World Journal of Cardiovascular Surgery
Vol.2 No.4(2012), Article ID:25675,4 pages DOI:10.4236/wjcs.2012.24015
End Organ Recovery and Survival with the QuadroxD Oxygenator in Adults on Extracorporeal Membrane Oxygenation
1Division of Cardiothoracic Surgery, Department of Surgery, Thomas Jefferson University, Philadelphia, USA
2Division of Pulmonary Medicine and Critical Care, Department of Medicine, Thomas Jefferson University, Philadelphia, USA
3Division of Cardiology, Department of Medicine, Thomas Jefferson University, Philadelphia, USA
Email: *Nicholas.Cavarocchi@jefferson.edu
Received October 9, 2012; revised November 15, 2012; accepted November 23, 2012
Keywords: Extracorporeal Membrane Oxygenation; Adults; End-Organ Recovery; Mechanical Circulatory Support
ABSTRACT
Introduction: Extracorporeal Membrane Oxygenation (ECMO) is used in selected patient with cardiogenic and/or respiratory shock. We report our experience with standardized management protocols and the application of the QuadroxD oxygenator with a centrifugal pump to maximize end-organ recovery and improve survival. Methods: This is an Internal Review Board (IRB) approved, single institution retrospective study of end-organ recovery and survival in patients who required ECMO for cardiogenic and/or respiratory shock between July 2010 and June 2011. Results: Sixteen patients (median age: 46 years) were initiated on either Veno-Arterial (VA) or Veno-Venous (VV) ECMO. Cardiogenic shock, acute respiratory distress syndrome (ARDS) and a combined respiratory and cardiogenic compromise were the primary indications for ECMO in 8 (50%), 5 (31%) and 3 (19%) patients respectively. The median time on ECMO was 8 days (range: 4 - 26 days). Twelve patients (75%) were successfully weaned off ECMO, of which four (25%) were bridged to a ventricular assist device (VAD) and eight (50%) were weaned to recovery. All eight patients (100%) that were weaned to recovery and two patients (50%) that were bridged to a VAD were successfully discharged from the hospital, resulting in a discharge rate of 63%. There was an improvement in prevs. post-ECMO AST (449 IU/L vs. 63 IU/L, p < 0.05) in 5 patients (31%) with liver injury; serum lactate (9.1 mmol/L vs. 1.9 mmol/L, p < 0.05) in 8 patients (50%); and PaO2/FiO2 ratio (87 to 161, p = 0.01) in 10 patients (62%) with ARDS. Patients with evidence of pulmonary edema (n = 8, 50%) and ARDS (n = 8, 50%) on chest X-ray showed radiographic evidence of complete resolution. Renal function was preserved in 15 patients (94%). Conclusion: ECMO using the QuadroxD oxygenator and a centrifugal pump, coupled with standardized management protocols is beneficial in carefully selected patients. Improvement or maintenance of end-organ function is associated with successful bridge to device therapy and/or increased survival.
1. Introduction
Extracorporeal Membrane Oxygenation (ECMO), a therapy first described in 1972 by Hill et al. [1], is a wellestablished form of temporary mechanical cardiopulmonary support for patients in severe life threatening cardiac and/or respiratory shock [2,3]. The positive outcomes associated with this intervention were historically low however major developments in mechanical circulatory support technology have been made over the past decade [4]. Subsequently, there has been an increase in its use and an improved survival-to-discharge rate [5-8].
Veno-Arterial (VA) and Veno-Venous (VV) ECMO are two basic types of ECMO. The former provides support for both the heart and lungs, whereas the latter provides support only for the lungs. The primary components of the ECMO circuit are the pump and the oxygenator/heat exchanger that assume the role of the heart and the lungs respectively [4].
A major contribution to ECMO technology over the past decade is the development of the polymethylpentene (PMP) membrane oxygenator. It combines the best aspects of its predecessors (the silicone membrane and the hollow fiber polypropylene membrane oxygenators) and when compared to them, has been reported to improve gas exchange and efficiency, increase longevity and results in less blood trauma [4,7-10]. Another critical development in ECMO therapy is the evolution of the centrifugal pump, driven by a magnetic impeller. This centrifugal pump is gaining popularity worldwide and has been reported to result in reduced hemolysis, blood transfusions, and circuit complications [11,12].
As technology develops, so does our understanding of the physiology of patients that are placed on ECMO. A concept that is often mentioned in the literature, but rarely scientifically analyzed and reported is that of “end-organ recovery”. An understanding of this concept is vital for successful extracorporeal therapy. Patients who present with multi-organ dysfunction secondary to failure of the cardiopulmonary systems require not only cardiopulmonary support through ECMO but also require a resolution of the end-organ injury. The restoration of organ function is the key to successful wean to recovery, bridge-to-decision (heart transplantation) or bridge-to-bridge (ventricular assist device [VAD] implantation) therapy. We sought to examine the evidence for improvement in endorgan function on patients on ECMO.
2. Methods
2.1. Patients
Data on all patients who received ECMO from July 2010 was collected and recorded in an IRB-approved ECMO database. Patients were mainly in-hospital referrals/consultations; however, in some instances, patients were cannulated and placed on ECMO at an outside hospital and were transported to our unit for further management. All patients placed on ECMO with a PMP hollow fiber membrane oxygenator and a centrifugal pump between June 2010 and July 2011 were included in this study. Patients receiving ECMO using a hollow fiber polypropylene membrane oxygenator were excluded from this study. The patients who were placed on ECMO as a form of salvage therapy (duration of ECMO <48 hours) and patients who required cardiopulmonary resuscitation >1 hour prior to initiation of ECMO were also excluded from this study.
2.2. Indications and Contraindications
Indications for VA-ECMO support at our institution are age <75 years, post-cardiotomy cardiopulmonary shock (failure to wean off cardiopulmonary bypass), cardiogenic shock defined as cardiac index <2 L/min, mean arterial pressure <60 mmHg, and a lactic acidosis refractory to >3 inotropic agents or other conventional circulatory support (i.e. intra-aortic balloon pump and/or Impella temporary cardiac assist device), acute decompensation of congestive heart failure refractory to cardiac support, as an adjunct to cardiopulmonary resuscitation, and in patients who require temporary support/clinical status improvement prior to initiation of a ventricular assist device. Indications for VV-ECMO are age <75 years, respiratory failure, hypoxia and/or acidosis despite optimal mechanical ventilator support. Contraindications for placement on VA-ECMO are age >75 years, sepsis, severe aortic regurgitation, terminal malignancy, irreversible neurologic injury, untreated aortic dissection, and severe atherosclerosis. Contraindications for VV-ECMO were similar except for severe aortic regurgitation and irreversible respiratory failure.
