Open Journal of Composite Materials
Vol.06 No.03(2016), Article ID:67932,13 pages
10.4236/ojcm.2016.63008
The Origin of the Giant Hall Effect in Metal-Insulator Composites*
Joachim Sonntag#
MEAS Deutschland GmbH a TE Connectivity LTD Company, Dortmund, Germany

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 April 2016; accepted 28 June 2016; published 1 July 2016
ABSTRACT
Near the metal-insulator transition, the Hall coefficient R of metal-insulator composites (M-I composite) can be up to 104 times larger than that in the pure metal called Giant Hall effect. Applying the physical model for alloys with phase separation developed in [1] [2] , we conclude that the Giant Hall effect is caused by an electron transfer away from the metallic phase to the insulating phase occupying surface states. These surface states are the reason for the granular structure typical for M-I composites. This electron transfer can be described by  [1] [2] , provided that long-range diffusion does not happen during film production (n is the electron density in the phase A.
[1] [2] , provided that long-range diffusion does not happen during film production (n is the electron density in the phase A. 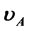 and
and 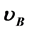 are the volume fractions of the phase A (metallic phase) and phase B (insulator phase). b is a measure for the average potential difference between the phases A and B). A formula for calculation of R of composites is derived and applied to experimental data of granular Cu1-y(SiO2)y and Ni1-y(SiO2)y films.
are the volume fractions of the phase A (metallic phase) and phase B (insulator phase). b is a measure for the average potential difference between the phases A and B). A formula for calculation of R of composites is derived and applied to experimental data of granular Cu1-y(SiO2)y and Ni1-y(SiO2)y films.
Keywords:
Metal-Insulator Composites, Granular Metals, Hall Coefficient, Conductivity, Electron Density

1. Introduction
Nanocomposites play a growing role in both scientific research and practical applications because of the possibility of combination of special properties which cannot be reached in classical materials [3] - [5] . A prominent example for both scientific challenge and practical application is the Giant Hall effect (GHE) in metal-insulator composites (M-I composites): Near the metal-insulator transition (M-I transition), the Hall coefficient can be up to 104 times larger than that in the pure metal [6] - [16] .
Applications of the GHE we find in magnetic field sensing elements, in read heads of magnetic recording devices and magnetic switching devices. Other examples for practical applications of nanocomposites are biomedical ones, materials with improved corrosion resistance, and thermoelectric materials with higher efficiency for energy harvesting, environmentally friendly refrigeration, direct energy transformation from heat into electricity, and temperature sensors.
As reasons for the GHE, quantum size effects and quantum interference effects on the mesoscopic scale have been discussed [8] [11] - [14] [17] . To our knowledge, until now, there is no explanatory model which can interpret the phenomenon of GHE. In the present paper, we present a discussion of the reasons for the GHE applying the electron transfer model [1] [2] developed for metal-metalloid alloys. This model can be summarized by three points1:
For large ranges of concentration there is
(1) Phase separation between two phases called phase A and phase B, where each phase has its “own” short-range order (SRO),
(2) The phase separation leads to band separation in the conduction band (CB) and valence band (VB) connected with the phases A and B, respectively, and the electrons are freely propagating and the corresponding wave functions are extended over connected regions of one phase as long as the phase forms an infinite (macroscopic) cluster through the alloy.
(3) Between the two coexisting phases there is electron redistribution (electron transfer) which can be described by
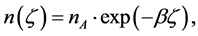 (1)
(1)
where 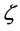 is the quotient of the volume or atomic fractions2 of the two coexisting phases.
is the quotient of the volume or atomic fractions2 of the two coexisting phases.  is the electron density in the phase A with
is the electron density in the phase A with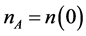 .
. 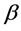 is a constant for a given alloy, which is determined by the average potential difference between the two phases.
is a constant for a given alloy, which is determined by the average potential difference between the two phases.
The points (1) and (2) imply the fact that each phase can be characterized by its own transport coefficients which can be calculated, in principle, by classical transport theory as done in [2] (conductivity) and [18] [19] (Seebeck coefficient).
Since M-I composites also consist of two separate phases with phase grains at the nanoscale, it is obvious to ask whether Equation (1) is reflected in the concentration dependence of the Hall coefficient R of M-I composites as well. Indeed, we have found that in the metallic regime of Cu1-y(SiO2)y and Ni1-y (SiO2)y thin films, the concentration dependence of R can be approximated by linear relations
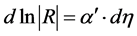 (2)
(2)
with constant slope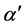 . For Cu1-y(SiO2)y and Ni1-y(SiO2)y it follows from Figure 1(a) and Figure 1(b),
. For Cu1-y(SiO2)y and Ni1-y(SiO2)y it follows from Figure 1(a) and Figure 1(b), 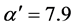 and
and 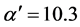 with the coefficient of determination
with the coefficient of determination 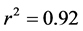 and
and


