apoptosis in human hepatoma cells. Biochemical Pharmacology, 60, 1865-1873. doi:10.1016/S0006-2952(00)00506-2
- Esteves-Souza, A., Silva T.M. and Alves, C.C.F. (2002) Cytotoxic activities against Ehrlich carcinoma and human K562 leukemia of alkaloids and flavonoid from two Solanum species. Journal of Brazil Chemical Society, 13, 838- 842. doi:10.1590/S0103-50532002000600017
- Solbec Pty Ltd. http://www.solbec.com.au
- Lee, K.R., Kozukue, N.J., Han, S.J., Park, H., Chang, E.Y., Back, E.J., Chang J.S. and Friedman, M. (2004) Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. Journal of Agricultural Food Chemistry, 52, 2832-2839. doi:10.1021/jf030526d
- Liu, L.F., Liang, C.H., Shiu, L.Y., Lin, W.L., Lin, C.C. and Kuo, K.W. (2004) Action of solamargine on human lung cancer cells-enhancement of the susceptibility of cancer cells to TNFs. FEBS Letters, 577, 67-74. doi:10.1016/j.febslet.2004.09.064
- Lipscombe, R.J., Carter, S.J. and Ruane, M. (2005) Rhamnose binding protein. US Patent No. 6930171.
- He, D.J. (2006) Structure and anticancer activity of glycoalkaloids. Master’s Thesis.
- Ono, M., Nishimura, K.K., Suzuki, T., Fukushima, K., Igoshi, H., Yoshimitsu, H., Ikeda, T. and Nohara, T. (2006) Steroidal glycosides from the underground parts of Solanum sodomaeum. Chemical Pharmacy Bulletin, 54, 230-233. doi:10.1248/cpb.54.230
- Shiu, L.Y., Chang, L.C., Liang, C.H., Huang, Y.S., Sheu, H.M. and Kuo, K. W. (2007) Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chemical Toxicology, 45, 2155-2164. doi:10.1016/j.fct.2007.05.009
- Liang, C.H., Shiu, L.Y., Chang, L.C., Sheu, H.M., Kuo, K.W. (2007) Solamargine upregulation of Fas, downregulation of HER2, and enhancement of cytotoxicity using epirubicin in NSCLC cells. Molecular Nutrition Research, 51, 999-1005. doi:10.1002/mnfr.200700044
- Shiu, L.Y., Liang, C.H., Chang, L.C., Sheu, H.M., Tsai, E.M. and Kuo, K.W. (2009) Solamargine induces apoptosis and enhances susceptibility to trastazumab and epirubicin in breast cancer cells with low or high expression levels of HER2/neu. Bioscience Reports, 29, 35-45. doi:10.1042/BSR20080028
- Sun, L., Zhao, Y., Li, X., Yuan, H., Cheng, A. and Lou, H. (2010) A lysosomal-mitochondrial death pathway is induced by solamargine in human K562 leukemia cells. Toxicology in Vitro, 24, 1504-1511. doi:10.1016/j.tiv.2010.07.013
- Sun, L., Zhao, Y., Yuan, H., Li, X., Cheng A. and Lou, H. (2010) Solamargine, a steroidal alkaloid glycoside, induces oncosis in human K562 leukemia and squamous cell carcinoma KB cells. Cancer Chemotherapy Pharmacology, 65, 1125-1130.
- Li, X., Zhao, Y., Wu, W.K.K., Liu, S., Cui, M. and Lou, H. (2011) Solamargine induces apoptosis associated with p53 transcription-dependent and transcription-independent pathways in human osteosarcoma U20S cells. Life Science, 88, 314-321. doi:10.1016/j.lfs.2010.12.006
- Wang, Y., Gao, J., Gu, G., Li, G., Cui, C., Sun, B. and Lou, H. (2011) In situ RBL receptor visualization and its mediated anticancer activity for solasodine rhamnosides. Chemistry Biochemistry, 12, 2418-2420. doi:10.1002/cbic.201100551
- Cham, B.E. and Chase, T.R. (2012) Solasodine rhamnosyl glycosides cause apoptosis in cancer cells. Do they also prime the immune system resulting in long-term protection against cancer? Planta Medica, 78, 349-353. doi:10.1055/s-0031-1298149
- Chataing, B., Concepcion, J.L., Buitrago de Cristancho, N. and Usubillaga, A. (1996) Clinical study of the effectiveness of extracts alkaloids obtained of the fruits of Solanum americanum miller on herpes simplex, herpes two zoster and genital herpes., 32, 18-25.
- Thorne, H.V., Clarke, G.F. and Skuce, R. (1985) The inactivation of herpes simplex virus by some Solanaceae glycoalkaloids. Antivirus Research, 5, 335-343. doi:10.1016/0166-3542(85)90003-8
- Ding, X., Zhu, F.S., Li, M. and Gao, S.G. (2012) Induction of apoptosis in human hepatoma SMMC-7721 cells by solamargine from Solanum nigrum L. Journal of Ethnopharmacology, 39, 599-604. doi:10.1016/j.jep.2011.11.058
- Li, X., Zhao, Y., Ji, M., Liu, S.S., Cui, M. and Lou, H.X. (2011) Induction of actin disruption and downregulation of HER2, and enhancement of cytotoxicity using epirubicin in NSCLC cells. Molecular Nutrition Research, 51, 999-1005.
