Open Journal of Anesthesiology
Vol.3 No.4(2013), Article ID:32554,6 pages DOI:10.4236/ojanes.2013.34050
Thromboelastometry during Labor and after Delivery
![]()
Department of Anesthesiology, Città di Roma Hospital, Rome, Italy.
Email: tola.gabriele@alice.it
Copyright © 2013 Gabriele Tola et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 15th, 2013; revised April 20th, 2012; accepted May 10th, 2013
Keywords: Pregnancy; Labor; Thromboelastometry
ABSTRACT
The normal values of thromboelastometry (MonoTEM-A®, Framar Hemologix) in pregnancy have not been determined. The aim of this study was to establish the reference ranges for the thromboelastometer in healthy pregnant women during labor and after delivery. After ethical institutional approval and informed consent, we collected blood samples for analysis from 95 healthy labouring women and 40 volunteers (non pregnant women, control group). A sample of 360 μL of whole native blood was tested using the MonoTEM-A® equipment and analyzed within 4 min, at 37˚C. We recorded: R = time to initial fibrin formation; K = time to initial clot formation; Alpha Angle = acceleration of clot formation and MA = strength of the blood clot. When compared to the control group, R and K values were lower in women during labor and after the delivery. The Alpha angle and MA values were higher in the laboring women and in the same women after delivery as compared to the control group. Our study determined the reference ranges for the MonoTEMA® in pregnancy during labor and immediately after the delivery. Data obtained from thromboelastometry confirm the hypercoagulability status in pregnancy and the puerperium. MonoTEM-A® thromboelastometry may be a very useful tool to assess the clotting activity in this patient setting.
1. Introduction
Thromboelastometry measures whole blood coagulation and provides information about the adequacy of the platelet function and other clotting factors, at the bedside and in a short time.
The traditional thromboelastographic instrument requires careful manual operations and attention that are time consuming in a situation where actions must be prompt.
The introduction of ThromboElastoMeter-Automated (MonoTEM-A®) has enabled the manual phases in thromboelastography to be automated so making the in vitro measurements faster and simpler.
Thromboelastometry parameters are interconnected and reflect the whole clotting activity, including clotting factors, platelets, fibrinogen, and their interaction, whereas coagulation profiles monitor an isolated portion of the coagulation cascade and do not reflect the interaction between clotting factors, platelets, and fibrinogen [1-3].
Thromboelastographic techniques have been previously used in the obstetric setting [4-15] but normal values for pregnant women have not been well established due to the paucity of studies dealing with this issue, the different thromboelastographic methods used and the different parturient population included in the studies [16- 21].
Unfortunately, there are no data available regarding pregnancy reference ranges specifically for the MonoTEMA®.
For this reason we decided to undertake this study in order to define the thromboelastometer reference ranges in healthy pregnant women during labor and after delivery. For the purpose of the study we also enrolled healthy female volunteers matching them with pregnant subjects.
2. Methods
The study was approved by the ethics committee of the hospital. All participants gave informed written consent to the procedure. Consecutively healthy at term, labouring women and a control group of healthy non pregnant women were enrolled in the study. The subjects of the control group were healthy volunteers with no contraceptive therapy, between 16 and 45 years. All subjects under anticoagulant or antiplatelet therapy and with haematological diseases were excluded. Laboring women who had received HES 6% as co-load for labor epidural analgesia were excluded [22,23]. In pregnant subjects blood samples were collected during labor and 24 hours after delivery. In the control group blood samples were collected once. From a dry tube 0.36 mL of whole native blood was pipetted into a disposable plastic cup within 4 min of blood sampling and then placed in the pre-warmed (37˚C) MonoTEM-A® (Framar Hemologix, Rome, Italy). All samples were analyzed in the labor ward by the same device. Venous blood was collected through a single 21 G cannula (Smiths Medical International Ltd., Rossendale, UK) by the same anesthesiologist. MonoTEMA® is a computerized, automated thromboe-lastometer instrument. Through a dedicated sensor during a mechanically-induced oscillatory movement, the blood clot undergoes a sophisticated analysis starting from its beginning to its possible lysis, thus allowing an in vitro check of the effective coagulative status of the patient.