Figure 1(a) and Figure 1(b) reflect immediately Equation (1) provided that 
The known EMT-formula for the Hall coefficient derived by Cohen and Jortner [21] is

Figure 1. Experimental Hall coefficient data at 5 K versus 



where 



As will be argued in Sec. 3.1, Equation (3) seems to be a good approximation for two-phase composites if


2. Derivation of the R Formula
Let us consider a non-magnetic two-phase composite, where the phase grains are spherical without preferred orientations and arranged in a symmetrical fashion and each phase i can be characterized by a set of transport coefficients. The local electric current density in a single grain of the phase i (i = A or B) can be written as

where 


where 

















where


with










At the interface between a single phase grain and its surroundings continuity of the normal components of the current density and the tangential components of the potential gradient are to be fulfilled. For the limiting case

following from the EMT-formula for

For the case



where the identities 


following from the tensor elements 








Substituting 



The same formalism can also be applied to composites with more than two phases leading to relatively complex formulae for R. A self-contained and more manageable description of these R formulae is given by

with

3. Discussion
3.1. Comparison between Equation (3) and Equation (13)
For three examples of two-phase composites, in Figure 2(b), Figure 2(d), and Figure 2(f), the concentration dependence of R related to its values at 



(1) The most striking difference appears in Figure 2(a) and Figure 2(c): The “C & J” curves decrease dramatically with increasing 









A possible interpretation for such dramatic decrease of 



The differences between Equation (13) and the curves “C & J” are the larger the larger the difference between 


(2) Another striking difference between Equation (13) and Equation (3) is represented by the boundary case “


and

Figure 2. 


respectively, and for

Starting at







These two differences, (1) and (2), suggest the fact that Equation (16) represents the physical situation better than Equation (17). Therefore, in the following, Equation (13), respectively Equation (16), will be applied in a discussion of the Hall coefficient in M-I composites.
3.2. The Giant Hall Effect in M-I Composites
For 

where 







where












Because the Fermi level lies in the energy gap between the valence band and conduction band of the insulator SiO2 phase, the transferred electrons occupy surface states on the SiO2 phase. This is the reason for the granular structure: spherical metal grains are embedded in the amorphous SiO2 phase (see, e.g., [29] , Figs. 13-16 therein). A minimum energy is realized if, firstly, the transferred (pinned) electrons are arranged on spherical surfaces and, secondly, the insulating phase forms very thin layers around the metal grains providing the largest possible surface to accommodate the large number of transferred electrons. This electron transfer from the metallic phase to the phase boundaries provides the logical explanation for the granular structure in M-I composites. Such a granular structure has been found in many M-I films [7] [13] [15] [29] . This proposal applies to magnetic M-I composites as well. For nonmagnetic M-I composites the parameter C in

(NFE approximation) is of the order of one, depending slightly on the magnetic field. [23] [24] 