- Cham, B.E. (2007) Review article. Solasodine rhamnosyl glycosides specifically bind cancer cell receptors and induce apoptosis and necrosis. Treatment for skin cancer and hope for internal cancers. Research Journal of Biological Sciences, 2, 503-514.
- Cham, B.E. (1994) Solasodine glycosides as anti-cancer agents: Pre-clinical and clinical studies. Asia Pacific Journal of Pharmacology, 9, 113-118.
- Cham, B.E., Daunter, B. and Evans, R. (1991) Topical treatment of malignant and premalignant skin cancers by very low concentrations of a standard mixture of solasodine glycosides. Cancer Letters, 59, 183-192. doi:10.1016/0304-3835(91)90140-D
- Cham, B.E. (2011) Topical solasodine rhamnosyl glycosides derived from the eggplant treats large skin cancers: Two case reports. International Journal of Clinical Medicine, 2, 473-477. doi:10.4236/ijcm.2011.24080
- Tavares, D.C., Munari, C.C., Araujo, M.G., Beltrame, M.C., Furtado, M.H., Goncalves, C.C., Tiossi, R.F., Bastos, J.K., Cunha, W.R. and Veneziani, R.C. (2011) Antimutagenic potential of Solanum lycocarpum against induction of chromosomal aberrations in V79 and micronuclei in mice by doxorubicin. Planta Medica, 77, 1489- 1494. doi:10.1055/s-0030-1270886
- Tang, Z., Zhang, Y., Li, N., Xu, L., Zhao, B., Xiao, W., Wang, Z. and Bi, Y. (2011) Extraction, purification technology and antineoplastic effects of solamargine. Zhongguo Zhong Yao Za Zhi, Journal of Chinese Material Medicine, 36, 2192-2195.
- Wu, C.H., Liang, C.H., Shiu, L.Y., Chang, L.C., Lin, T.S., Lan, C.E.R., Tsai, J.C., Wong, T.W., Wei, K.J., Lin, T.K., Chang, N.S. and Sheu, H.M. (2011) Solanum incanum extract (SR-T100) induces human cutaneous squamous cell carcinoma apoptosis through modulating tumor necrosis factor receptor signaling pathway. Journal of Dermatological Science, 63, 83-92.
- Millward, M., Powell, A., Daly, P., Tyson, R., Ferguson, R. and Carter, S. (2006) Results of phase I clinical trials of coramsine in patients with advanced solid tumours. Journal of Clinical Oncology, 24, 2070.
- Millward, M., Powell, A., Tyson, S., Daly, P., Ferguson, R. and Carter, S. (2005) Phase I trial of coramsine (SBP002) in patients with advanced solid tumours. Journal of Clinical Oncology, 23, 3105.
- Cham, B.E. (2008) Cancer intralesion chemotherapy with solasodine rhamnosyl glycosides. Research Journal of Biological Science, 3, 1008-1017.
- Cham, B.E. (2012) Intralesion and Curaderm BEC5 topical combination therapies of solasodine rhamnosyl glycosides derived from the eggplant or devil’s apple result in a rapid removal of large skin cancers. Methods of treatment compared. International Journal of Clinical Medicine, 3, 115-124.
- Cham, B.E. (2013) Inspired by nature, proven by science. The new generation cancer treatment that causes cancer cells to commit suicide. Submitted.
- Cham, B.E. and Meares, M.M. (1987) Glycoalkaloids from Solanum sodomaeum L. are effective in the treatment of skin cancers in man. Cancer Letters, 36, 111-118. doi:10.1016/0304-3835(87)90081-4
- Cham, B.E., Daunter, B. and Evans, R. (1991) Topical treatment of malignant and premalignant skin cancers by very low concentrations of a standard mixture of solasodine glycosides. Cancer Letters, 59, 183-192. doi:10.1016/0304-3835(91)90140-D
- Cham, B.E. (2013) Solasodine glycosides: A topical therapy for actinic keratosis. A single-blind, randomized, placebo-controlled, parallel group study. Submitted.
- Punjabi, S., Cook, L.J., Kersey, P., Marks R. and Cerio, R. (2008) Solasodine glycoalkaloids: A novel topical therapy for basal cell carcinoma. A double-blind, randomized, placebo-controlled, parallel group, multicentre study. International Journal of Dermatology, 47, 78-82.
- Cham, B.E. (2007) Solasodine rhamnosyl glycosides in a cream formulation is effective for treating large and troublesome skin cancers. Research Journal of Biological Sciences, 2, 749-761.
- Cham, B.E. (2011) Topical solasodine rhamnosyl glycosides derived from the eggplant treats large skin cancers: Two case reports. International Journal of Clinical Medicine, 2, 473-477. doi:10.4236/ijcm.2011.24080
- Cham, B.E. (2007) Solasodine rhamnosyl glycosides specifically bind cancer cell receptors and induce apoptosis and necrosis. Treatment for skin cancer and hope for internal cancers. Research Journal of Biological Sciences, 2, 503-514.
- Chase, T.R. (2011) Curaderm BEC5 for skin cancers, is it? An overview. Journal of Cancer Therapy, 2, 728-745. doi:10.4236/jct.2011.25099
- Goldberg, L.H., Landau, J.M., Moody, M.N. and VergilisKalner, I.J. (2011) Treatment of Bowen’s disease on the penis with low concentration of a standard mixture of solasodine glycosides and liquid nitrogen. Dermatologic Surgery, 37, 858-861. doi:10.1111/j.1524-4725.2011.02014.x

