Open Journal of Composite Materials
Vol.07 No.02(2017), Article ID:75807,6 pages
10.4236/ojcm.2017.72006
Microwave Synthesis and Photoluminescence Properties of  Nanocomposites
Nanocomposites
Qiuci Li, Xiaomei Zeng, Shuibin Yang, Xuehong Liao
Hubei Key Laboratory for Processing and Application of Catalytic Materials, The College of Chemical Engineering, Huanggang Normal University, Huanggang, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 27, 2017; Accepted: April 27, 2017; Published: April 30, 2017
ABSTRACT
In this paper, using calcium chloride and sodium molybdate as raw material, polyethylene glycol (PEG2000) as surfactant, the nanocomposites of CaMoO4: Eu3+ were prepared by a direct feeding microwave synthesis method. The as- prepared sample was characterized by X-ray diffraction (XRD), scanning electron micrograph (SEM) and photoluminescence spectrum (PL). The XRD Pattern showed that the samples are scheelite structure of CaMoO4. The SEM image showed that the majority of as-prepared sample is a relatively flake structure, and some fine particles attached to it. PL spectra showed that as- prepared samples have strong luminescence properties; it had purity red emission at 615 nm. The effects of different Eu3+ ions doping amount and surface active agent on the photoluminescence properties were studied. The results showed that when the molar ratio of Eu3+ was 0.10, PEG2000 as surfactant, the luminescence intensity of as-prepared sample was maximum.
Keywords:
Calcium Molybdate, Eu3+ Ion, Doping, Nanocomposite, Microwave Sythesis, Photoluminescence

1. Introduction
Molybdate has superior optical, electrical, magnetic properties, in terms of scintillator, light soldering, sensors and catalysts has a wide application prospect. Metal molybdate as luminescent materials in important family, due to its excellent luminescence has been widely attention. Molybdate system because of it’s in the near UV region has wide and strong charge transfer absorption band. After UV excitation energy can be through non radiative transition is passed to the activator ion. So we often used molybdate as matrix material doped with rare earth ions prepared in near ultraviolet excitation efficient red phosphor, used for white light emitting diode to arouse people’s great interest [1] - [11] . At present, several techniques were used to synthesize CaMoO4:Eu3+ red phosphors, such as high temperature solid-state, hydrothermal, sol-gel, chemical co-precipitation, combustion, microwave radiation method and so on.
In this study, we report on a direct feeding microwave synthesis method to synthesize CaMoO4:Eu3+ red phosphors. The calcium molybdate as matrix, using Eu3+ ion as activator, by changing the concentration of Eu3+ ion, the choice of different surfactant, to seek the strongest luminescence.
2. The Experiment
2.1. Synthesis of 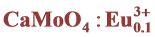 Nanocomposite
Nanocomposite
All chemicals were analytical grade and used without further purification. nanocomposites of CaMoO4:Eu3+ were prepared by a direct feeding microwave synthesis method. In a typical procedure, at a molar ratio of Ca:Mo:Eu of 1:1:0.1, 1.1 g of CaCl2 was dissolved in 50 ml of 2% PEG aqueous solution, dispersed and dissolved with ultrasonic waves, add 1 mmol of Eu3+ reserve liquid, mixed uniform for A solution. 2.42 g of Na2MoO4・2H2O was dissolved in 50 mL of 2% PEG aqueous solution, the dispersion was dissolved by ultrasonic mixing, for B solution. The A, B solutions were mixed rapidly transferred into 250 ml of flask, then the mixed solution was placed in a microwave refluxing system to react for 20 min with a power microwave radiation of 40% and cool down naturally to the room temperature. Then the precipitate was centrifuged, washed with the deionized water for several times and dried at 60˚C in the vacuum for 8 h, The final product was collected for the characterization.