2.3. ECMO Cannulation and Circuitry
In patients on VA-ECMO, femoral cannulation was preferred, whilst those who received VV-ECMO were cannulated via the neck into the right internal jugular vein using the Avalon bicaval dual-lumen cannula (Avalon Laboratories, LLC, Rancho Dominguez, CA). All patients were cannulated peripherally via percutaneous insertion using a modified Seldinger technique. The heparin coated Heart and Lung cannula (HLS cannula, Maquet Cardiovascular LLC, San Jose, CA) was used for percutaneous cannulation and it was connected to the closed crystalloid primed circuit (~300 ml). The ECMO circuit consisted of the QuadroxD PMP membrane oxygenator (Maquet Cardiovascular LLC, San Jose, CA) and the Rota flow centrifugal pump (Maquet Cardiovascular LLC, San Jose, CA) from September 2010 to July 2011. Prior to that, the Medtronic Bio-Medicus centrifugal pump (Medtronic, Minneapolis, MN) was used instead of the Rotaflow pump in two patients.
2.4. Tissue Perfusion Monitoring
In patients on VA-ECMO, a distal perfusion catheter was placed prophylactically in all patients to avoid distal extremity ischemia. All patients were externally monitored using near-infrared spectroscopy (NIRS, INVOSTM, Somanetics, Troy, MI). NIRS sensor pads were placed bilaterally on patients’ foreheads (cerebral oximetry) and on their distal extremities (femoral/extremity oximetry) to monitor adequacy of tissue perfusion and to detect developing ischemic changes.
2.5. ECMO Heparin Protocol
At our institution, regardless of whether a VA or a VV circuit was used, a bolus of 5000 - 7500 units of heparin was given just after placement of the arterial and/or venous guide wires. Heparin was then held for 24 hours after ECMO was initiated unless clots were observed in the circuit. After 24 hours, a heparin drip is started with a low goal therapeutic PTT range of 45 - 55. The PTT goal was lowered to a 40 - 45 range if there was a high risk of bleeding (i.e. due to an extensive surgical procedure or ongoing gastrointestinal/retroperitoneal bleed). The presence of small thrombi or fibrin is common in the ECMO circuit and their presence in the circuitry and the oxygenators must be continuously monitored. As long as oxygenation and flow are adequate, exchange of the circuitry is not necessary. Rapid formation of thrombus or a rapid decline in oxygenation should prompt a change of the circuit at the bedside. No additional heparin was used if a circuit change was necessary at out institution. If procedures were performed while patients were on ECMO, anticoagulation was held for 6 hours preand post ECMO for minor procedures. For major procedures, it was held for 6 hours pre-procedure and 24 hours post-procedure.
2.6. End-Organ Data Analysis
Data regarding hepatic, renal, metabolic and pulmonary function on patients included in this study were collected. Liver function was evaluated using serum aspartate transaminase (AST) and alanine transaminase (ALT), and bilirubin; renal function with serum creatinine; metabolic parameters with serum lactic acid; and pulmonary function by PaO2/FiO2 ratios and imaging via serial chest X-rays. Pre-ECMO data was collected up to 12 hours prior to the initiation of ECMO. In cases where ECMO therapy was required emergently, data was collected immediately after ECMO initiation. Post-ECMO data was collected within 24 hours after the patient was weaned off ECMO. The pre-and post-ECMO data was compared. Patients were stratified in regards to presence of a particular organ dysfunction and comparisons of organ function indicators were made preand post-ECMO therapy.
2.7. Definition of Survival and Complications
Patients were recorded as either being successfully weaned off ECMO or died on ECMO. In the group that was successfully weaned, we further stratified them into patient who were weaned to a device (e.g. a ventricular assist device) or patients who were weaned to medical management. As the ability to be weaned off ECMO did not automatically confer survival to hospital discharge, we also recorded survival-to-discharge data. Major surgical site bleeding was defined as bleeding that required surgical exploration for resolution. Neurologic injury was defined as the occurrence of a hemorrhagic or ischemic infarct, and/or diffuse anoxic brain injury that was confirmed by neuroimaging such as MRI, CT scan, nuclear brain perfusion scan, or EEG), or by serial neurologic exams conducted by two independent neurologists 24 hours apart.
2.8. Statistical Analysis
Demographic and clinical characteristics were demonstrated using medians, ranges for continuous variables, and percentages for categorical variables. Preand postECMO data were compared using paired Wilcoxon analyses. P value less than 0.05 was considered to be significant.
3. Results
3.1. Demographics
From July 2010 to June 2011, a total of sixteen patients met the inclusion criteria. Their demographics are shown in Table 1. Twelve (75%) patients were supported with VA-ECMO and the remaining four (25%) with VVECMO. The median duration of ECMO therapy was 8 days (range: 4 - 26 days). The indications for placement on ECMO are as follows; primary cardiogenic shock/ cardiac arrest (8 patients, 50%), primary respiratory failure/ARDS (5 patients, 31%) and combined cardiac and respiratory failure (3 patients, 19%). One patient with ARDS was supported using VAinstead of VV-ECMO due to an inability to gain access to the necessary blood vessels and maintain good flow.
