American Journal of Analytical Chemistry
Vol.3 No.10(2012), Article ID:23622,5 pages DOI:10.4236/ajac.2012.310091
Measurement of Arsenic Species in Infant Rice Cereals by Liquid Chromatography Inductively Coupled Plasma Mass Spectrometry
University of Missouri-Columbia, Research Reactor, St. Louis, USA
Email: brockmanjd@missouri.edu
Received August 11, 2012; revised September 23, 2012; accepted September 30, 2012
Keywords: Arsenic Speciation; Epidemiology; Infant Rice Cereal; Liquid Chromatography Inductively Coupled Plasma Mass Spectrometry
ABSTRACT
Infant rice cereals were analyzed for total arsenic, inorganic arsenic (i-As) and the organic arsenic species monomethylarsonoic acid (MMA) and dimethylarsinic acid (DMA) using liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP-MS). Total arsenic concentrations in the samples ranged from 110 ng/gup to 420 ng/g. The i-As in the rice cereals accounted for 33% to 77% of the total arsenic. The observed variability between infant rice cereals makes a dietary survey approach to accessing arsenic exposures difficult.
1. Introduction
Exposure to arsenic has been linked to immune system dysfunction, cancer, skin legions and cardiovascular disease in adults [1-3]. The inorganic arsenic species arsenite (AsIII) and arsenate (AsV) are the most toxic forms. Both inorganic arsenic species are water soluble and readily absorbed in the gastrointestinal tract. Humans and other organisms metabolize inorganic arsenic (i-As) by successive oxidative methylation and reduction steps into monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) which are considered less toxic than i-As. DMA is also known as cacodylic acid and was widely used at a pesticide until it was banned by the US EPA in 2009.
Consumption of rice and rice products may be a significant source of arsenic exposure, particularly in individuals who regularly consume rice products and have low exposure from drinking water. In a pilot study Gilbert-Diamond et al. observed that total arsenic, inorganic arsenic (i-As), MMA and DMA in measured urine samples collected from 229 pregnant women were correlated with rice consumption [4]. Food products made from rice may also contribute to arsenic exposure. Elevated levels of total arsenic and i-As have been reported in brown rice syrup, rice based energy bars, infant rice cereal and infant rice based formulas [5-8]. The chemical form and total concentration of arsenic in rice and rice products depends on many variables including the species and genetic variation of rice, arsenic concentration in the soil and irrigation water, flooded or non-flooded fields, and historical use of the As containing pesticides DMA [9- 11]. The processing of the rice may also affect the As content, for example brown rice contains higher levels of arsenic than white rice [12].
In this paper we used Instrumental Neutron Activation Analysis (INAA) to measure total arsenic and anion exchange liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP-MS) to measure i-As, DMA and MMA in infant rice cereals purchased in a US market. For INAA measurement of total As, the infant rice cereal was encapsulated in high purity polyethylene vials and irradiated at the University of Missouri Research Reactor (MURR). Arsenic species were extracted from infant rice cereal using a simple hot water extraction. Hydrogen peroxide was added to the extract to quantitatively convert the arsenitespecies into arsenate in order to measure total i-As. A similar procedure was used by Loorente-Mirandes et al. to measure arsenic species in rice and infant rice products [5]. We expected the infant rice cereal made from white rice flour would contain the least amount of total arsenic and i-As since both are known to accumulate in the rice bran; which is removed in the processing to produce white rice [13]. We also expected the organic brown rice to have the lowest levels of DMA since it would be grown without use of pesticides.
