American Journal of Analytical Chemistry
Vol.07 No.08(2016), Article ID:69575,6 pages
10.4236/ajac.2016.78057
Determination of Lead(II) in Liver Corpse of a Slaughtered Cattle with Preconcentration on a Chelating Sorbent
R. A. Aliyeva1, N. S. Huseynova2, Ulviya M. Abilova1*, G. B. İskandarov2, F. M. Chiragov1
1Department of Analytical Chemistry, Faculty of Chemistry, Baku State University, Baku, Azerbaijan
2Department of the General and Toxicological Chemistry, Faculty of Pharmacology, Azerbaijan Medical University, Baku, Azerbaijan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 19 February 2016; accepted 5 August 2016; published 8 August 2016
ABSTRACT
The copolymer of the maleic anhydride-styrene is modified at the presence of 4-amino-2-thiouracil and formaldehyde and the new polymeric sorbate with spatial structure is received. The received sorbate is identified by the IR-spectroscopy method. The complete static sorption capacitance was studied ( = 7.8 mmol/g) and the ionization constants of ionic groups in a sorbate link was defined by electrometric method. Ionization constants were determined by potentiometric titration of the sorbent (
= 7.8 mmol/g) and the ionization constants of ionic groups in a sorbate link was defined by electrometric method. Ionization constants were determined by potentiometric titration of the sorbent (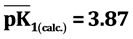 ,
, 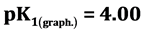 ,
, 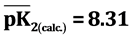 ,
,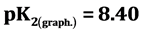 ). Sorption and desorption of the received sorbent with a lead ion (ІІ) are investigated and optimum concentration conditions are defined: рНopt. = 5, ionic force µ = 0.8 pier/l, full sorption balance 4 hours, optimum eluent 5 ml of 0.5 M HCl.
). Sorption and desorption of the received sorbent with a lead ion (ІІ) are investigated and optimum concentration conditions are defined: рНopt. = 5, ionic force µ = 0.8 pier/l, full sorption balance 4 hours, optimum eluent 5 ml of 0.5 M HCl.
Keywords:
Sorption Capacity, Preconcentration, Polymeric Sorbate, Lead(II)

1. Introduction
Slight lead content in animal and human organs necessitates its selection and concentration before subsequent determination. The intense way of restoring the amino acids necessary for proper formation and tissue growth is possible only with consumption of animal protein. Animal protein sources in Azerbaijan are beef, poultry and lamb. Messy dumping on land and in water of bodies, illegal extraction of ore and painting houses, the use of tetra ethyl lead as an anti-knock additive to improve asoline quality in Azerbaijan and in many other developing countries leads to lead poisoning animals. Cattle and other ruminants graze freely in these conditions, and drink water from ponds, streams, rivers and other possible sources of pollution, which are hazardous to human and animal health. These metals bio-accumulate in the organs and other tissues of these animals. When these animals are killed for human consumption, lead can bio-accumulate in human tissues and organs. This explains why the lead level in animal products continues to be the center of attention of health workers [1] - [5] . Lead can negatively affect many organs and life-support systems, causing high blood pressure, anemia, kidney damage, hearing loss and mental retardation [6] . This research was conducted to determine the level of lead residue in the cattle’s liver in Baku. In case of determination of small amounts of metals in various sites, preconcentration is often necessary.
These include methods of using of sorptive chelate adsorbents. Their high selectivity allows you to retrieve hard metals from solutions of complex composition [7] . Determination of trace amounts of lead in the environment is possible only after pre-concentration. To increase the selectivity and effectiveness of lead concentration azo containing sulfur, sorbents are often used and have high practical interest in determining its complex objects in [8] . Chelating properties of selective sorbents caused by the presence of complexing groups depend on the experimental conditions of pH, saline background and the presence of the masking agents. Previously, it was shown that chelate sorbent-copolymer of maleic anhydride with styrene, modified with ternary amines in the presence of formaldehyde, effectively absorbed acidic and alkaline environment ions of Cu, Fe, Cd, U, Zn and other elements [9] .
The main objective in the present study is to investigate the sorption and desorption of Pb(II) obtained by the sorbent and to determine the optimal concentration condition.
The sorption capacity of sorbent to the amount of lead is −497 mg/g (2.40 mmol/g). This indicator is not inferior in such well-known complexing sorbents as PSTM-3T (CE = 160 mg/g), fibrous sorbents with nitrogen-con- taining functional groups, the Academy of Sciences 1 (1.33 mmol/g) AL-1 (0.43 mmol/g), KH-1 (0.21 mmol/g), AN-3 (0.035 mmol/g) [6] - [8] . Potentiometric method defined ionization constants of ionic groups in a part of the sorbent. The methodology was used for the sorption-photometric determination of lead in the liver of cattle.