3.2. Patient Survival and Outcomes
Figures 1 and 2 describe patient survival and outcomes on VAand VV-ECMO respectively. Twelve (75%) of the 16 patients placed on ECMO were successfully weaned
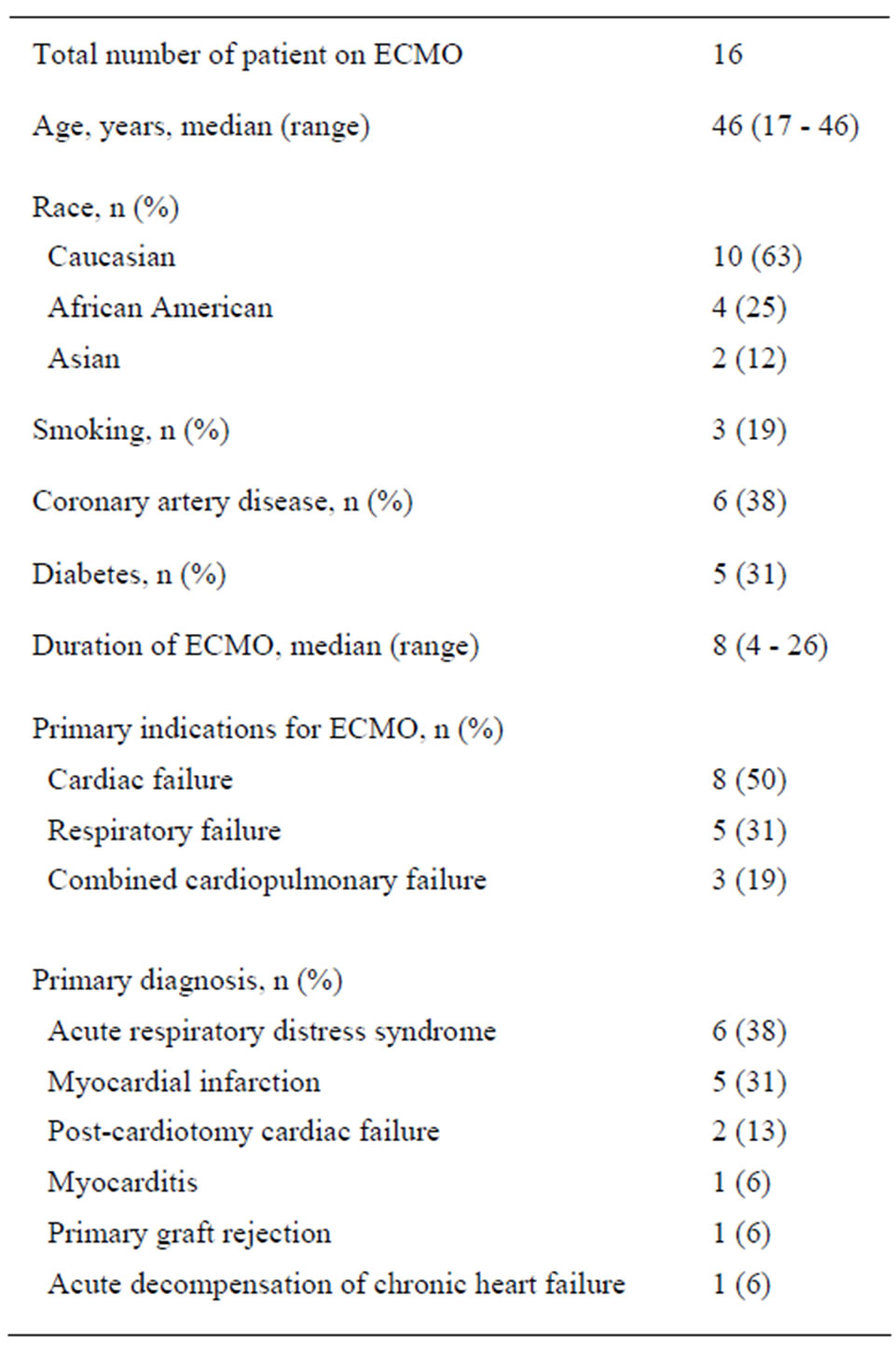
Table 1. Patient demographics.
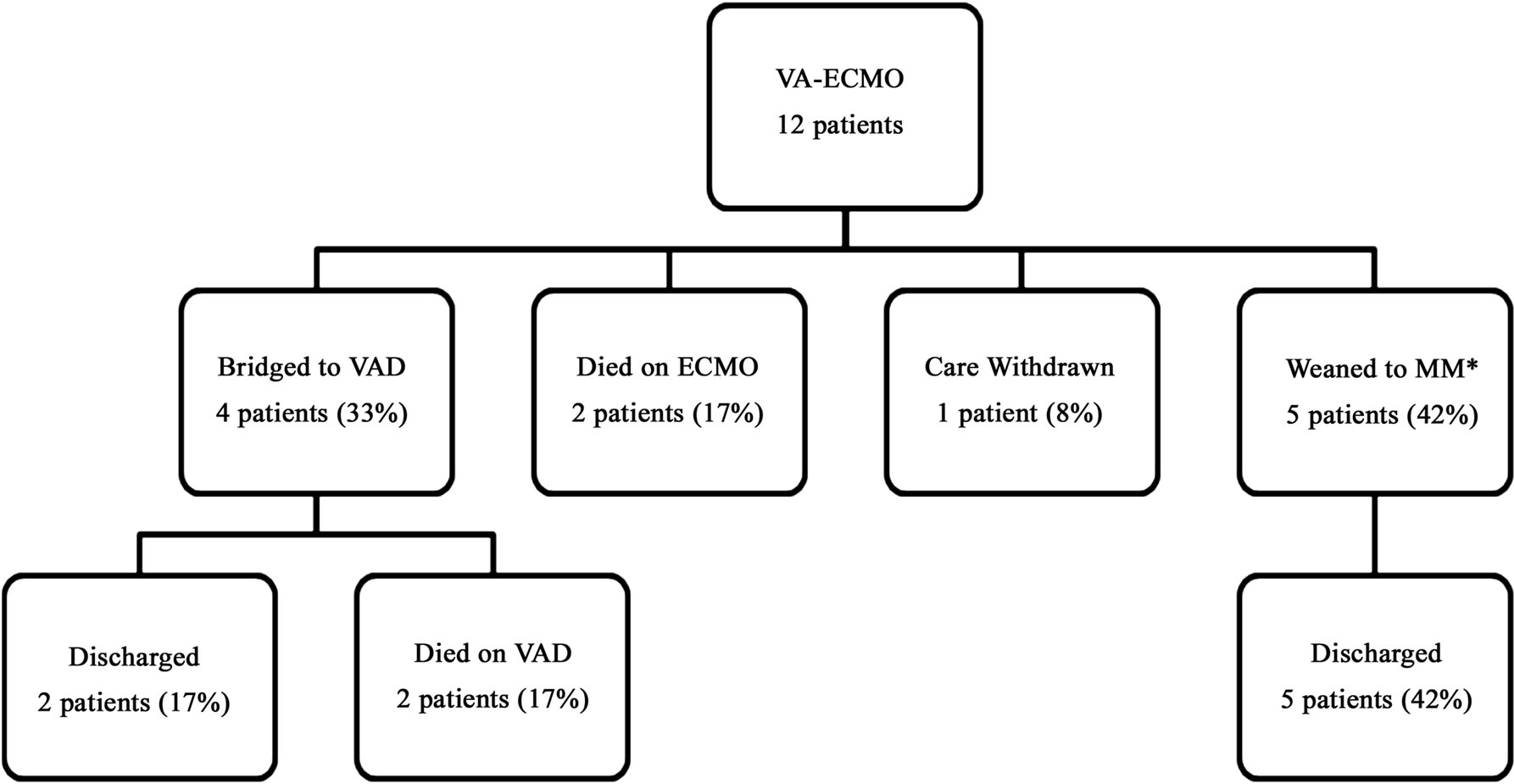
Figure 1. Survival and outcomes of patients who were placed on veno-arterial ECMO (VA-ECMO). *MM: medical management; VAD: ventricular assist device.