It consists of a cup containing a fresh sample of blood and constantly oscillating in a known repeatable arc and for a known repeatable time, so that the blood is submitted to a situation similar to that in the vascular system. Immersing a pendulum which is suspended from a thin torsion wire, of known resistance to torsion, into the blood sample will cause the blood to act as an intermediary between the oscillating cup and the pendulum: as long as the blood remains fluid and provides no linking strength, the pendulum will remain stable in its zero position, despite the oscillation of the cup, due to the resistance of the torsion wire. But as soon as the first monomers and polymers organize to form the clot, these will create strains linking the two, of sufficient strength to overpower the resistance to the torsion of the wire, and the oscillation will drag the pendulum into its course, in an arc that increases proportionally to the strength of the link. The MonoTEM-A® software displays its results either as numerical data or as easily interpretable curves (thromboelastometer). The following data were obtained: time to initial fibrin formation (R) (min); time to initial clot formation (K) (min); acceleration of clot formation (Alpha Angle) (degrees); strength of the blood clot (Maximum Amplitude, MA) (mm). K time is the measurement of the speed at which a clot achieves this level of strength or firmness. Alpha Angle (α) reflects the speed of fibrin accumulation and polymerization and is closely related to K-time. MA—Maximum Amplitude is the highest vertical amplitude of the tracing and is the reflection of both the platelet and plasmatic coagulation part of the clotting process and fibrinolytic activity and of the clot strength. Unless otherwise indicated all data are expressed as mean ± SD. The percentiles method was used to estimate the reference limits in labouring women and in the postpartum period. With regard to the comparison between the pregnant and non pregnant subjects, the sample size of at least 40 for each group provided estimates of the precision (95% CI) of the 95% reference limits which were less than the SD for all measurements. The unpaired t-test was used to compare data in the two groups. A P-value of <0.05 was considered to be significant.
3. Results
Of the initial 135 women (pregnant and non pregnant) included in the study, 28 women were excluded, and the remaining 67 women (pregnant women) and 40 (nonpregnant women) were analyzed. 7 pregnant women were excluded after delivery (24 h): 5 for unplanned caesarean section, 1 for anticoagulant therapy and 1 for blood analysis error.
Demographic and obstetric characteristics of the groups are reported in Table 1. The reference values tests for MonoTEM-A® in the general population are reported in Table 2. Results are reported in Tables 3 and 4 and Figures 1-7. Our results show that R and K values are lower in women during labor and after the delivery when compared to the control group of non pregnant volunteers. Alfa angle and MA values are higher in the labouring women group and after delivery as compared to the control group values.
4. Discussion
To the best of our knowledge, this is the first study to analyze the coagulative status during labor and immediately after delivery in a controlled cohort of healthy

Table 1. Demographic and obstetric characteristics. Values are mean ± SD (95% confidence intervals).

Table 2. Time to initial fibrin formation (R); time to initial clot formation (K); acceleration of clot formation (Alfa Angle); strength of the blood clot (Maximum Amplitude, MA) in the general population.
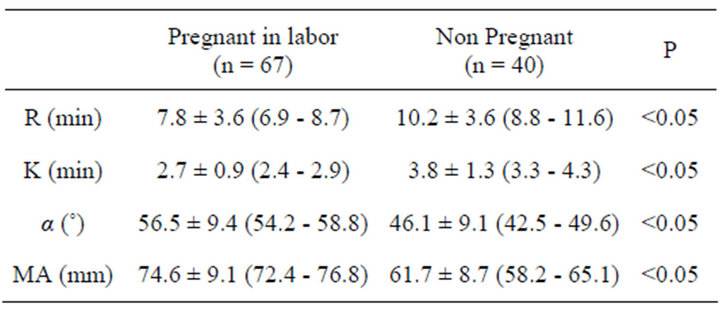
Table 3. Time to initial fibrin formation (R); time to initial clot formation (K); acceleration of clot formation (Alfa Angle); strength of the blood clot (Maximum Amplitude, MA) in labouring patients and in non pregnant control group. Values are mean ± SD (95% confidence intervals).