2. Experimental Section
2.1. Reagents and Apparatus
All the chemicals and reagents used in this work are of analytical grade and standart solution of lead(II) ion was prepared by dissolving Pb(CH3COO)2 in water. Lead(2+) solution with concentration 1.0 × 10−2 mg−1∙l−1 is used in this work. The calculated amount of KCl was introduced to maintain a constant ionic strength. Caustic potassium hydroxide used for potentiometric titration of solutions was prepared by dissolving KOH (c.p.) in distilled water, the concentration of solution was determined by titration with standard solution of HCl. The necessary pH values were created by using fiksanal HCl (pH 1 - 2) and ammonium acetate buffer solution (pH 3 - 11). pH of solutions were measured by ionomer I-130 with glass electrode. Optical density of solutions were measured by using of photocolorimeter KFK-2 (l = 1 sm). For photometric determination of lead, we used xylene orange as a reagent. Concentration of Pb(II) was calculated by using of calibration curve and the results were worked up by math statistic methods [10] . The investigation of sorption was made in static conditions.
2.2. The Method of Preparation of Chelating Polymer
In this work, we applied a new polymer chelateforming sorbent with fragments of 4-amino-2-thiouracil. Radical copolymerization of maleic anhydride with styrene was carried out by the technique [11] [12] . Counted quantity of formalin and 4-amino-2-thiourasil is added to the received copolymer. The sorbents was synthesized by adding suitable amine and formaldehyde onto gotten copolymer. Reaction was performed on sand bath with continuously mixing. The reaction was performed in water medium. Therefore, copolymer’s anhydride groups were hydrolyzed. Reaction lasts 30 - 40 min. under 333.15 - 343.15 K of temperature for removal of remaining parts of reaction product sorbent has been rinsed several times with distilled water. Then constant mass was dried in vacuum desiccators at 323 K and skipped through sieve with 0.14 mm of pore diameter. The synthesis reactions are shown below.
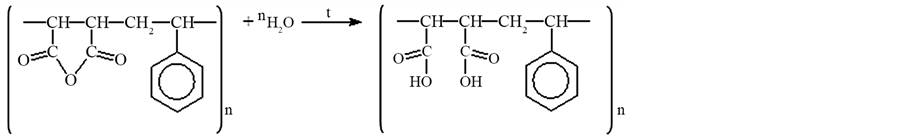
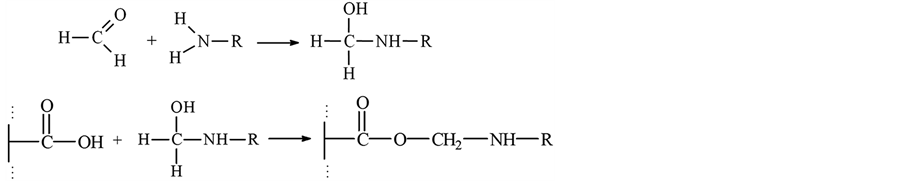
The resulting product was investigated by IR-spectroscopy.
3. Results and Discussion
3.1. Absorption Spectra
It is known that sorption properties of the sorbent depend on the dissociation constants of ionogenic groups contained in their composition. To study the acid-base properties of produced sorbent by a known method the complete static sorption capacitance was studied (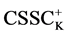 = 7.8 mmol/g) and then verified by potentiometric titration [13] . Based on the results neutralization degree (α) of individual acidic groups and the magnitude of lgα/1-α were calculated as a function of pH for the construction of the potentiometric curve, which is characterized by two inflection points. From these data, using the modified equation Ganderson-Gasselbakh dissociation constants of alkaline groups
= 7.8 mmol/g) and then verified by potentiometric titration [13] . Based on the results neutralization degree (α) of individual acidic groups and the magnitude of lgα/1-α were calculated as a function of pH for the construction of the potentiometric curve, which is characterized by two inflection points. From these data, using the modified equation Ganderson-Gasselbakh dissociation constants of alkaline groups  and detachment of protons from non-reactive carboxyl groups [14] [15] . Ionization constants were determined by potentiometric titration of the sorbent. (
and detachment of protons from non-reactive carboxyl groups [14] [15] . Ionization constants were determined by potentiometric titration of the sorbent. (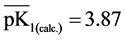 ,
, 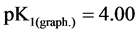 ,
, 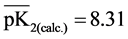 ,
,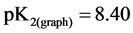 ).