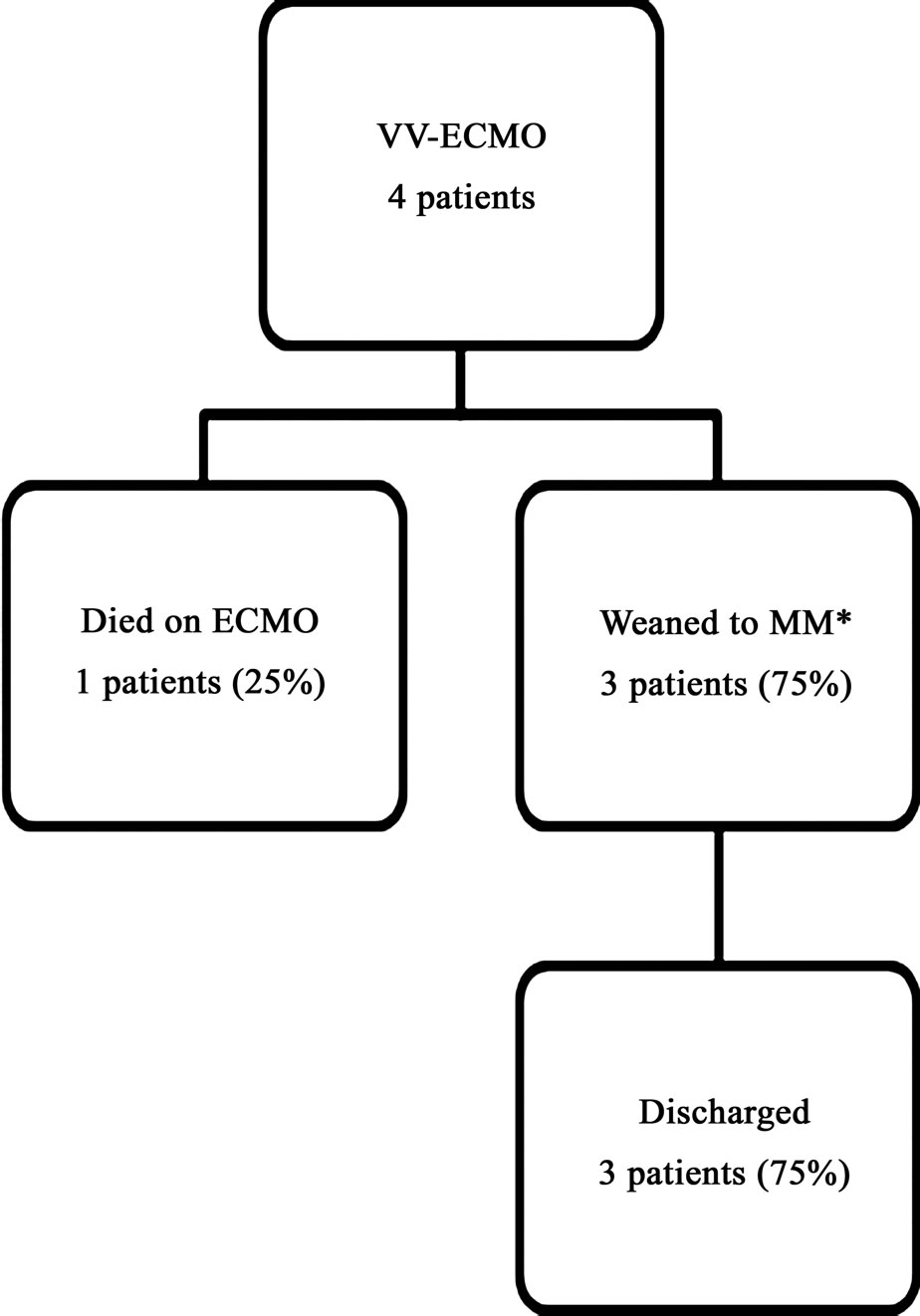
Figure 2. Survival and outcomes of patients who were placed on veno-venous ECMO (VV-ECMO). *MM: medical management.
off extracorporeal therapy. Of these 12, 10 were eventually discharged from the hospital resulting in an overall discharge rate of 63%. When stratifying based on type of ECMO received, of the twelve patients that were placed on VA-ECMO support, nine (9/12, 75%) were successfully weaned off ECMO; four (4/12, 33%) to a VAD and the remaining five (5/12, 42%) to recovery. Of the four patients that were placed on VV-ECMO, three (75%) were successfully weaned off extracorporeal therapy. There were a total of 6 in-hospital patient deaths; 3 of these occurred while patients were on ECMO. The causes of death were major stroke (2 patients), diffuse anoxic brain injury (2 patients), care withdrawal due to biopsy proven active end-stage leukemia (1 patient) and non-candidacy to receive either a VAD or transplant (1 patient).