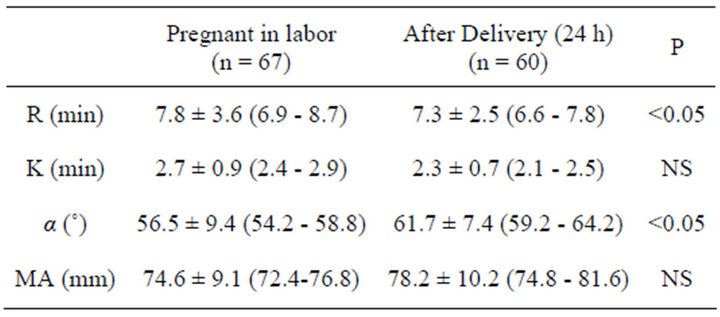
Table 4. Time to initial fibrin formation (R); time to initial clot formation (K); acceleration of clot formation (Alfa Angle); strength of the blood clot (Maximum Amplitude, MA) in labouring patients and during their after delivery. Values are mean ± SD (95% confidence intervals).
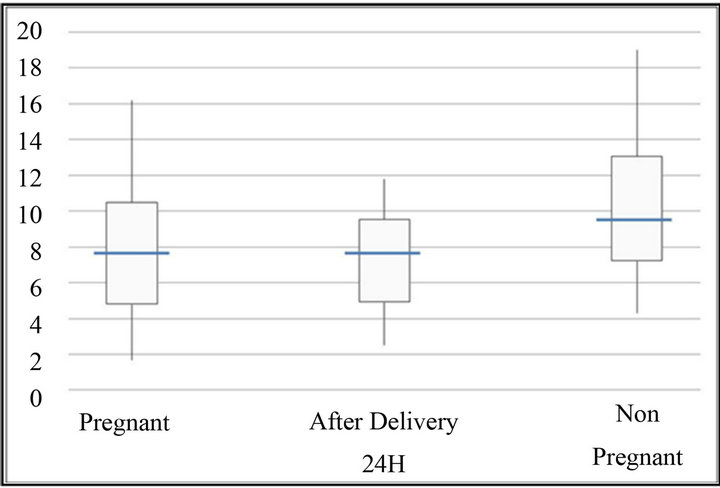
Figure 1. Time R (min). The bottom and top of the box represent the 25th and 75th percentile. The band inside the box shows the median. The ends of the whiskers represent the minimum and maximum of all the data except extreme value.
women by using the MonoTEM-A®. The presence of a healthy control group of well-matched non pregnant women allowed us to determine the reference ranges for the thromboelastometer in pregnancy. Our findings confirm a hypercoagulative state in pregnancy as suggested by lower R and K values, as well as higher alpha angle and MA values in the laboring women group as compared to the control group.
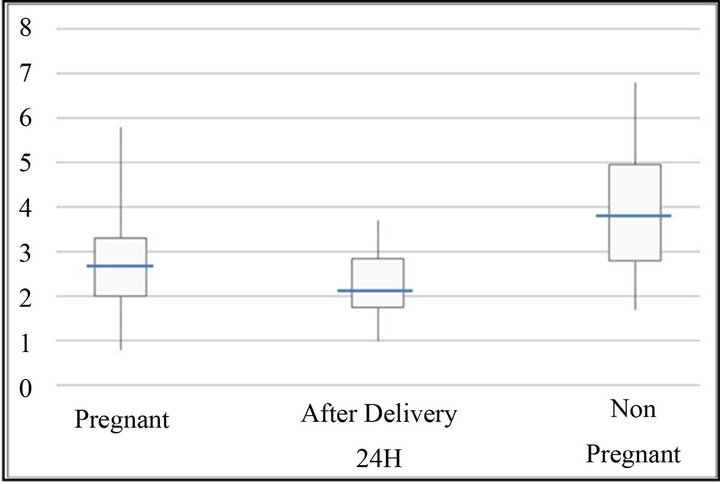
Figure 2. Time K (min). The bottom and top of the box represent the 25th and 75th percentile. The band inside the box shows the median. The ends of the whiskers represent the minimum and maximum of all the data.