This assumption is supported by the experimental finding by Xiong et al. [31] that (for not too small fields H), in the granular Co-Ag system, the Hall resistivity 
If the metallic phase of a M-I composite is a noble metal, the NFE approximation is a good one for the metallic phase, above all as the Fermi surface moves away from the Brillouin zone boundary as n decreases. For the metallic phase in Ni-SiO2 the NFE approximation is surely also a good one, because Ni has only 0.55 4s valence electrons per Ni atom ( [30] , p. 271).
If the metallic phase of a M-I composite is a transition-metal, the electron transfer is expected to be composed of both the d and s electrons. As the d density of states at the Fermi level is essentially larger than the s density of states, the principal share of electrons transferred to the insulating phase, is made up of d electrons, that is, the s electron density in the metallic phase remains relatively large. Because the electronic transport is determined by the s valence electrons in the A phase, the effect of the electron transfer on the electronic transport in the metallic phase is expected to be relatively small, and the increase of 







Now the question arizes: why do we find an exponential dependence of 

In summary, for M-I composites containing a noble metal, we expect an exponential 

Comparing granular M-I composites with amorphous transition-metal―metalloid alloys ( [1] ), we state that the exponential increase of R and the exponential decrease of 


Our electron transfer model is compatible with a series of other experimental findings:
1) The GHE occurs both in magnetic M-I composites and non-magnetic ones suggesting a mechanism independent from magnetism [13] .
2) In M-I composites, 





Figure 3. (a) 





where 



The only exception in Figure 3, where such an exponential concentration dependence of

3) With increasing y the temperature coefficient of resistivity, TCR, decreases and changes sign from positive to negative. [6] [11] [12] [14] [15] [34] The reason is an activation of localized electrons to the conduction band of the metallic phase. This conductivity contribution by activation is in competition with the positive contribution to the TCR due to scattering. The activation contribution is the larger the larger the amount of transferred electrons, i.e., the larger y, in correspondence to Equation (1).
In earlier papers it was suggested “that the GHE is a result of the drastic reduction of both the effective electron density and (in case of EHE) the effective carrier mobility”8 (Pakhomov et al. [11] ) or a drastic reduction of carrier density (Jing et al. [35] ). These two suggestions [11] [35] correspond to our physical model summarized in Sec. I. We emphasise, however, that it is not any effective electron density or carrier density (electrons or holes), but it is the real electron density which is reduced in the M-I composites.
3.3. The Effect of the Grain Size on the GHE
Approaching the M-I transition, the charging energy arising from the positively charged metal ions grows more and more and one could assume that such ‘metal’ phase cannot exist, because the electrostatic contribution by the positive ions increases more and more as n decreases. However, the growth of the electrostatic energy is not unbounded; decrease of n is accompanied with a decrease of the sizes of the metal grains. For granular Al1-yGey films, with increasing y the sizes of the metal grains decrease from 10 - 20 nm (on the metallic-rich side) to sizes <2 nm beyond the MIT (Rosenbaum et al. [36] [37] ). This decrease of 





We suppose that the electron transfer described by Equation (1), respectively Equation (20), holds also beyond the M-I transition. This assumption correlates with the concentration dependence of
As mentioned earlier ( [18] , Sec. IVA therein), Equation (1), is part and result of a complex energy balance realized during solidification of the alloy, where the sizes of the phase grains are part of this balance. Equation (1) holds for situations, where atomic diffusion does practically not play a role because of the high cooling rate during the film deposition process. Because of this suppression of the long-range diffusion, the EMT provides a more realistic description of the electrical properties of disordered alloys with phase separation than any percolation description. This is justified in [2] (Sec. IVA therein).
On the other hand, at sufficiently high temperatures, appreciable diffusion can take place leading to additional growth of

Therefore, the GHE decreases or disappears by annealing at sufficiently high temperatures [14] . This phenomenon is also reflected by the concentration dependences of 







This can also explain the experimental finding [14] that the maximum of the enhancement of R in Zn1-y(SiO2)y is about 60, but 700 in Cu1-y(SiO2)y: the size of the granules in Zn1-y(SiO2)y is much larger (