3.3. End-Organ Recovery Data
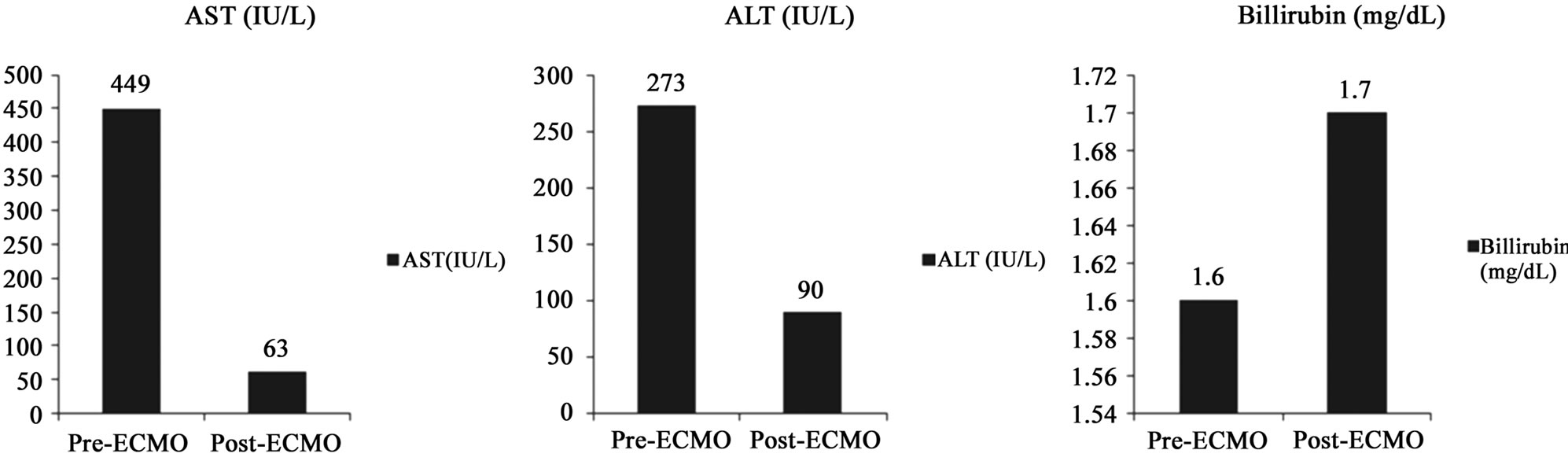
Five patients had an abnormal AST, ALT and bilirubin levels prior to initiation of ECMO. There was an improvement in median AST for these patients (prevs. post-ECMO median AST: 449 IU/L vs. 63 IU/L, p < 0.05) (Figure 3(a)). Similarly, these five patients had elevated pre-ECMO ALT levels that improved from 273 IU/L to 90 IU/L after therapy (Figure 3(b)), although this failed to achieve statistical significance (p = 0.07). There was a minor deterioration in prevs. post-ECMO bilirubin from 1.6 to 1.7 (Figure 3(c)). However, this too failed to achieve statistical significance (p = 0.7). In the remaining 11 patients without evidence of liver injury prior to ECMO, there was only one patient who had a deterioration (AST: 58 IU/L to 92 IU/L, ALT: 44 IU/L to 162 IU/L and bilirubin: 0.8 mg/dL to 1.1 mg/dL) while the rest maintained normal liver function.
Ten patients (62%) had abnormal PaO2/FiO2 ratios that were below 200. There was an improvement in prevs.
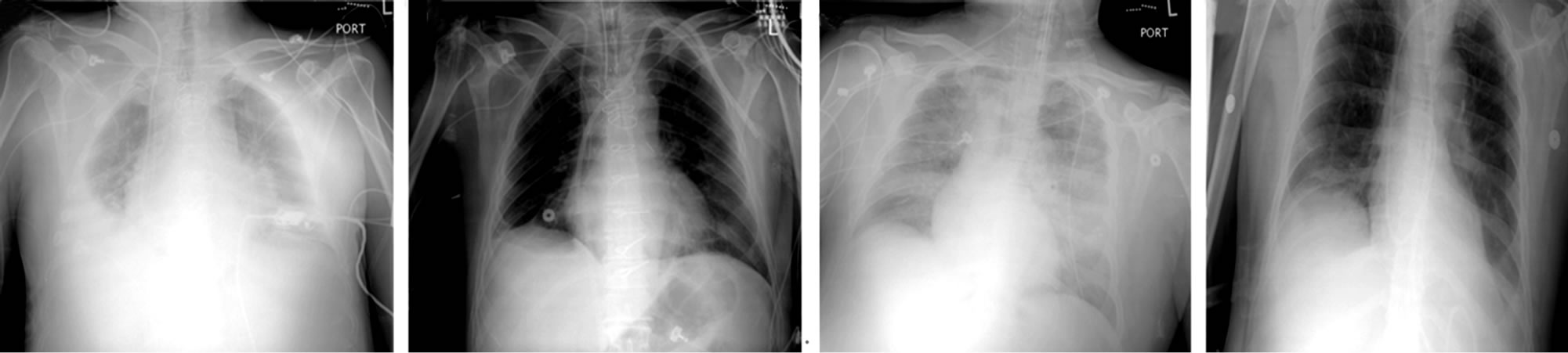
post-ECMO PaO2/FiO2 ratios in all these patients from 87 to 161 (p = 0.01) (Figure 3(d)). Eight patients (50%) had varying degrees of pulmonary edema as demonstrated by a pre-ECMO chest x-ray. In all these patients, there was a resolution of chest X-ray abnormalities upon discontinuation of ECMO. ARDS was also diagnosed in eight patients (50%), six (75%) showed a resolution of the bilateral infiltrates on chest x-ray after ECMO therapy (Figure 4).
Metabolic derangement as reflected by abnormally elevated serum lactate levels were seen in 8 patients (50%).
In these patients, the median pre-ECMO serum lactate was 9.1 mmol/L, which improved to 1.9 mmol/L postECMO (p < 0.05) (Figure 3(e)). The remaining eight patients (50%) maintained normal metabolic function and did not have an increase in lactate levels.
Only one patient had a significantly elevated creatinine level at 3.6 mg/dL prior to the initiation of ECMO support, this improved to 0.8 mg/dL post-ECMO. There was no kidney injury in the remaining fifteen patients prior to starting ECMO.