Figure 3. Alpha angle (˚). The bottom and top of the box represent the 25th and 75th percentile. The band inside the box shows the median. The ends of the whiskers represent the minimum and maximum of all the data.
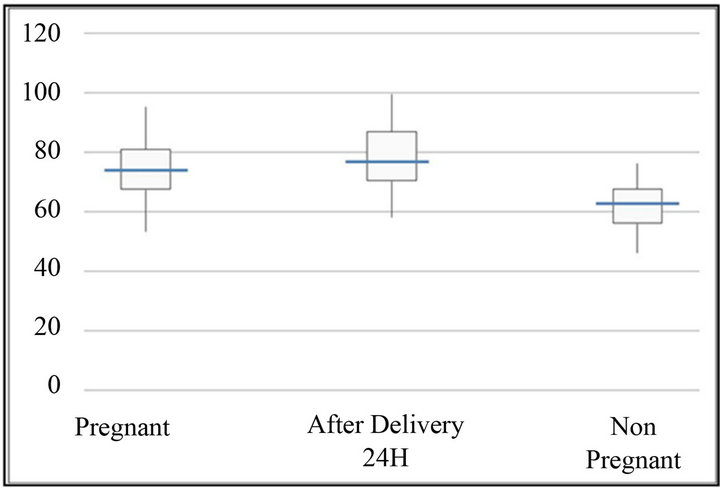
Figure 4. Maximum amplitude (mm). The bottom and top of the box represent the 25th and 75th percentile. The band inside the box shows the median. The ends of the whiskers represent the minimum and maximum of all the data.
The analysis of our data suggests that in laboring women, fibrin and clot initiation starts earlier and advances faster than in non-pregnant subjects. The strength

Figure 5. Graph of MonoTEM-A. Pregnant laboring women.
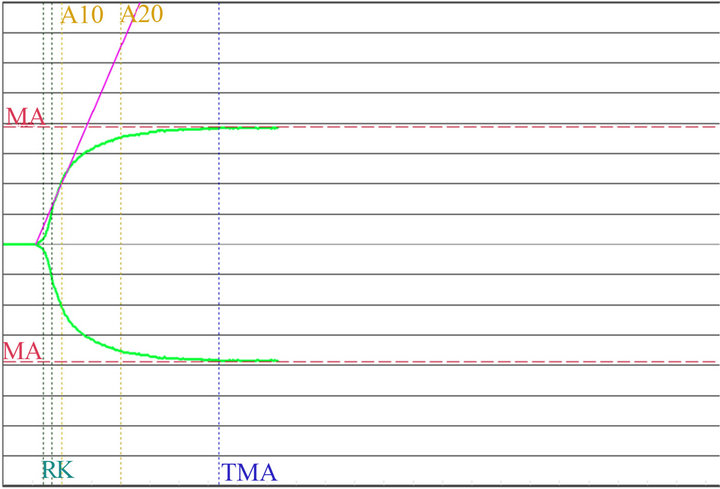
Figure 6. Graph of MonoTEM-A. 24 h after delivery.
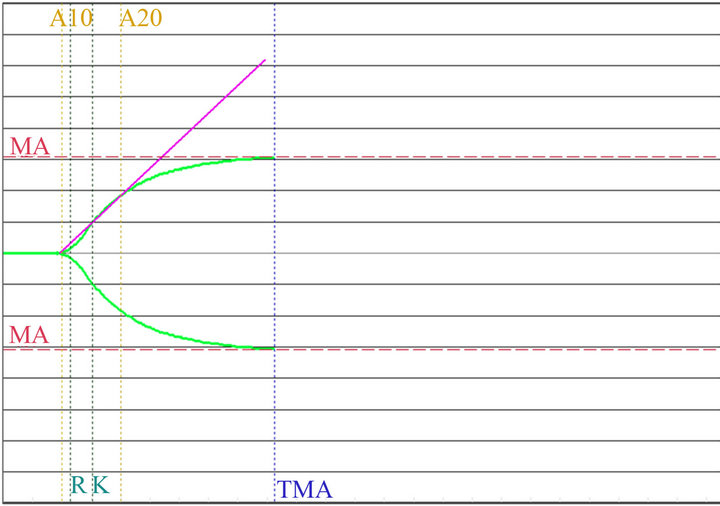
Figure 7. Graph of MonoTEM-A. Non pregnant women.
of the blood clot is also higher than in non-pregnant women. This hypercoagulability state is further increased 24 hours after delivery.