).
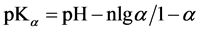
We can see that the sorbent contains 2 different ionogenic groups. So the ionization of the sorbent goes on in 2 stages:
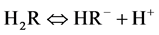

3.2. Effect of pH
The effect of pH on the preconcentration of Pb(II) ions in static conditions, on chelating sorbents is in the pH range of 1 - 8. The influence of pH is shown in Figure 1. As can be seen, quantitative recovery of metal ions is achieved in the pH range of 4 - 6.
For small values (pH 1 - 4) of the liquid phase low degree of recovery may be associated with protonation of functional groups in the sorbent phase and with a low swelling degree of the polymer. For these values of pH Pb(II) ions are in the form of Pb2+ [16] . With increasing of pH of the liquid phase (pH 5 - 6) the swelling degree of polymer sorbents increases [16] .
3.3 Effect of Initial Concentration of Pb2+ Ions on Sorbent’s Sorption Capacity
The effect of initial Pb(II) ions concentration on adsorption was studied at the range of 0.2 × 10−3 mol∙l−1 to 8.0 × 10−3 mol∙l−1. The percentage removal of Pb2+ ions increased with increasing of Pb2+ concentration. Investigation has shown that the maximum adsorption is observed when the metal ion concentration is 8.0 × 10−3 mol∙l−1. Results are illustrated on the diagram and table below (Table 1, Figure 2).
Figure 1. Effect of pH on recovery degree (R, %) of Pb(II) ions (concentration of Pb(II) 200 mg/l, solution volume 20 ml, msorp. = 100 mg).
Figure 2. Effect of Pb2+ ions concentration on sorption capacity msorb. = 30 mg, Vtotal = 20 ml, 
Table 1. Removal efficiency percentage of Pb(II) ions by the investigated sorbent.
Sc―sorption capacity.
Table 1 shows that removal percentage of Pb(II) sorption reaches its maximal value when the metal ion concentration is 2.0 × 10−3 mol∙l−1. As we see at the range of concentration 0.2 × 10−3 - 1.0 × 10−3 mol∙l−1 full sorption of Pb(II) ions by investigated sorbent is observed. Therefore to determine sorption capacity volume of metal ion solution should be increased.
3.4. Effect of Eluent
Various acids-HClO4, H2SO4, HCl, HNO3 were tested to select a suitable eluent. HClO4 shows the greatest influence on the desorption degree of lead ion (ІІ). Various concentrations of 0.2 - 1.5 M HCl were tested to select a suitable tested eluent (Table 2).
In further experiments, 5 ml of 0.5 M HCl was used as eluent. The adsorbent does not lose its sorption properties after regeneration and can be reused.
The optimal conditions of sorption (Table 3) were determined while studying sorption equilibrium of obtained new chelating sorbent with Pb2+ ions (exploring the influence of pH of solutions, ionic strength, Pb2+ ion concentration on the sorption, the dependence on time).
It may be concerned with the arrangement and stability of remaining chelat cycles. Adsorption and desorption processes has been taken place in static condition.
3.5. Using Method to Object
Total 100 grams fresh samples of liver were collected from killed cattle’s in Baku. Samples were packed in sterile plastic bags, labeled with a marker, and stored frozen in freezer. Then frozen samples were transported to the research laboratory of the Baku State University for further processing and analysis. For the analysis took three liver samples, respectively 23.9387 g (I sample) 36.8022 g (ІІ sample), 28.1416 g (III sample) of mass. To the first and the second samples 1 g. Pb(CH3COO)2 was added. Samples were dissolved in a graphite crucible under heating in 55 ml of HNO3 (conc.). Within an hour, the liver was dissolved in acid. The solution was then calcined in the muffle furnace. Obtained weight of the ashes of the first sample is 4.6511, 5.1930-second sample, a third sample −5.5223 g. The resulting ash is dissolved in aqua regia. After the resulting mixture was dissolved in distilled water, the insoluble material was separated by filtration. The resulting solution was transferred into a flask of 100 ml, pH = 6 adjusted to the desired value, added to the mark with distilled water and mixed. In the prepared liver, lead (Pb) residue was determined pre-concentration with chelating polymer. Then photometric determination of lead we used xylene orange as a reagent [17] . Data were presented on Table 4.
The concentration of lead in the liver samples ranged to 1.087 mg/kg with mean and standard deviation of 0.0067 mg/kg.
Table 2. Effect of concentration and volume of eluent on recovery degree (R, %) of determined Pb(II) ions (n = 3).
Table 3. Optimal conditions of sorption (*msorp. = 50 mg; Vlic.ph. = 20 ml).