 (a)
(a)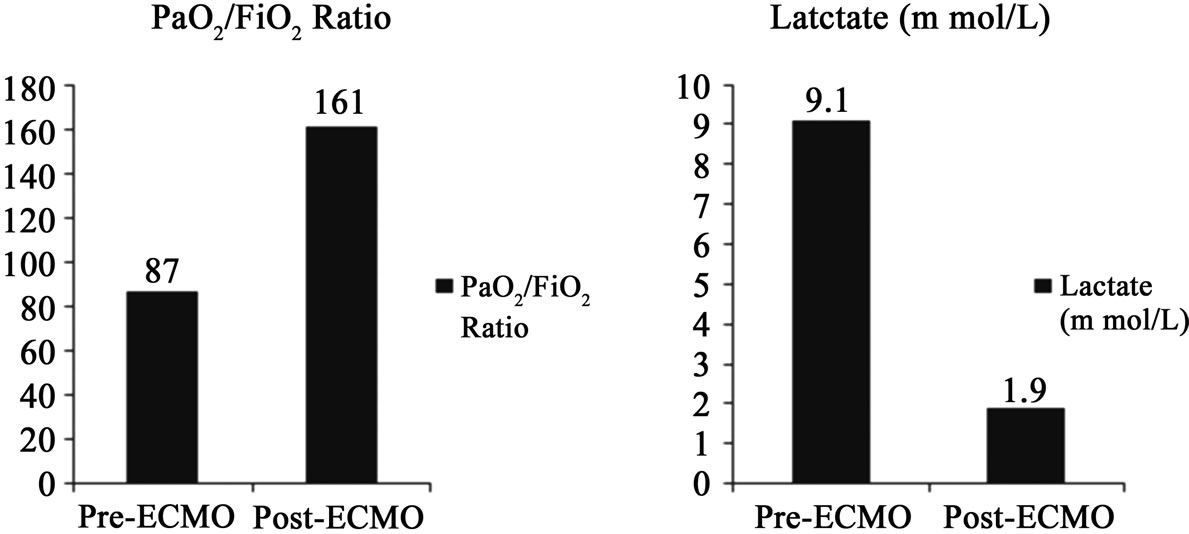 (b)
(b)
Figure 3. Comparisons between preand post-ECMO values. Improvements in median AST (a) (p < 0.05) and ALT (b) (p = 0.7). Mild elevation in serum bilirubin (c) (p = 0.7). Improvement in PaO2/FiO2 ratios (d) (p = 0.01). Improvement in serum lactate levels (e) (p < 0.05).
 (a) (b) (c) (d)
(a) (b) (c) (d)
Figure 4. Chest X-ray showing pulmonary edema (a) with resolution after ECMO (b) Similarly, pulmonary infiltrates (c) in the setting of ARDS also resolved (d) after ECMO.
3.4. Complications
Complications occurred in nine patients (56%) while on ECMO. These complications are illustrated in Table 2. The most common complication observed was major surgical site bleeding (31%), bacteremia (19%) and ireversible neurologic injury (13%). Of note, no patients developed an ECMO-induced systemic coagulopathy (e.g. disseminated intravascular coagulation). Other complications include sepsis, acute liver injury and clostridium difficile colitis. There were three instances on three different patients (19%) that required circuitry change. None of these changes were due to circuitry malfunction or clinical evidence of hemolysis; instead these were all due to the formation of clots in the circuitry while off heparin.
4. Discussion
ECMO was first described being used in a patient with ARDS in 1971 [1]. Since then, its use is increasing in the adult population and the indications for initiation of ECMO therapy has grown to encompass cardiac failure as well [5,6]. Although this is true, much uncertainty still remains regarding ECMO technology. Different centers across the United States employ varying management strategies (i.e. anticoagulation protocols, monitoring systems and weaning protocols) and often place patients on this form of support for a variety of indications where ECMO may not truly benefit the patient.
The substantial complication risks that are reported in patients placed on ECMO also deter many from employing ECMO therapy. Bleeding, irreversible neurologic injury, and infection are the most common complications observed in this critically ill population [4-6,13,14], all of which may result in death especially in such vulnerable patients. Despite all this, clinicians still press on in their research for better and more efficient ECMO circuit technology, improved tissue perfusion monitoring, and a better understanding of the indications for and management of ECMO.
A definitive advancement in extracorporeal technology that has occurred over the past decade is the development
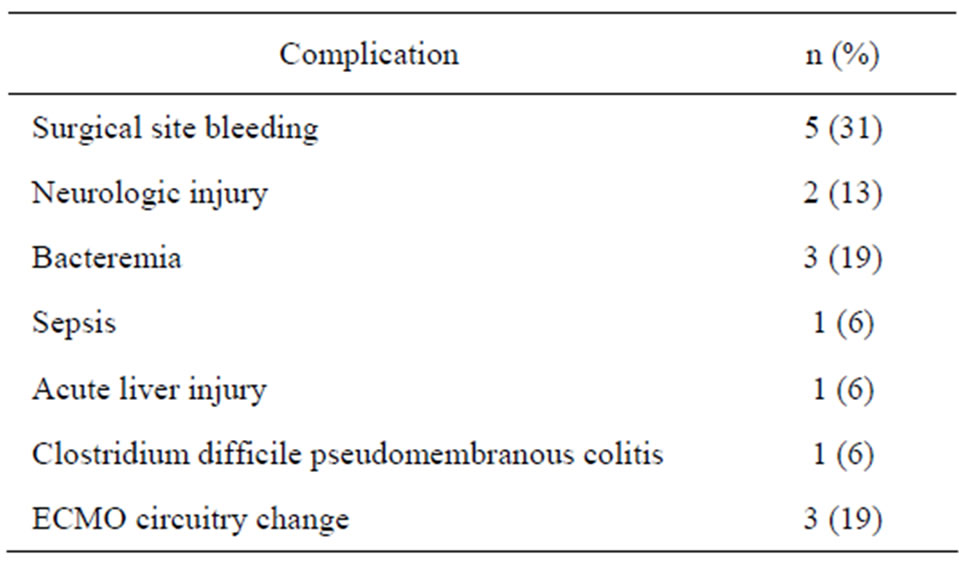
Table 2. Complications.
of more efficient and less traumatic oxygenators and blood pumps. The centrifugal pumps, particularly the newer second generation pumps (Rotaflow, Cobe Revolution) were reported to result in reduced transfusion requirements, hemolysis and complications when compared to the roller pumps [11,12]. Benefits of the QuadroxD PMP hollow fiber membrane oxygenator that have been reported are improved longevity, efficiency (gas exchange), hemolysis and platelet consumption [7-10]. One of the first few reports in the literature regarding the use of a PMP hollow fiber membrane oxygenator was in 2002 by Peek et al. They reported lower platelet transfusion rates and no oxygenator failure in six patients that were placed on ECMO for 151.7 ± 75.6 hours [8]. Toomasian et al. in an animal study in 2005 compared the PMP membrane oxygenator to its predecessor, the silicone membrane oxygenator and found that the PMP oxygenators demonstrated better oxygen and carbon dioxide exchange; lower pressure drops in across the PMP device and lower platelet consumption [9]. Lastly, Formica et al. in 2008 studied the use of the PMP membrane oxygenator with a centrifugal pump, in adults with cardiogenic shock. The authors reported a survival rate of 27.8%, a bleeding complication rate of 61.1% and noted that only one patient in his study required a circuit change. They further used serum lactate and cardiac enzymes as markers of end-organ injury and saw improvements in these markers after the intiation of ECMO [10].