2.2. Characterization
The crystal sttructure of nanocomposites of CaMoO4:Eu3+ was measured by XRD on a Shimadzu XRD-6100 X-ray diffractometer (Cu Kα radiation, λ = 0.15418 nm). The morphology and size of products were determined by SEM. The SEM images were recorded on a Quanta 200 FEG field emission scanning electron microscope. The optical property was obtained by Cary Eclipse fluorescence spectrometer (USA Varian Company).
3. Results and Discussion
3.1. XRD and SEM Analysis
Figure 1 shows the XRD pattern graph of as-prepared sample. The XRD pattern (peak 2θ: 18.75, 28.82, 31.36, 34.39, 47.13, 49.37, 54.14, 58.11) showed that the product is the Tetragonal system of scheelite structure of CaMoO4 (JCPDS File No. 29-0351). The diffraction peak is strong and sharp, which indicates that the sample has a high degree of crystallinity.
Figure 2 shows the SEM image of as-prepared sample. It shows that the majority of the catalyst is a relatively flake structure, parts overlap each other, and some particles attached to it. The size of most of the flakes is 50 to 500 nm.
Figure 1. X-ray diffraction pattern of as-prepared samples.
Figure 2. Scanning electron micrograph image of as-prepared sample.
3.2. Photoluminescence Properties of  Nanocomposite
Nanocomposite
Figure 3 is photoluminescence spectrum of as-prepared sample. The excitation wavelength is 258 nm. It can be seen in the 430 - 450, 590 - 600, 610 - 620, 700 - 710 nm have a certain luminescence, which is the strongest at 615 nm. 430 - 450 nm belongs to calcium molybdate luminescence. The luminescence mechanism of calcium molybdate is due to transition induced by electrons in  ion within the groups, belonging to the intrinsic emission. 516 nm has a very high peak, this is a frequency doubling peak of excitation light. At 590 - 600 nm, 610 - 620 nm and 700 - 710 nm, luminescence by Eu3+ (5D0 → 7F1), (5D0 → 7F2), (5D0 → 7F4) electronic energy level transition, respectively. Among them, 615 nm (5D0 → 7F2) electric dipole transition emission is the main, it has high emission efficiency, the strongest luminescence properties, and pure red light. So, as-prepared
ion within the groups, belonging to the intrinsic emission. 516 nm has a very high peak, this is a frequency doubling peak of excitation light. At 590 - 600 nm, 610 - 620 nm and 700 - 710 nm, luminescence by Eu3+ (5D0 → 7F1), (5D0 → 7F2), (5D0 → 7F4) electronic energy level transition, respectively. Among them, 615 nm (5D0 → 7F2) electric dipole transition emission is the main, it has high emission efficiency, the strongest luminescence properties, and pure red light. So, as-prepared  nanocomposite is an ideal red light-emitting material.
nanocomposite is an ideal red light-emitting material.
3.3. Effect of Eu3+ Doping on Photoluminescence Properties
The effect of Eu3+ ions doping on the luminescence properties was studied. Figure 4 is photoluminescence spectra of samples with different Eu3+ doping. It can be seen that when the doping amount of Eu3+ ion is less than 0.10 (molar ratio), the luminescence intensity of CaMoO4:Eu3+ composite increases with the increase of the amount of Eu3+ ions. When the doping amount of Eu3+ ion is 0.10, the luminescence properties are the best. When the doping amount of Eu3+ ion is more than 0.10, the amount of Eu3+ ions doping will continue to increase, the intensity of the luminescence will decrease obviously due to the concentration of the particle, the luminescence intensity of the CaMoO4:Eu3+ nanocomposite decreases with the increase of Eu3+ doping amount.
Figure 3. Photoluminescence spectrum of as-prepared sample.
3.4. Effect of Different Surfactants on Photoluminescence Properties
We also investigated the effect of different surfactants on the luminescence pro- perties. Figure 5 is photoluminescence spectra of samples with different surfactants. It can be seen that the nonionic surfactant PEG is the best, next is the anionic surfactant sodium dodecyl sulfate (SDS), the cationic surfactant cetyltrimethyl ammonium bromide (CTAB) is worst.