4. Conclusions
A formula is derived for the Hall coefficient R of composites and applied to a discussion of the concentration dependence of R in M-I composites. From the empirical relation 
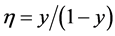
insulating phase which obeys
atomic diffusion does practically not play a role during the film deposition process. It is part and result of a complex energy balance realized during solidification of the alloy, where the sizes of the phase grains are part of this balance.
In M-I composites, the decrease of electron density n in the metallic phase occurs as interface charges occupying surface states on the insulating phase which is responsible for the granular structure.
Acknowledgements
The author is appreciative to MEAS Deutschland GmbH a TE Connectivity LTD company for supporting this work. He also would like to thank Professor Stolze from the University of Dortmund for a critical reading of the manuscript and Stefan Lange for technical support.
Cite this paper
Joachim Sonntag, (2016) The Origin of the Giant Hall Effect in Metal-Insulator Composites. Open Journal of Composite Materials,06,78-90. doi: 10.4236/ojcm.2016.63008
References
- 1. Sonntag, J. (1989) Disordered Electronic Systems: Concentration Dependence of the dc Conductivity in Amorphous Transition-Metal—Metalloid Alloys (Metallic Regime). Physical Review B, 40, 3661-3671. http://dx.doi.org/10.1103/PhysRevB.40.3661
- 2. Sonntag, J. (2005) Disordered Electronic Systems: II. Phase Separation and the Metal-Insulator Transition in Metal-Metalloid Alloys. Physical Review B, 71, 115114. http://dx.doi.org/10.1103/physrevb.71.115114
- 3. Rowe, D.M. (2006) Thermoelectrics Handbook: Macro to Nano. CRC Press, Taylor and Francis Group, Boca Raton, London, New York.
- 4. Minnich, A.J., Dresselhaus, M.S., Ren, Z.F. and Chen, G. (2009) Bulk Nanostructured Thermoelectric Materials: Current Research and Future Prospects. Energy & Environmental Science, 2, 466-479. http://dx.doi.org/10.1039/b822664b
- 5. Reddy, B. (2011) Nanotechnology and Nanomaterials. Advances in Diverse Industrial Applications of Nanocomposites. InTech.
- 6. Zhao, B. and Yan, X. (1997) Giant Hall Effect in Granular Fe-SiO2 Film. Journal of Applied Physics, 81, 4290-4292. http://dx.doi.org/10.1063/1.364804
- 7. Wu, Y.N., Li, Z.Q. and Lin, J.J. (2010) Giant Hall Effect in Nonmagnetic Mo/SnO2 Granular Films. Physical Review B, 82, Article ID: 092202. http://dx.doi.org/10.1103/PhysRevB.82.092202
- 8. Wen, J.F., Wang, J.F., Zou, W.Q., Zhang, F.M. and Du, Y.W. (2005) Investigation on the Giant Hall Effect of (FexSn100-x)1-y(SiO2)y Granular Films. Journal of Alloys and Compounds, 393, 77-80. http://dx.doi.org/10.1016/j.jallcom.2004.10.021
- 9. Miao, G.X. and Xiao, G. (2004) Giant Hall Resistance in Pt-Based Ferromagnetic Alloys. Applied Physics Letters, 85, 73-75. http://dx.doi.org/10.1063/1.1757645
- 10. Pakhomov, A.B., Yan, X. and Zhao, B. (1995) Giant Hall Effect in Percolating Ferromagnetic Granular Metal-Insulator Films. Applied Physics Letters, 67, 3497-3499. http://dx.doi.org/10.1063/1.115259
- 11. Pakhomov, A.B., Yan, X., Wang, N., Jing, X.N., Zhao, B., Fung, K.K., Xhie, J., Hung, T.F. and Wong, S.K. (1997) On the Origin of the Giant Hall Effect in Magnetic Granular Metals. Physica A, 241, 344-349. http://dx.doi.org/10.1016/S0378-4371(97)00105-2
