Journal of Materials Science and Chemical Engineering
Vol.06 No.08(2018), Article ID:87011,16 pages
10.4236/msce.2018.68003
Study on the Mechanism of Heterogeneous Catalysis (4)
―Electron Cyclic Donate-Accept Catalysis Mechanism-ECDAM or Electron Orbital Deformation-Reversion Cyclic Catalysis Mechanism-EODRM
Jiamin Jin, Weifang Bao
Shanghai Research Institute of Materials, Shanghai, China
Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 29, 2018; Accepted: August 28, 2018; Published: August 31, 2018
ABSTRACT
This paper has expounded the derivation of the Electron Cyclic Donate-Accept Catalysis Mechanism-ECDAM or Electron Orbital Deformation-Reversion Cyclic Catalysis Mechanism-EODRM and its three main arguments as well as three argument verifications. These three main arguments are: 1) There is a demarcation between catalysts and poisons, or promoters and poisons. 2) The relative activities of catalysts or poisons of poisons are closely related to the Electrical Negativity Values-ENV of catalysts or poison. 3) The activities or ENVs of catalysts are closely related to the chemical states of substance being added. The ECDAM or EODRM can also be extended to iron or metal base catalyst for selecting promoter, support and judging poison. It can also be extended to the study of the fire retardant of carbon materials. The author holds that the catalytic phenomenon should be physical phenomenon rather than chemical phenomenon or not completely chemical phenomenon at least.
Keywords:
Heterogeneous Catalysis, Mechanism, ECDAM, EODRM

1. Introduction
Over the past two years, three articles are published on the mechanism of heterogeneous catalysis and reported the experimental results for verifying ECDAM or EODRM and Chemical Reaction Model Mechanism-CRMM on the
2. Electron Cyclic Donate-Accept Catalysis Mechanism-ECDAM or Electron Orbital Deformation-Reversion Cyclic Catalysis Mechanism-EODRM
Derivation of ECDAM or EODRM
It is well known that the carbon gasification reaction (CO2 + C = 2CO) or Boudouard reaction is the rate-determining step in the process of the carbon thermic reduction of Fe2O3. This conclusion has also been proved by author for many times [4] . In 1970s, the EODRM or ECDAM has been proposed by author to be used to account for the test results on the effects of impurities in carbon on the reduction process of iron oxides with carbon.
Many scholars have studied the mechanism of carbon gasification reaction, and in this paper, it is to be consider as most reasonable reaction mechanism.
The derivation of EODRM or ECDAM is as follows:
1) The first step of interaction between CO2 and solid carbon is that the direct-type structure CO2 molecule in gas phase diffuses to the surface of solid carbon, and is then actively adsorbed by carbon atom. As a result, the strength of carbon-oxygen bond in CO2 molecule is weakened, and subsequently is broken, and then the Ketone-group (>C=O) and Ethenone-group (>C=C=O) have formed. The reaction equation is represented as follows:
Scheme 1 is an imagination reaction process of C + CO2 = 2CO.
2) The next step is the thermal dissociation of Ethenone-group (C(s)・CO(ads)),
and then a CO molecule has formed and released out it. Because the thermal dissociation of Ethenone-group does not break up the C-C bond in carbon lattice, hence the energy required is small, while the reaction rate is great.
3) The next step of reaction is the dissociation of Ketone-group (C(s) ∙O(ads)).
There are two dissociation forms for Ketone-group, namely thermal dissociation and collision mechanism. No matter what is the dissociation form for it, the C-C bond will eventually be to break off to release a CO molecule. Now, let Ea be the activation energy of dissociation of Ethenone-group, and Eb be the activation energy of dissociation of Ketone-group. Table 1 gives the values of Ea and Eb.
From Table 1, it is clear that:
1) In general, Eb values are always greater than Ea.