Figure 4. Effect of Eu3+ doping on photoluminescence properties.
Figure 5. Effect of different surfactants on photoluminescence properties.
4. Conclusions
CaMoO4:Eu3+ nanocomposites were successfully prepared by a direct feeding microwave synthesis method. This method is sample.
As-prepared samples have strong luminescence properties; it had purity red emission at 615 nm. When the molar ratio of Eu3+ was 0.10, PEG2000 as surfactant, the luminescence intensity of as-prepared sample was maximum.
Cite this paper
Li, Q.C., Zeng, X.M., Yang, S.B. and Liao, X.H. (2017) Microwave Synthesis and Photolumine- scence Properties of
References
- 1. Li, X., Wang, X. and Wu, H.Y. (2007) Research of Three-Dimensional Fluorescence Spectra of RE3+ (RE=Eu,Tb) and CaWO4 Co-Doped Silica Luminescence Materials. Journal of Rare Earths, 25, 5-8.
- 2. Wang, X., Bo, S., Qi, X., et al. (2009) Preparation and Luminescent Properties of CaMoO4:Eu3+. Chinese Journal of Inorganic Chemistry, 25, 350-353.
- 3. Zhang, Z.J., Chen, H.H., Yang, X.X., et al. (2007) Preparation and Luminescent Properties of Eu3+ and Tb3+ Ions in the Host of CaMoO4. Materials Science and Engineering B, 145, 34-40.
https://doi.org/10.1016/j.mseb.2007.09.091 - 4. Yang, Y., Li, X., Feng, W., et al. (2011) Synthesis and Characterization of CaMoO4: Eu3+ Red Phosphors for W-LED by Co-Precipitation. Journal of Inorganic Materials, 25, 115-119.
- 5. Zhu, F., Xiao, Z.S., Yan, L., et al. (2010) The Influence on Intrinsic Light Emission of Calcium Tungstate and Molybdate Powders by Multivalence Pr Co-Doping. Applied Physics A, 101, 689-693.
https://doi.org/10.1007/s00339-010-5950-3 - 6. Kang, M., Liu, J., Yin, G.F., et al. (2009) Preparation and Characterization of Eu3+-Doped CaCO3 Phosphor by Microwave Synthesis. Rare Metals, 28, 439-444.
https://doi.org/10.1007/s12598-009-0085-4 - 7. Zhai, Y.Q., You, Z.J., Liu, Y.H., et al. (2012) Properties of Red-Emitting Phosphors Sr2MgSi2O:Eu3+ Prepared by Gel-Combustion Method Assisted by Microwave. Journal of Rare Earths, 30, 114-117.
https://doi.org/10.1016/S1002-0721(12)60005-2 - 8. Zhao, C., Meng, Q. and Sun, W. (2015) Luminescence Properties of Eu3+ Doped CaMoO4 Micron Phosphors. Acta Physica Sinica, 10, 283-291.
- 9. Haque, M.M., Lee, H.I. and Kim, D.K. (2009) Luminescent Properties of Eu3+-Activated Molybdate-Based Novel Red-Emitting Phosphors for LEDs. Journal of Alloys and Compounds, 481, 792-796.
https://doi.org/10.1016/j.jallcom.2009.03.083 - 10. Yoon, J.W., Ryu, J.H. and Shim, K.B. (2006) Photoluminescence in Nanocrystalline MMoO4 (M=Ca,Ba) Synthesized by a Polymerized Complex Method. Materials Science and Engineering: B, 127, 154-158.
https://doi.org/10.1016/j.mseb.2005.10.015 - 11. Yang, Z., Dong, H., Liu, P., et al. (2014) Photoluminescence Properties of Sm3+ Doped LiY(MoO4)2 Red Phosphors. Journal of Rare Earths, 32, 404-408.
https://doi.org/10.1016/S1002-0721(14)60085-5