- 12. Zhang, X.X., Liu, H. and Pakhomov, A.B. (2000) Observation of Giant Hall Effect in Non-Magnetic Cermets. Physica B, 279, 81-83. http://dx.doi.org/10.1016/S0921-4526(99)00674-2
- 13. Zhang, X.X., Wan, C., Liu, H., Li, Z.Q., Sheng, P. and Lin, J.J. (2001) Giant Hall Effect in Nonmagnetic Granular Metal Films. Physical Review Letters, 86, 5562-5565. http://dx.doi.org/10.1103/PhysRevLett.86.5562
- 14. Liu, H., Zheng, R.K., Wen, G.H. and Zhang, X.X. (2004) Giant Hall Effect in Metal/Insulator Composite Films. Vacuum, 73, 603-610. http://dx.doi.org/10.1016/j.vacuum.2003.12.076
- 15. Denardin, J.C., Knobel, M., Zhang, X.X. and Pakhomov, A.B. (2003) Giant Hall Effect in Superparamagnetic Granular Films. Journal of Magnetism and Magnetic Materials, 262, 15-22. http://dx.doi.org/10.1016/S0304-8853(03)00012-X
- 16. Denardin, J.C., Pakhomov, A.B., Knobel, M., Liu, H. and Zhang, X.X. (2000) Giant Hall Effect in Co-SiO2 Nano- composites. Journal of Physics: Condensed Matter, 12, 3397-3399. http://dx.doi.org/10.1088/0953-8984/12/14/315
- 17. Wan, C. and Sheng, P. (2002) Quantum Interference and the Giant Hall Effect in Percolating Systems. Physical Review B, 66, 075309. http://dx.doi.org/10.1103/PhysRevB.66.075309
- 18. Sonntag, J. (2006) Disordered Electronic Systems: III. Thermoelectric Power in Alloys with Phase Separation. Physical Review B, 73, 045126. http://dx.doi.org/10.1103/physrevb.73.045126
- 19. Sonntag, J. (2009) Thermoelectric Power in Alloys with Phase Separation (Composites). Journal of Physics: Condensed Matter, 21, 175703. http://dx.doi.org/10.1088/0953-8984/21/17/175703
- 20. Savvides, N., Alister, S.P., Hurd, C.M. and Shiozaki, I. (1982) Localization in the Metallic Regime of Granular Cu- SiO2 Films. Solid State Communications, 42, 143-145. http://dx.doi.org/10.1016/0038-1098(82)90370-2
- 21. Cohen, M.H. and Jortner, J. (1973) Effective Medium Theory for the Hall Effect in Disordered Materials. Physical Review Letters, 30, 696-698. http://dx.doi.org/10.1103/PhysRevLett.30.696
- 22. Czycholl, G. (2008) Theoretische Festkorperphysik. 3rd Edition, Springer-Verlag, Berlin.
- 23. Kirejew, P.S. (1974) Physik der Halbleiter. Akademie-Verlag, Berlin.
- 24. Kirejew, P.S. (1978) Semiconductor Physics. Mir Publishers, Moscow.
- 25. Harman, T.C. and Honig, J.M. (1967) Thermoelectric and Thermomagnetic Effects and Applications. McGraw-Hill Book Company, New York.
- 26. Cohen, M.H. and Jortner, J. (1974) The Inhomogeneous Transport Regime and Metal-Nonmetal Transitions in Disordered Material. Journal de Physique. Colloques, 35, 345-366. http://dx.doi.org/10.1051/jphyscol:1974467
- 27. Odelevskii, V.I. (1951) Calculation of the Generalized Conductivity of Heterogeneous Systems. Journal of Technical Physics (USSR), 21, 678-685.
- 28. Landauer, R. (1952) The Electrical Resistance of Binary Metallic Mixtures. Journal of Applied Physics, 23, 779-784. http://dx.doi.org/10.1063/1.1702301
- 29. Abeles, B., Sheng, P., Coutts, M.D. and Arie, Y. (1975) Structural and Electrical Properties of Granular Metal Films. Advances in Physics, 24, 407-461. http://dx.doi.org/10.1080/00018737500101431
- 30. Schulze, G.E.R. (1967) Metallphysik. Akademie-Verlag, Berlin.