Steer and colleagues [24], and Koh and colleagues [25], evaluated differences between laboring women and healthy fertile women but they accelerated the coagulation process through different additive substances which may have, in turn, affected the results.
Sharma et al. [19] used the thromboelastography to show that the hypercoagulable changes associated with pregnancy were present until 12 - 24 h postpartum. Unfortunately they compared a miscellaneous group of women scheduled for caesarean section mixed with women 24 hr after vaginal delivery and this might represent a possible bias. In fact, the unique feature of our study is that we determined the normal values of thromboelastography by using the same sample of parturients, in each patient during their labor and 24 hr after their delivery, and we compared them with a control group of wellmatched non pregnant women.
Polak and colleagues [17] conducted a study on a cohort of pregnant women at their third trimester, to compare thromboelastography coagulation parameters in pregnant and non-pregnant women in order to establish new reference ranges for pregnant women in their third trimester.
Della Rocca et al. [20] investigated the effect of pregnancy on coagulation assessed by thromboelasto-graphy and established the normal ranges of TEG® for citrated/ recalcifed blood.
Huissoud [21] reported the first reference values of ROTEM® during pregnancy and demonstrated a significant correlation between the results obtained with ROTEM® and those from standard coagulation. Unlike us, Huissoud used coagulation activators to speed up the analysis and to help the study of the intrinsic and extrinsic pathway. Also Armstrong [18] reported reference ranges for ROTEM® by using specific activators and after platelet inactivation to demonstrate the hypercoagulability of pregnancy. Despite the fact that both thromboelastography and ROTEM® devices evaluate the same process, the reference values are different. Not only technical differences, such as variations of cup size or the material of the cup, but also differences of the coagulation activators should also be considered when using algorithms developed with one system while analyzing blood samples with the other device.
Adopting the original Hartert method of measurement, a torsion wire freely suspended in an oscillating cup, and a precise maintenance-free electromagnetic transducer, MonoTEMA® provides dynamic data on the interaction of the blood components as they produce the clot. These components are the time required to begin coagulation, the velocity of hardening, the maximum hardness, the percentage of fibrinolysys and other measurements/indexes used to determine how the components quantified by the routine lab analysis interact to produce the quality of the final haemostasis. The ROTEM® system used a rotating pin, fixed on a steel axis which is stabilized by a unique ball bearing and the precise optical detection method.
Blood samples can be treated with other reagents to investigate platelet aggregation, procedures that can be performed knowledgeably in the laboratory as they involve measurements and calculations made on several analysis on samples treated with different reagents. Treating the sample with reagent would stimulate the intrinsic and extrinsic coagulation pathways, producing a fast and reliable response on the MA (Maximum Amplitude) that expresses the functionality of platelets. The reliability of other TEM-A thromboelastography parameters would, however, be compromised. The aim was to define the thromboelastometer reference ranges in native blood in order to analyze the global coagulation.
The common finding of the thromboelastrographic studies is that they consistently confirm a hypercoagulative state in pregnancy even with different techniques.
We believe this study may make some further contribution to better determine the references values for the thromboelastometric techniques in pregnancy.
In addition, given that we have specifically evaluated the labor period, our data would also be of special anaesthesiological interest because of the spread of local anaesthetic techniques in this patient setting [6,8,10]. Indeed worldwide laboratory parameters such as PT and PTT do not truly mirror the effective coagulative status and other tests, such as those to evaluate the platelets function, are much too complex and difficult to obtain in an emergency setting.
Our findings, however, show the MonoTEM-A® to be a sensitive laboratory test to indicate the effective coagulative status during the labor period, an especially interesting result in this setting of patients who have a high risk of bleeding [4,6-10]. This laboratory equipment allows for an easily interpretable coagulative cascade analysis through a small blood sample even if we must acknowledge some limitations such as the need for quailfied staff for the analysis process and a slight elaborating slowness (30 to 60 minutes).