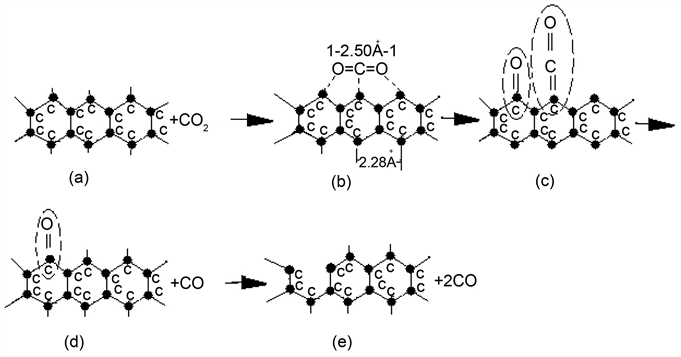
Scheme 1. The reaction process of C + CO2 = 2CO.
Table 1. Ea and Eb [5] .
2) Ea values are almost not related to the kinds of carbon being employed as well as the kinds of impurity being added, and these values are almost the same, whereas Eb values are closely related to the kinds of carbon being adopted and kinds of impurity being added. The Eb values containing 7% iron catalysts are almost 1/2 of Eb values that contain no iron catalysts.
According to the analysis above, we can get two conclusions, namely:
1) The dissociation of Ketone-group is the rate-controlling step of Boudouard reaction.
2) The function of catalyst Fe should be ascribed to a decrease in energy required to break off the C-C bonds.
Scheme 2 [6] is a scheme on the breakup of Ethenone-group (a) and Ketone-group (b).
Because the ENV of oxygen (χo = 3.44, Pauling value) that is a strong electron acceptor is greater than that of carbon (χc = 2.55), once the Ketone-group has been formed on the surface of carbon, the periphery or hang electron of carbon particle or matrix has to move towards the atom of oxygen. As a result of move, the originally stable, symmetrical, balanceable and lower energy potential electron orbitals of the carbon matrix or particle are distorted,or regrouped, or leapt to higher energy potential orbitals, the hang or peripheral electronic movement must be affected by the internal electronic hamper and restricting each other.

Scheme 2. A scheme of the breakup of Ethenone-group (a) and Ketone-group (b).
That is to say, the movements of the periphery electrons or the suspended electrons are definitely not free, it is constrained by an internal atomic group of particle or matrix. The larger the particle size, the greater the drag force, and the slower reaction rate, on the contrary, the faster the reaction rate. This is common sense. Because of the distortion or regrouping implies an increase in the internal energy of carbon matrix, so it becomes more unstable network orbitals or higher energy potential orbitals. To maintain the original structure and lower energy potential network orbitals, the carbon matrix must be strongly to bind the peripheral electron that moved towards oxygen atom all the time. As a result, a fierce scramble for electrons between carbon and oxygen atoms has appeared. At this moment, any chemical or physical method that can donates the electron towards carbon matrix will facilitates the restoration of orbitals being distorted, and therefore reduces the energy required to break up C-C bond, and therefore consequently enhances the reaction rate of carbon with CO2. Based on this reasoning, any substances that can donate electron towards carbon should be a catalyst. Conversely, any substances that can seize the electron from carbon must be a poison.
Scheme 3 [6] is an imaginative picture; (a) is a catalyzing process, and (b) is a poisoning process. The picture shows clearly that the catalyst can make the recovery of orbital deformed, and the poison can make the orbits deformed further deformation.