2. Experimental
2.1. Reagents and Standards
Optima grade ammonium hydroxide and trace metal grade 85% phosphoric acid were purchased from Fisher Scientific. Trace select 30% Fluka Hydrogen Peroxide was obtained from Sigma Aldrich. Methylarsonic acid (MMA) was purchased from WACO Pure Chemicals, dimethylarsinic acid (DMA) was purchased from Sigma Aldrich. Certified solutions of arsenate and arsenite were purchased from elemental scientific (ESI). Certified As standard solutions for ICPMS were purchased from High Purity Standards. Eluent 1 was prepared from 20 mM phosphoric acid adjusted to a pH of 9.6 using 22% ammonium hydroxide. Eluent 2 was prepared from 150 mM phosphoric acid adjusted to pH 8.6 with 22% ammonium hydroxide.
2.2. Rice Cereal
Samples were purchased from local (Columbia Mo) supermarkets and stored in their original packaging, at room temperature.
2.3. Analysis of Total As by INAA
Samples of rice cereal from each package were prepared in triplicate for INAA of total arsenic. The INAA procedure has been described previously [14]. For total arsenic by neutron activation analysis 50 mg of each sample was weighed into a polyethylene vial. Standards that contained 100 ng of As were prepared from a certified ICP standard solution. Samples were irradiated for 30 minutes in the row 2 pneumatic tube position at the University of Missouri Research Reactor at a flux of 6.5 E + 13 n/cm2/s. Following irradiation the samples were allowed to decay for 24 hours to reduce the background from decay of 24Na. The 559.1 keV gamma ray from beta decay of 76As was measured using a high purity germanium gamma ray spectrometer.
2.4. Rice Cereal Extraction Procedure
Arsenic species were extracted from infant rice cereal using a hot water extraction procedure. 1 gram of infant rice cereal was transferred gravimetrically into pre-cleaned 15 mL polypropylene tube. We added 10 mL of 18.2 MΩ de-ionized water gravimetrically to each tube and heated the samples to 98˚C using a hot-block (Environmental Express) for 3 hours and cooled to room temperature. Once cool, each sample was centrifuged for one hour at 4400 RPM. A 0.5 mL aliquot of each sample was transferred to a Millipore 10 kilo Dalton centrifuge filter (0.5 mL) and centrifuged for 1 hour at 4400 RPM. The resultant filtered extract was diluted 1:25 with eluent 1 and 100 µL of 30% hydrogen peroxide to oxidize arsenite to arsenate. The sample solutions were stored in a refrigerator until the time of analysis.
2.5. Analysis of Rice Cereal Extracts for Total As and Arsenic Species
Total arsenic in rice cereal extracts was measured using a Nexion Quadrapole ICP-MS (Perkin Elmer). A four point matrix matched calibration curve was constructed in eluent 1 for analysis of total As in the diluted extract. To eliminate interference at m/z 76 from 40Ar36Cl we used oxygen gas in the ICP-MS Dynamic Reaction Cell (DRC) to measure 76As16O at m/z 91. The instrument parameters are reported in Table 1.
|
3. Results and Discussion
Six different brands of infant rice cereal and NIST SRM 1568 rice flour were analyzed for total arsenic by instrumental neutron activation analysis.