- 31. Xiong, P., Xiao, G., Wang, J.Q., Xiao, J.Q., Jiang, J.S. and Chien, C.L. (1992) Extraordinary Hall Effect and Giant Magnetoresistance in Granular Co-Ag System. Physical Review Letters, 69, 3220-3224. http://dx.doi.org/10.1103/PhysRevLett.69.3220
- 32. Tanaka, K., Saito, T., Suzuki, K. and Hasegawa, R. (1985) Role of Atomic Bonding for Compound and Glass Formation in Ni-Si, Pd-Si, and Ni-B Systems. Physical Review B, 32, 6853-6860. http://dx.doi.org/10.1103/PhysRevB.32.6853
- 33. Priestley, E.B., Abeles, B. and Cohen, R.W. (1975) Surface Plasmons in Granular Ag-SiO2. Physical Review B, 12, 2121-2124. http://dx.doi.org/10.1103/PhysRevB.12.2121
- 34. Ren, S.L., You, B., Du, J., Bai, X.J., Zhang, J., Zhang, W., Hu, A., Zhang, B. and Zhang, X.X. (2007) Magnetic Transport Properties in Iron/Iron-Oxide Films. Physica B: Condensed Matter, 400, 185-189. http://dx.doi.org/10.1016/j.physb.2007.07.005
- 35. Jing, X.N., Wang, N., Pakhomov, A.B., Fung, K.K. and Yan, X. (1996) Effect of Annealing on the Giant Hall Effect. Physical Review B, 53, 14032-14035. http://dx.doi.org/10.1103/PhysRevB.53.14032
- 36. Rosenbaum, R.L., Slutzky, M., Mobius, A. and McLachlan, D.S. (1994) Various Methods for Determining the Critical Metallic Volume Fraction phic at the Metal-Insulator Transition. Journal of Physics: Condensed Matter, 6, 7977-7992. http://dx.doi.org/10.1088/0953-8984/6/39/018
- 37. Lereah, Y., Deutscher, G. and Grünbaum, E. (1991) Formation of Dense Branching Morphology in the Crystallization of Al-Ge Amorphous Thin Films. Physical Review A, 44, 8316. http://dx.doi.org/10.1103/PhysRevA.44.8316
- 38. Abeles, B., Pinch, H.L. and Gittleman, J.I. (1975) Percolation Conductivity in W-Al2O3 Granular Metal Films. Physical Review Letters, 35, 247-250. http://dx.doi.org/10.1103/PhysRevLett.35.247
- 39. Edwards, A.M., Fairbanks, M.C., Singh, A., Newport, R.J. and Gurman, S.J. (1989) An Investigation of the Structure of Amorphous Si1-xNix through the Metal-Insulator Transition. Physica B: Condensed Matter, 158, 600-601. http://dx.doi.org/10.1016/0921-4526(89)90402-X
- 40. Edwards, A.M., Fairbanks, M.C. and Newport, R.J. (1991) Structural Studies of Amorphous Ge-Au Alloys. Philosophical Magazine Part B, 63, 457-463. http://dx.doi.org/10.1080/13642819108205950
- 41. Lorentz, R.D., Bienenstock, A. and Morrison, T.I. (1994) Structural Studies of the Phase Separation of Amorphous FexGe1-x Alloys. Physical Review B, 49, 3172-3182. http://dx.doi.org/10.1103/PhysRevB.49.3172
- 42. Regan, M.J., Rice, M., van Raap, M.B.F. and Bienenstock, A. (1994) Anisotropic Phase Separation through the Metal-Insulator Transition in Amorphous Alloys. Physical Review Letters, 73, 1118-1121. http://dx.doi.org/10.1103/PhysRevLett.73.1118
- 43. Van Raap, M.B.F., Regan, M.J. and Bienenstock, A. (1995) Evidence of Phase Separation in Amorphous FexSi1-x Films. Journal of Non-Crystalline Solids, 191, 155-163. http://dx.doi.org/10.1016/0022-3093(95)00286-3
- 44. Yoshizumi, S., Mael, D., Geballe, T.H. and Greene, R.L. (1985) The Metal-Insulator Transition and Superconductivity in Amorphous Molybdenum-Germanium Alloys. In: Fritzsche, H. and Adler, D., Eds., Localization and Metal- Insulator Transitions, Plenum Press, Berlin, 77-88. http://dx.doi.org/10.1007/978-1-4613-2517-8_7