We found that patients who received the PMP hollow fiber membrane oxygenator (QuadroxD) and a centrifugal pump as part of the ECMO circuit had low complication rates and acceptable overall outcomes. Prior to this change in circuitry at our institution, there was 100% mortality on ECMO couple with high incidences of neurologic and bleeding complications.
Major surgical site bleeding occurred in 31% of patients and was mainly attributed to the additional surgical procedures that were performed on patients while still on ECMO. The rates of surgical site bleeding is lower than that reported in the literature [4,10,14-16], however, it is difficult to compare them as there have been varying definitions of “a major bleeding complication” in regards to patients on ECMO. Sidebotham et al. in his review of ECMO reported a bleeding rate between 5.3% and 79% in existing literature [4]. This vast range illustrates that there is major variation between institutions when reporting or defining complications. At our institution, we believe that our success in terms of low major surgical bleeding complications rates is attributed to our established heparin protocol coupled with the lower hemolytic properties of our ECMO circuit.
Circuit complications necessitating an exchange of circuitry occurred in only 3 patients (19%) and did not result in any patient morbidity. These circuit changes were necessary due to the formation of blood clots in the oxygenator as a result of our holding anticoagulation prophylactically in anticipation of a surgical procedure being performed. In 2005, the Extracorporeal Life Support Organization (ELSO) registry reported a 27% oxygenator failure and a 36% pump malfunction in patients above the age of 16 years [17]. This was in contrast to our experience where there was no instance in all sixteen of out patients where either the pump and/or the oxygenator malfunctioned or failed. Formica et al. reported similar findings in their experience with the QuadroxD oxygenator and centrifugal pump where only one oxygenator change was required due to the presence of persistent sepsis [10].
We report a higher patient survival rate compared to existing literature [5,6,10,14,15], with an overall survival to discharge of 62%. We attributed these outcomes to advancements in ECMO circuitry, clinician experience and careful patient selection. More importantly, our data suggests that ECMO support does restore and objectively improve end-organ function in patients with end-organ injury. Patients that are placed on ECMO are often critically ill and a large majority of them have some form of ischemic injury to their major organs. If the precipitating pathology is reversible, these patients are supported until their heart and lungs are able to resume perfusion and oxygenation of end-organ tissues, and thus weaned from ECMO therapy. In the event of irreversibility, patients are stabilized and supported to improve their overall clinical status to increase the likelihood of successful bridge-to-bridge (ECMO-VAD) or bridge-to-transplant (ECMO-Heart Transplant) therapy [18-20].
Our data showed that all patients with liver injury recovered liver function, all patients with evidence of endorgan injury by metabolic parameters (metabolic injury) recovered metabolic injury, and all patients except for one patient who had acute renal failure prior to initiation of ECMO preserved renal function while being supported by ECMO. Additionally, all patients without end-organ injury pre-ECMO, with the exception of one patient that developed liver injury on ECMO, maintained normal endorgan function throughout their course on ECMO.
There are several limitations to this study; firstly, our sample size consisted of 16 patients and is relatively small. There was a lack of a comparison group thus making it difficult to definitively attribute the improvement in clinical outcomes and reduced complications in patients placed on the QuadroxD oxygenator and centrifugal pump. Lastly, there is a lack of hard definitions of complications in patients placed on ECMO.
Improvement of end-organ injury while on ECMO carries importance in two clinical scenarios. Firstly, patients who were in multi-organ failure who were not candidates for VAD implantation due to their poor clinical status stand to gain much by being supported on ECMO as outcomes for VAD implantation in patients with severe hemodynamic instability and multi-organ dysfunction is poor [21]. Therefore an improvement in organ function and overall predicted mortality in these patients will result in better outcomes after VAD placement. Secondly, the overall improvement in organ function in patients places in extracorporeal support with the intent on weaning off ECMO will increase the survival rates after ECMO wean. It is noteworthy that in our experience 100% of patients that were successfully weaned off ECMO survived to discharge.
5. Conclusion
In conclusion, ECMO is an important therapeutic measure for patients in severe cardiopulmonary failure. New technology and an increased institutional experience has vastly improved outcomes and reduced complications in patients requiring such support. It is our opinion that a combination of the QuadroxD PMP membrane oxygenator and a centrifugal pump improved clinical outcomes and decreased complication rates.
REFERENCES
- J. D. Hill, T. G. O’Brien, J. J. Murray, L. Dontigny, M. L. Bramson, J. J. Osborn and F. Gerbode, “Prolonged Extracorporeal Oxygenation for Acute Post-Traumatic Respiratory Failure (Shock-Lung Syndrome). Use of the Bramson Membrane Lung,” The New England Journal of Medicine, Vol. 286, No. 12, 1972, pp. 629-634. doi:10.1056/NEJM197203232861204
- D. Brodie and M. Bacchetta, “Extracorporeal Membrane Oxygenation for ARDS in Adults,” The New England Journal of Medicine, Vol. 365, No. 20, 2011, pp. 1905- 1914. doi:10.1056/NEJMct1103720