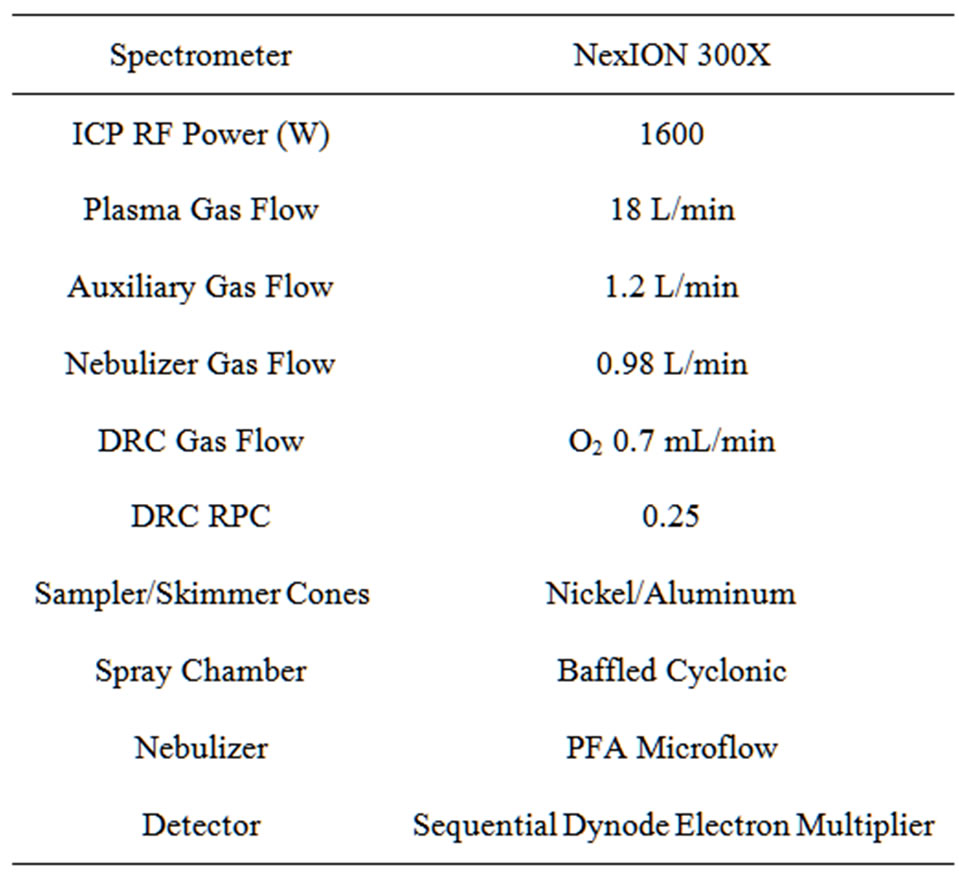
Table 1. Operating conditions for ICP-MS.
The total arsenic concentration in infant rice cereal ranged from 110 ng/g up to 421 ng/g. The arsenic concentration measured in NIST SRM 1568 rice flour was 410 ± 5 ng/g and the certified value is 410 ± 50 ng/g.
Total arsenic was measured in the rice extracts using ICP-MS. A matrix matched calibration curve was constructed using extract solutions spiked with a certified arsenic calibration standard. The R2 value of the calibration curve was 0.999. The extraction recovery, determined using the ratio of the total extracted arsenic and the total arsenic measured in the rice cereal, ranged from 78% up to 108%. The data is reported in Table 2. This measure of the extraction recovery does not account for the variability of arsenic in the rice cereal since the total arsenic and extracted arsenic were not measured in the same sample. The extracted arsenic was analyzed for total i-As, DMA and MMA using anion exchange liquid chromatography coupled with ICP-MS detection. The arsenite was quantitatively oxidized to arsenate by addition of 30% hydrogen peroxide to the extract samples. The oxidation step was tested by adding hydrogen peroxide to a sample spiked with arsenite, DMA and MMA.
The spike recovery of DMA and MMA in the spiked sample was 97% and 99%, respectively, indicating that these species were not altered by addition of hydrogen peroxide. The recovery of arsenite, converted to arsenate, in the sample was 107% indicating quantitative oxidation of arsenite. Calibration curves for arsenic speciation analysis were constructed using matrix matched extract solutions spiked with each arsenic species. The calculated R2 values of the DMA, MMA and As(V) calibration curves was greater than 0.999. The data is presented in Table 3. Equation (1) gives the instrumental limit of detection (IDL) for DMA, MMA and As(V) using the standard deviation of the y intercept (σy–intercept) of the regression analysis and the slope of the calibration curve (m) as [5].
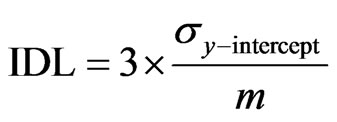 (1)
(1)
The chromatograph of a typical sample is shown in Figure 1. The rise in background starting at 220 seconds corresponds to the step gradient change to elute MMA and arsenate.