*Mass of sorbent; **Sorption capacity; ***Ionic strength of the adductor to a decrease in sorption.
Table 4. Results of the analysis (100 ml sample volume, the volume of eluent to 5 ml, msorb = 100 mg; P = 0.95; n = 5).
4. Conclusion
The present study has shown that maleic anhydride styrene copolymer based sorbents can be used for removal of Pb2+ ions from natural and industrial objects. Moreover, the functionally active groups of amine of 4-amino-2- thiouracil show high selectivity over Pb2+ ions. The current method was successfully used for determination Pb2+ ions in the liver of murdered large cattle. Lead concentration was determined in liver samples of slaughtered cattle at Baku. There was a significant different in the concentration of lead in liver of the samples.
Cite this paper
R. A. Aliyeva,N. S. Huseynova,Ulviya M. Abilova,G. B. İskandarov,F. M. Chiragov, (2016) Determination of Lead(II) in Liver Corpse of a Slaughtered Cattle with Preconcentration on a Chelating Sorbent. American Journal of Analytical Chemistry,07,617-622. doi: 10.4236/ajac.2016.78057
References
- 1. Bala, A., Junaidu, A.U., Salihu, M.D., Onifade, K.I., Magaji, A.A., Faleke, O.O., Saulawa, M.A., Musawa, A.I., Mohammed, M, Muhammad, L.U., Pewan, S.B., Anzaku, S.A. and Emenna, P. (2012) Journal of Veterinary Advances, 2, 132.
- 2. Blakley, B.R (1984) Veterinary and Human Toxicology. No. 26. 505.
- 3. Bolter, E., Butz, T.R. and Arseneau, J.F. (1975) Iranian Journal of Veterinary Research. Toronto Canada, 10, 28.
- 4. Bratton, G.R. and Zmudski, J. (1984) Veterinary and Human Toxicology. 26, 387.
- 5. Wagner, H.P. (1995) Journal of America Society. Brewery Chemistry, 53, 141.
- 6. Maxodoeva, O.B., Myasoedova, Q.V. and Kubrakova, I.V. (2007) JAХ, B62, 454
- 7. Losev, V.N, Barash, A.N., Volkova, Q.B., Baxvalova, I.P. and Jarova, L.A. (1999) Chemistry and Chemical Technology, P42, 41
- 8. Arnautov, N.V. (1987) Standard Samples of the Chemical Composition of Natural Minerals: Method. Recommendations. IQiQSO ANASSSR, Novosibirsk, 204 p.
- 9. Vasileva, I.E., Pojidaev, Y.N., Vlasova, N.N., Voronkov, M.Q. and Filipcenko, Y.A. (2010) Sorption-Atomic- Emission Determination of Gold, Platinum and Palladium in Rocks and Ores Using Sorbent PSTM-3T. Analitical and Kontrol, P.14, 16.
- 10. Wagner, H.P. (1995) Determination of Lead in Beer Using Zeeman Background Corrected Graphite Furnace Atomic Absorption Spectrometry. Journal of America Society. Brewery Chemistry, 53, 141-144.
- 11. Akperov, O.N. and Akperov, E.O. (2002) Workshop on High-Molecular Chemistry. Baku, 231.
- 12. Aliyeva, R.A., Hamidov, S.Z. and Chiragov, F.M. (2007) The Study of Sorption of Zn(II) Ions with Chemically Modified Synthetic Sorbent. Journal of Chemical Problems, No. 2, 28.
- 13. Basargin, N.N. and Isayev, E.I. (1986) Correlation and Prediction of Analytic Properties of Organic Reagents and Chelating Sorbents. Nauka, Moscow, 199 p.
- 14. Cepalev, S.V. (2011) Concentration of Metal Ions Polymer Complexing Sorbents in the Presence of Ligands Monodentantnyh: Extraction and Identification of Pb (II), Cd (II), Ni (II) in Natural Objects. Abstract of the Thesis for the Degree of Candidate of Chemical Sciences Moskva.
- 15. Myasoedova, G.V. and Savvin, S.B. (1984) Chelating Sorbents. Nauka, Moscow, 173.
- 16. Nazarenko, V.A., Antonovich, V.P. and Nevskaya, E.M. (1979) The Hydrolysis of Metal Ions in Dilute Solutions. M. Atomizdat, 192 p.
- 17. Bulatov, M.I. and Kalinkin, I.P. (1972) Practical Book on Photometric and Spectrometric Methods of Analysis. L. Chemistry, 407 p.
NOTES
*Corresponding author.