In conclusion our findings confirm the hypercoagulability reported in pregnancy and the puerperium with a significant increase 24 h after the delivery. Our experience identifies the MonoTEM-A® thromboelastometry as a potentially useful piece of laboratory equipment in this patient setting. Furthermore our study supplies the formal reference ranges for MonoTEM-A® that may be potentially useful in labouring women, as well as in those 24 h after delivery and in childbearing age.
REFERENCES
- D. Woźniak and B. Adamik, “Thromboelastography,” Anestezjologia Intensywna Terapia, Vol. 43, No. 4, 2011, pp. 244-247.
- R. J. Luddington, “Thromboelastography/Thromboelastometry,” Clinical & Laboratory Haematology, Vol. 27, No. 1, 2005, pp. 81-90. doi:10.1111/j.1365-2257.2005.00681.x
- H. Gorton and G. Lyons, “Is It Time to Invest in a Thromboelastograph?” International Journal of Obstetric Anesthesia, Vol. 8, No. 3, 1999, pp. 171-178. doi:10.1016/S0959-289X(99)80133-2
- G. Rajpal, J. M. Pomerantz, M. V. Ragni, J. H. Waters and M. C. Vallejo, “The Use of Thromboelastography for the Peripartum Management of a Patient with Platelet Storage Pool Disorder?” International Journal of Obstetric Anesthesia, Vol. 20, No. 2, 2011, pp. 173-177. doi:10.1016/j.ijoa.2010.09.014
- S. K. Sharma and J. Philip, “The Effect of Anesthetic Techniques on Blood Coagulability in Parturients as Measured by Thromboelastography,” Anesthesia & Analgesia, Vol. 85, No. 1, 1997, pp. 82-86.
- E. Cuvillon, M. Bonnetty, J. P. Favereau, P. Grandchamp and N. Nathan, “Epidural Analgesia in a Pregnant Woman with Essential Thrombocythaemia,” Annales Fran- çaises d’Anesthésie et de Réanimation, Vol. 22, No. 5, 2003, pp. 453-456. doi:10.1016/S0750-7658(03)00091-1
- S. K. Sharma, J. Philip, C. W. Whitten, U. B. Padakandla and D. F. Landers, “Assessment of Changes in Coagulation in Parturients with Preeclampsia Using Thromboelastography,” Anesthesiology, Vol. 90, No. 2, 1999, pp. 385-390. doi:10.1097/00000542-199902000-00009
- S. K. Backe and G. R. Lyons, “High-Dose Tinzaparin in Pregnancy and the Need for Urgent Delivery,” British Journal of Anaesthesia, Vol. 89, No. 2, 2002, pp. 331-334. doi:10.1093/bja/aef189
- J. R. Davies, R. Fernando and S. P. Hallworth, “Hemostatic Function in Healthy Pregnant and Preeclamptic Women: An Assessment Using the Platelet Function Analyzer (PFA-100) and Thromboelastograph,” Anesthesia & Analgesia, Vol. 104, No. 2, 2007, pp. 416-420. doi:10.1213/01.ane.0000253510.00213.05
- A. A. Hanke, O. Elsner and K. Görlinger, “Spinal Anaesthesia and Caesarean Section in a Patient with Hypofibrinogenaemia and Factor XIII Deficiency,” Anaesthesia, Vol. 65, No. 6, 2010, pp. 641-645. doi:10.1111/j.1365-2044.2010.06324.x
- S. K. Sharma, R. L. Vera, W. C. Stegall and C. W. Whitten, “Management of a Postpartum Coagulopathy Using Thrombelastography,” Journal of Clinical Anesthesia, Vol. 9, No. 3, 1997, pp. 243-247. doi:10.1016/S0952-8180(97)00026-3
- A. Butwick and S. Harter, “An in Vitro Investigation of the Coagulation Effects of Exogenous Oxytocin Using Thromboelastography in Healthy Parturients,” Anesthesia & Analgesia, Vol. 113, No. 2, 2011, pp. 323-326. doi:10.1213/ANE.0b013e3182222a82
- C. A. Wong, S. Liu and R. Glassenberg, “Comparison of Thrombelastography with Common Coagulation Tests in Preeclamptic and Healthy Parturients,” Regional Anesthesia, Vol. 20, No. 6, 1995, pp. 521-527.