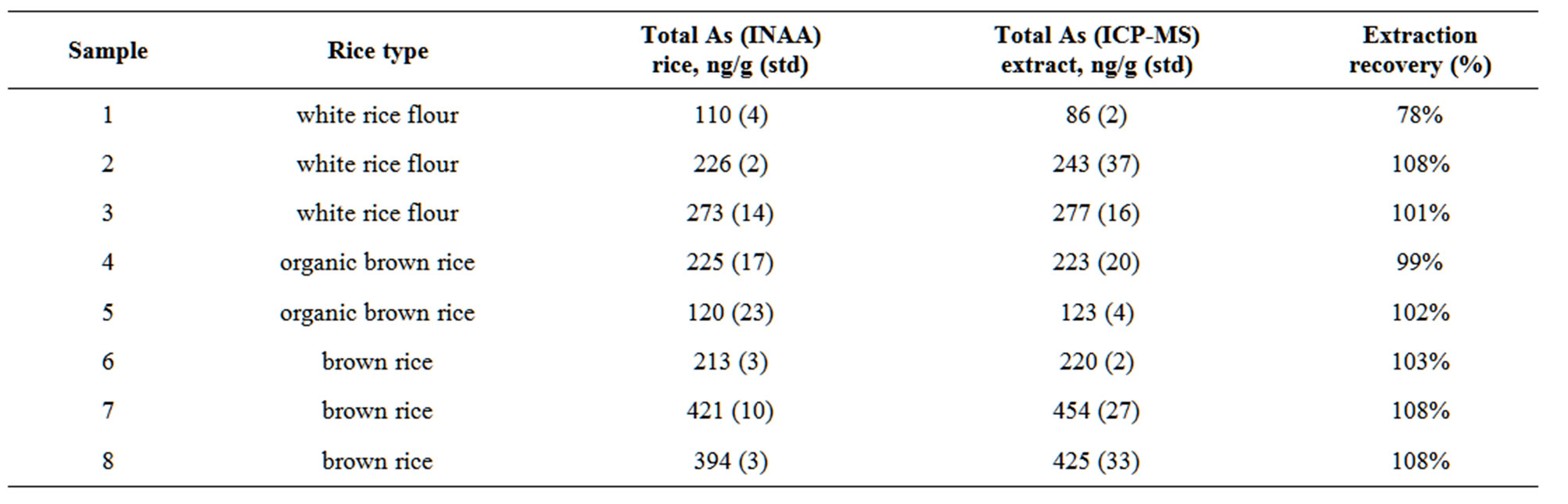
Table 2. Total arsenic measured in rice cereal, rice cereal extract and the measured extraction recovery.
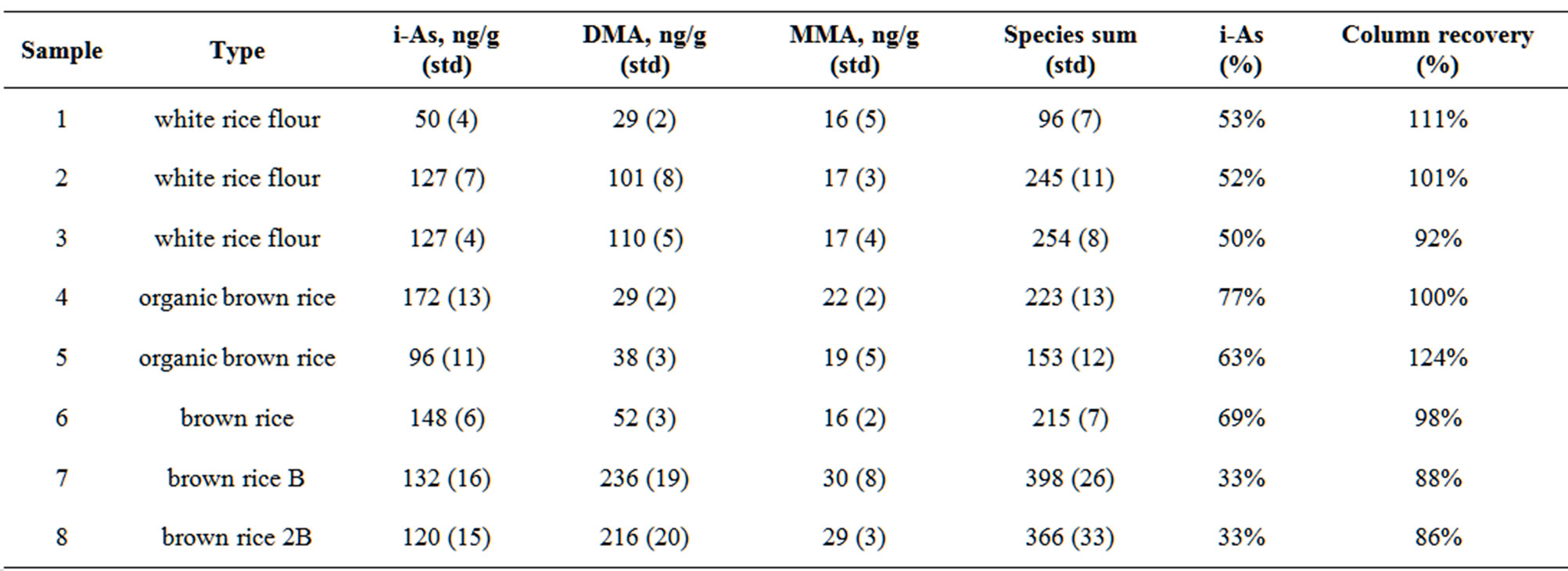
Table 3. Inorganic As (i-As), DMA, MMA measured in rice cereal extracts. The species sum is the sum of i-As, DMA and MMA.