- D. Sidebotham, A. McGeorge, S. McGuinness, M. Edwards, T. Willcox and J. Beca, “Extracorporeal Membrane Oxygenation for Treating Severe Cardiac and Respiratory Disease in Adults: Part 1—Overview of Extracorporeal Membrane Oxygenation,” Journal of Cardiothoracic and Vascular Anesthesia, Vol. 23, No. 6, 2009, pp. 886-892. doi:10.1053/j.jvca.2009.08.006
- D. Sidebotham, A. McGeorge, S. McGuinness, M. Edwards, T. Willcox and J. Beca, “Extracorporeal Membrane Oxygenation for Treating Severe Cardiac and Respiratory Failure in Adults: Part 2—Technical Considerations. Journal of Cardiothoracic and Vascular Anesthesia, Vol. 24, No. 1, 2010, pp. 164-172. doi:10.1053/j.jvca.2009.08.002
- N. G. Smedira, N. Moazami, C. M. Golding and P. M. McCarthy, C. Apperson-Hansen, E. H. Blackstone, et al. “Clical Experience with 202 Adults Receiving Extracorporeal Membrane Oxygenation for Cardiac Failure: Survival at Five Years,” The Thoracic and Cardiovascular Surgeon, Vol. 122, No. 1, 2001, pp. 92-102. doi:10.1067/mtc.2001.114351
- F. Hei, S. Lou, J. Li, K. Yu, J. Liu, Z. Feng, J. Zhao, S. Hu, J. Xu, Q. Chang, Y. Liu, X. Wang, P. Liu and C. Long, “Five-Year Results of 121 Consecutive Patients Treated with Extracorporeal Membrane Oxygenation at Fu Wai Hospital,” Artifical Organs, Vol. 35, No. 6, 2011, pp. 572-578. doi:10.1111/j.1525-1594.2010.01151.x
- E. Khoshbin, N. Roberts, C. Harvey, D. Machin, H. Killer, G. J. Peek, A. W. Sosnowski and R. K. Firmin, “Polymethyl Pentene Oxygenators Have Improved Gas Exchange Capability and Reduced Transfusion Requirements in Adult Extracorporeal Membrane Oxygenation,” ASAIO Journal, Vol. 51, No. 3, 2005, pp. 281-287. doi:10.1097/01.MAT.0000159741.33681.F1
- G. J. Peek, H. M. Killer, R. Reeves, A. W. Sosnowski and R. K. Firmin, “Early Experience with a Polymethyl Pentene Oxygenator for Adult Extracorporeal Life Support,” ASAIO Journal, Vol. 48, No 5, 2002, pp. 421-480. doi:10.1097/00002480-200209000-00007
- J. M. Toomasian, R. J. Schreiner, D. E. Meyer, M. E. Schmidt, S. E. Hagan, G. W. Griffith, R. H. Bartlett and K. E. Cook, “A Polymethylpentene Fiber Gas Exchanger for Long-Term Extracorporeal Life Support,” ASAIO Journal, Vol. 51, 2005, pp. 390-397. doi:10.1097/01.mat.0000169111.66328.a8
- F. Formica, L. Avalli, A. Martino, E. Maggioni, M. Muratore, O. Ferro, A. Pesenti and G. Paolini, “Extracorporeal Membrane Oxygenation with a Poly-Methylpentene Oxygenator (QuadroxD). The Experience of a Single Italian Center in Adult Patients with Refractory Cardiogenic Shock,” ASAIO Journal, Vol. 54, No. 1, 2008, pp. 89-94. doi:10.1097/MAT.0b013e31815ff27e
- A. M. Horton and W. Butt, “Pump-Induced Haemolysis: Is the Constrained Vortex Pump Better or Worse Than the Roller Pump,” Perfusion, Vol. 8, No. 3, 1993, pp. 103- 108.
- D. S. Lawson, R. Ing, I. M. Cheifetz, R. Walczak, D. Craig, S. Schulman, F. Kern, I. R. Shearer, A. Lodge and J. Jaggers, “Hemolytic Characteristics of Three Commercially Available Centrifugal Blood Pumps,” Pediatric Critical Care Medicine, Vol. 6, No. 5, 2005, pp. 573-577. doi:10.1097/01.PCC.0000163282.63992.13
- F. J. Mateen, R. Muralidharan, R. T. Shinohara, J. E. Parisi, G. J. Schears and E. F. Wijdicks, “Neurological Injury in Adults Treated with Extracorporeal Membrane Oxygenation,” Archives of Neurology, Vol. 68, No. 12, 2011, pp. 1543-1549. doi:10.1001/archneurol.2011.209
- G. J. Magovern Jr. and K. A. Simpson, “Extracorporeal Membrane Oxygenation for Adult Cardiac Support: The Allegheny Experience,” The Annals of Thoracic Surgery, Vol. 68, No. 2, 1999, pp. 655-661. doi:10.1016/S0003-4975(99)00581-0
- C. A. Bermudez, R. V. Rocha, Y. Toyoda, D. Zaldonis, P. L. Sappington, S. Mulukutla, O. C. Marroquin, C. Toma, J. K. Bhama and R. L. Kormos, “Extracorporeal Membrane Oxygenation for Advanced Refractory Shock in Acute and Chronic Cardiomyopathy,” The Annals of Thoracic Surgery, Vol. 92, No. 6, 2011, pp. 2125-2131. doi:10.1016/j.athoracsur.2011.07.029
- M. R. Hemmila, S. A. Rowe, T. N. Boules, J. Miskulin, J. W. McGillicuddy, D. J. Schuerer, J. W. Haft, F. Swaniker, S. Arbabi, R. B. Hirschl and R. H. Bartlett, “Extracorporeal Life Support for Severe Acute Respiratory Distress Syndrome in Adults,” The Annals of Thoracic Surgery, Vol. 240, No. 4, 2004, pp. 595-605.
- S. A. Conrad, P. T. Rycus and H. Dalton, “Extracorporeal Life Support Registry,” ASAIO Journal, Vol. 51, No. 1, 2005, pp. 4-10. doi:10.1097/01.MAT.0000151922.67540.E9
- D. Hoefer, E. Ruttmann, G. Poelzl, J. Kilo, C. Hoermann, R. Margreiter, G. Laufer and H. Antretter, “Outcome Evaluation of the Bridge-to-Bridge Concept in Patients with Cardiogenic Shock,” The Annals of Thoracic Surgery, Vol. 82, No. 1, 2006, pp. 28-33. doi:10.1016/j.athoracsur.2006.02.056
- F. D. Pagani, W. Lynch, F. Swaniker, D. B. Dyke, R. Bartlett, T. Koelling, M. Moscucci, G. M. Deeb, S. Bolling, H. Monaghan and K. D. Aaronson, “Extracorporeal Life Support to Left Ventricular Assist Device Bridge to Heart Transplant: A Strategy to Optimize Survival and Resource Utilization,” Circulation, Vol. 100, No. 19, 1999, pp. 206-210.
- S. S. Wang, W. J. Ko, Y. S. Chen, R. B. Hsu, N. K. Chou and S. H. Chu, “Mechanical Bridge with Extracorporeal Membrane Oxygenation and Ventricular Assist Device to Heart Transplantation,” Artifical Organs, Vol. 25, No. 8, 2001, pp. 99-602. doi:10.1046/j.1525-1594.2001.025008599.x
- M. C. Oz, D. J. Goldstein, P. Pepino, A. D. Weinberg, S. M. Thompson, K. A. Catanese, R. L. Vargo, P. M. McCarthy, E. A. Rose and H. R. Levin, “Screening Scale Predicts Patients Successfully Receiving Long-Term Implantable Left Ventricular Assist Devices,” Circulation, Vol. 92, No. 9, 1995, pp. 169-173. doi:10.1161/01.CIR.92.9.169
NOTES
*Corresponding author.