- 45. Mael, D., Yoshizumi, S. and Geballe, T.H. (1986) Specific Heat of Amorphous MoxGe1-x through the Metal-Insulator Transition. Physical Review B, 34, 467-470. http://dx.doi.org/10.1103/PhysRevB.34.467
- 46. Fischer, J. and Loneysen, H.V. (1993) Electronic Properties and Superconductivity of Amorphous Si1-xAux Alloys near the Metal-Insulator Transition. Annalen der Physik, 2, 635-646. http://dx.doi.org/10.1002/andp.19935050705
- 47. Mizutani, U., Ishizuka, T. and Fukunaga, T. (1997) Interrelations of Atomic Structures, Electronic Structures, Electron Transport, and Magnetic Properties across the Metal-Insulator Transition for Amorphous VxSi100-x (7 ≤ x ≤ 74) Alloys. Journal of Physics: Condensed Matter, 9, 5333-5354. http://dx.doi.org/10.1088/0953-8984/9/25/004
- 48. Rogatchev, A.Y., Takeuchi, T. and Mizutani, U. (2000) Comparison of the Specific Heat and the Conductivity of Amorphous TixSi100-x Alloys across the Metal-Insulator Transition. Physical Review B, 61, 10010-10014. http://dx.doi.org/10.1103/PhysRevB.61.10010
- 49. Abkemeier, K.M., Adkins, C.J., Asal, R. and Davis, E.A. (1992) Conductivity and Magnetoresistance of Hydrogenated Amorphous Silicon-Nickel Alloys near the Metal-Insulator Transition. Journal of Physics: Condensed Matter, 4, 9113- 9130. http://dx.doi.org/10.1088/0953-8984/4/46/017
- 50. Abkemeier, K.M., Adkins, C.J., Asal, R. and Davis, E.A. (1992) Hopping Conduction in Hydrogenated Amorphous Si1-yNiy. Philosophical Magazine Part B, 65, 675-680. http://dx.doi.org/10.1080/13642819208204902
NOTES
*PACS numbers: 71.23.-k 71.55.Jv 72.10.Bg 72.15.-v.
#Corresponding author.
1The points (1) and (2) are now confirmed experimentally or supported by independent authors [39] - [50] (details in [2] , Sec. I therein). Support for point (3) comes from the fact that it is successfully applied for a quantitative description of the concentration dependence of both conductivity and Seebeck coefficient in [18] [19] and of the M-I transition and structural features of metal-metalloid alloys and M-I composites in [2] .
2In [1] , the available experimental data were not sufficient to decide this question. This question was discussed in [2] with the result that 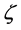 is to be interpreted as volume quotient.
is to be interpreted as volume quotient.
3Equation (3) is a comprehensive formulation of the Equations (16)-(20) of [21] .
4RA calculated by Equation (17) can also be approximated by Equation (19) with the slopes β′ = 7.5 and β′ = 9.9 and r2 = 0.92 and r2 = 0.96, for Cu1−y(SiO2)y and Ni1−y(SiO2)y, respectively.
5For large ranges of composition, amorphous transition-metal―metalloid alloys are composed of different amorphous phases [39] - [43] with Di ~ 1 - 2 nm [42] [43] .
6The potential difference ∆V is identical with the difference of the electrochemical potentials of the phases, as long as they are not in contact to each other. Only, when a contact is realized, a common electrochemical potential is realized by electron transfer between the phases.
7In Mo1−y(SnO2)y the carriers are holes [7] ; electron transfer away from the metallic phase can lead to an increase of the hole density p, but also to a decrease of it depending on the position of the Fermi surface in relation to the Brillouin zones.
8EHE is applied in [11] for the extraordinary Hall effect in magnetic M-I composites.