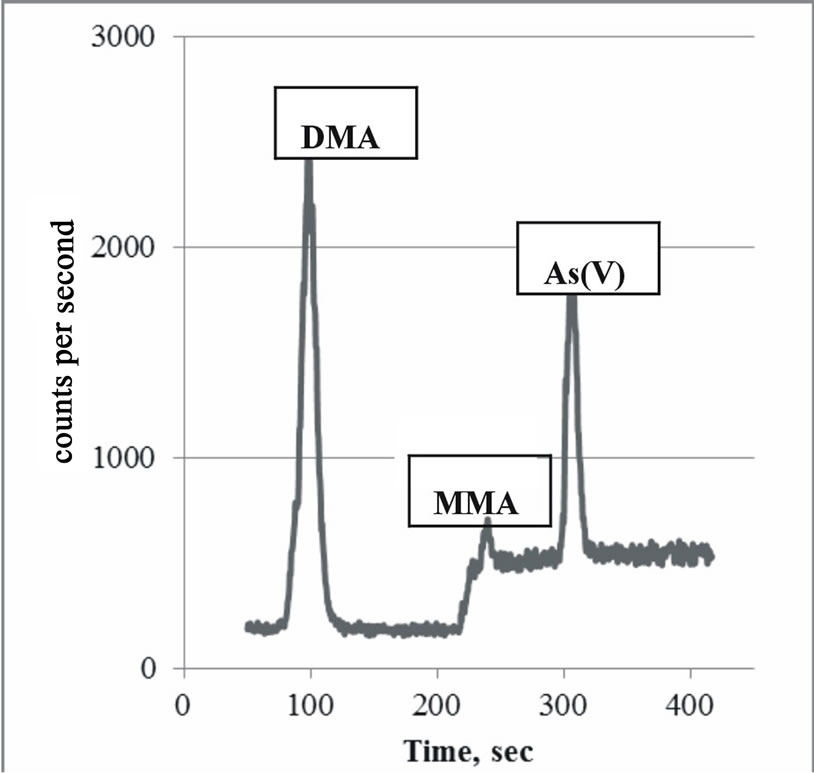
Figure 1. Chromatograph showing the arsenic species DMA, MMA and As(V). The As(III) species has been quantitatively oxidized to As(V) and does not appear on the chromatogram. The rise in background at 220 seconds corresponds with the step gradient change from eluent 1 to eluent 2.