- F. M. Miall, P. S. Deol, T. A. Barnes, K. Dampier, C. C. Watson, C. A. Oppenheimer, et al., “Coagulation Status and Complications of Pregnancy,” Thrombosis Research, Vol. 115, No. 6, 2005, pp. 461-467. doi:10.1016/j.thromres.2004.09.019
- O. Karlsson, T. Sporrong, A. Hillarp, A. Jeppsson and M. Hellgren, “Prospective Longitudinal Study of Thromboelastography and Standard Hemostatic Laboratory Tests in Healthy Women during Normal Pregnancy,” Anesthesia & Analgesia, Vol. 115, No. 4, 2012, pp. 890-898. doi:10.1213/ANE.0b013e3182652a33
- B. Macafee, J. P. Campbell, K. Ashpole, M. Cox, F. Matthey, L. Acton and S. M. Yentis, “Reference Ranges for Thromboelastography (TEG) and Traditional Coagulation Tests in Term Parturients Undergoing Caesarean Section under Spinal Anaesthesia,” Anaesthesia, Vol. 67, 2012, No. 10, pp. 741-747. doi:10.1111/j.1365-2044.2012.07101.x
- F. Polak, I. Kolnikova, M. Lips, A. Parizek, J. Blaha and M. Stritesky, “New Recommendations for Thromboelastography Reference Ranges for Pregnant Women,” Thrombosis Research, Vol. 128, No. 1, 2011, pp. 14-17. doi:10.1016/j.thromres.2011.04.007
- S. Armstrong, R. Fernando, K. Ashpole, R. Simons and M. Columb, “Assessment of Coagulation in the Obstetric Population Using ROTEM® Thromboelastometry,” International Journal of Obstetric Anesthesia, Vol. 20, No. 4, 2011, pp. 293-298. doi:10.1016/j.ijoa.2011.05.004
- S. K. Sharma, J. Philip and J. Wiley, “Thromboelastographic Changes in Healthy Parturients and Postpartum Women,” Anesthesia & Analgesia, Vol. 85, No. 1, 1997, pp. 94-98.
- G. Della Rocca, T. Dogareschi, T. Cecconet, S. Buttera, A. Spasiano, P. Nadbath, et al., “Coagulation Assessment in Normal Pragnancy: Thrombelastography with Citrated Non Activated Samples,” Minerva Anestesiologica, Vol. 67, No. 7, 2012, pp. 741-747.
- C. Huissoud, N. Carrabin, M. Benchaib, O. Fontaine, A. Levrat, D. Massignon, et al., “Coagulation Assessment in Normal Pregnancy,” Journal of Thrombosis and Haemostasis, Vol. 101, No. 4, 2009, pp. 755-761.
- A. Butwick and B. Carvalho, “The Effect of Colloid and Crystalloid Preloading on Thromboelastography Prior to Cesarean Delivery,” Canadian Journal of Anesthesia, Vol. 54, No. 3, 2007, pp. 190-195. doi:10.1007/BF03022639
- G. Turker, T. Yilmazlar, E. B. Mogol, A. Gurbet, S. Dizman and H. Gunay, “The Effects of Colloid Pre-Loading on Thromboelastography Prior to Caesarean Delivery: Hydroxyethyl Starch 130/0.4 versus Succinylated Gelatin,” Journal of International Medical Research, Vol. 39, No. 1, 2011, pp. 143-149. doi:10.1177/147323001103900115
- P. L. Steer, B. E. Finley and L. A. Blumenthal, “Abruptio Placentae and Disseminated Intravascular Coagulation: Use of Thrombelastography and Sonoclot Analysis,” International Journal of Obstetric Anesthesia, Vol. 3, No. 4, 1994, pp. 229-233. doi:10.1016/0959-289X(94)90074-4
- S. C. L. Koh, et al., “Thrombelastography in Normal Subjects and in Various Clinical States,” Journal of Medical Laboratory Science, Vol. 7, No. 1, 1993, pp. 29-34.