The column recovery was determined by taking the ratio of the sum of the arsenic species and the total arsenic measured in the extract solution. The column recovery ranged from 86% to 124%. The arsenic species DMA, MMA and arsenate were measured above the IDL in all extract samples. A sample of NIST 1568 rice flour was analyzed using the extraction procedure. The total i-As was measured at 90 ± 5 ng/g, the DMA was measured at 253 ± 7 ng/g and MMA was measured at 39 ± 4 ng/g. The sum of the arsenic species in the rice flour is 381 ± 9 ng/g and is 93% of the total arsenic measured by INAA, which is in good agreement with the certified total As concentration.
In this small sample of infant rice cereals we found that i-As accounted for between 33% and 77% of the total arsenic. Carbonell-Barrachina et al. analyzed i-As in rice based infant foods from Spain, UK, China and the USA and reported that i-As accounted for at least 60% of the total arsenic in all products [15]. In their work a strong trend is observed between the level of i-As and total arsenic in infant rice products. This trend was not observed in the present study. We observed the highest total arsenic in the brown rice cereals samples 7 and 8. These two samples originate from different lots of the same brand of infant rice cereal. Sample 6 which is also a brown rice cereal contained 50% of the total arsenic and 25% of the DMA measured in samples 7 and 8 but had equivalent levels of i-As. As expected the organic brown rice cereals, samples 4 and 5, contained the least amount of DMA but surprisingly they had the highest percentage of i-As. The observed differences between our results in this limited study and those published by CarbonellBarrachine et al. may be explained by regional differences in arsenic concentration, soil conditions, genetics and farming methods in rice growing areas [15]. Because of the observed variability of total arsenic and arsenic species in infant rice cereals arsenic exposure would be best estimated using a biomonitoring approach that monitors arsenic in toenail or urine samples.
4. Conclusion
Conventional LC-ICP-MS can be used to measure arsenic species in extracts of infant rice cereal. The infant rice cereals measured in this study contained elevated levels of total arsenic similar to what has been previously reported. However, we measured a greater variability in the percentage of the more toxic i-As than previous studies. The observed variability in the concentration of arsenic species in the infant rice cereals would complicate a dietary survey approach to accessing human arsenic exposures.
5. Acknowledgements
The authors would like to thank Vickie Spate and Stacy Crane, the Trace Element Epidemiology Research Group and the University of Missouri Research Reactor for support in making the INAA measurements of total As.
REFERENCES
- A. A. Duker, E. J. Carranza and M. Hale, “Arsenic Geochemistry and Health,” Environment International, Vol. 31, No. 5, 2005, pp. 631-641. doi:10.1016/j.envint. 2004.10.020
- J. C. States, S. Srivastava, Y. Chen and A. Barchowsky, “Arsenic and Cardiovascular Disease,” Toxicological Science, Vol. 107, No. 2, 2009, pp. 312-323. doi:10.1093/toxsci/kfn236
- H. Y. Chiou, Y. M. Hsueh, K. F. Liaw, S. F. Horng, M. H. Chiang, Y. S. Pu, J. S. N. Lin, C. H. Huang and C. J. Chen, “Incidence of Internal Cancers and Ingested Inorganic Arsenic: A Seven-Year Follow-Up Study in Taiwan,” The Journal of Cancer Research, Vol. 55, No. 6, 1995, pp. 1296-1300.
- D. Gilbert-Diamond, K. L. Cottingham, J. F. Gruber, T. Punshon, V. Sayarath, A. J. Gandolfi, E. R. Baker, B. P. Jackson, C. L. Folt and M. R. Karagas, “Rice Consumption Contributes to Arsenic Exposure in US Women,” Proceedings of the National Academic Science of the United States of America, Vol. 108, No. 51, 2011, pp. 20656-20660. doi:10.1073/pnas.1109127108
- T. Llorente-Mirandes, J. Calderón, J. F. López-Sánchez, F. Centrich and R. Rubio, “A Fully Validated Method for the Determination of Arsenic Species in Rice and Infant Cereal Products,” Pure and Applied Chemistry, Vol. 84, No. 2, 2012, pp. 225-238. doi:10.1351/PAC-CON-11-09-30
- B. P. Jackson, V. F. Taylor, T. Punshon and K. L. Cottingham, “Arsenic Concentration and Speciation in Infant Formulas and First Foods,” Pure and Applied Chemistry, Vol. 84, No. 2, 2012, pp. 215-223.
- B. P. Jackson, V. F. Taylor, M. R. Karagas, T. Punshon and K. L. Cottingham, “Arsenic, Organic Foods and Brown Rice Syrup,” Environmental Health Perspect, Vol. 120, No. 5, 2012, pp. 623-626.
- G. X. Sun, P. N. Williams, Y. G. Zhu, C. Deacon, A. M. Carey, A. Raab, J. Feldmann and A. A. Meharg, “Survey of Arsenic and Its Speciation in Rice Products Such as Breakfast Cereals, Rice Crackers and Japanese Rice Condiments,” Environment International, Vol. 35, No. 3, 2009, pp. 473-475. doi:10.1016/j.envint. 2008.07.020
- Y. Takahashi, R. Minamikawa, K. H. Hattori, K. Kurishima, N. Kihou and K. Yuita, “Arsenic Behavior in Paddy Fields during the Cycle of Flooded and Non-Flooded Periods,” Environmental Science and Technology, Vol. 38, No. 4, 2004, pp. 1038-1044. doi:10.1021/es034383n
- G. J. Norton, S. R. M. Pinson, J. Alexander, et al., “Variation in Grain Arsenic Assessed in a Diverse Panel of Rice (Oryza Sativa) Grown in Multiple Sites,” New Phytologist, Vol. 193, No. 3, 2012, pp. 650-664. doi:10.1111/j.1469-8137.2011.03983.x
- S. Quazi, D. Sarkar and R. Datta, “Changes in Arsenic Fractionation, Bioaccessibility and Speciation in OrganoArsenical Pesticide Amended Soils as a Function of Soil Aging,” Chemosphere, Vol. 84, No 11, 2011, pp. 1563- 1571.
- A. A. Meharg, E. Lombi, P. N. Williams, et al., “Speciation and Localization of Arsenic in White and Brown Rice Grains,” Environmental Science and Technology, Vol. 42, No. 4, 2008, pp. 1051-1057. doi:10.1021/es702212p
- A. M. Carey, E. Lombi, E. Donner, et al., “A Review of Recent Developments in the Speciation and Location of Arsenic and Selenium in Rice Grain,” Analytical and Bioanalytical Chemistry, Vol. 402, No. 10, 2012, pp. 3275- 3286. doi:10.1007/s00216-011-5579-x
- J. D. Brockman and L. Schell, “A Radiochemical Method for Neutron Activation Analysis of Arsenic in Biological Samples and Its Potential Use in Epidemiology Studies,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 291, No. 2, 2012, pp. 473-478. doi:10.1007/s10967-011-1196-6
- A. A. Carbonell-Barrachina, X. Wu, A. Ramírez-Gandolfo, et al., “Inorganic Arsenic Contents in Rice-Based Infant Foods from Spain, UK, China and USA,” Environmental Pollution, Vol. 163, 2012, pp. 77-83. doi:10.1016/j.envpol.2011.12.036