Once the Ketone-group has dissociated, the carbon bond C-C has broken, then the gaseous CO molecule has formed and escaped out, an extra electron will arisen in the carbon matrix, the unnecessary electron results in the decrease of ENV of the carbon. Consequently, the extra electron which is donated by catalyst has to return to the catalyst, so that the electronic orbitals being distorted of both catalyst and carbon matrix can restore to the original orbitals, and the catalytic cycle has completed. The electrons of catalyst flow continuously to circulate back and forth between the catalyst and carbon. The electron orbitals in the carbon matrix or particle undergoes continuously the deformation-recovery
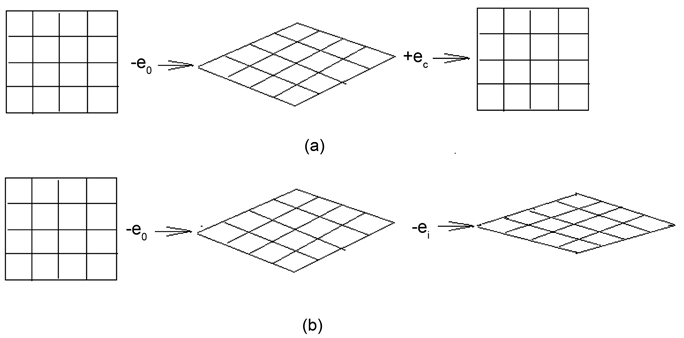
Scheme 3. The imaginative catalyzing and posting model: (a) catalyzing; (b) poisoning; -e0, -ei―electron is seized by oxygen or inhibitor. +ec―electron is donated by catalyst or promotor.
cycle. This is the essence of EODRM or ECDAM.
For iron-based ammonia synthesis catalysts, during the production process, when the nitrogen molecules in gas phase diffuse to the surface of the iron and have been absorbed by iron, because of the iron has the ability to tear nitrogen molecule bonds, the nitrogen molecules are torn apart by iron to form a complex ( ) on the iron surface.
When the complex has been formed on the iron surface and absorbed by iron. Because the ENV of nitrogen is greater than that of iron, the electrons of the iron have to move in the direction of the nitrogen atom. As a result, the electron orbital in the iron crystals or matrix has been distorted, the potential energy rises and becomes unstable. In order to maintain the original low energy state of the electronic motion orbital, the iron crystals or the iron matrix must be trying to pull back electrons which have moved to the nitrogen atom. As a result, a pull electron situation each another is appeared between the iron-nitrogen atoms. At this moment, any chemical or physical method that can donate the electrons toward the iron matrix or crystal, which is beneficial to the recovery of the electron orbital distorted in the iron matrix, is beneficial to the destruction of the complex and to increase the reaction speed. The promoter can donate its electron towards the iron, and the poisons are the opposite.
The difference between the catalytic reaction of carbon gasification and that of ammonia is that, for carbon-catalyzed gasification, the cyclic electron donate-accepting phenomenon occurs between carbon and catalyst, whereas for the iron-based ammonia synthesis catalysis reaction, the cyclic electron donate-accepting phenomenon occurs between iron and promoter, and after reaction the former appears that the carbon bond has been broken, while the latter is that the iron bond of catalyst does not be broken. But the direction of electron movement is the same, namely, the electron moving direction in these catalyzing processes is from catalyst towards reactants or reaction products.
From the above, the electronic orbital deformation-recovery cycle caused by the electron movement is the core idea of EODRM. The power that causes the electrons to move is the ENVs difference (C-O, Fe-N). The same is true for noble metal catalysts. Hence the ECDAM or EODRM also applies to other heterogeneous catalytic reactions. The author considers that ECDAM or EODRM is a basic theory about heterogeneous catalysis maybe.
It is obvious that the EODRM is completely different from the Electron Transfer Mechanism-ETM which was used in literature, the ETM only considers the electronic mobile of peripheral electron, but it doesn’t take into account the electronic mobile influence on electron orbital deformation of matrix, and it is more without to consider to electron cyclic donate-accept at the catalyzing process.
The electronic movement orbital within crystal just as like the sun, the moon, and the star, has a fixed orbit, different orbital, have different crystal forms such as Fe, C, Al2O3 allotropicity. The peripheral electron move can lead to deformation of the electron orbital in the crystal or matrix. As for the electron orbit shape, that is quantum chemistry.
Based on the inference above, naturally, three main arguments can be achieved.
3. Three Main Arguments about EODRM or ECDAM
3.1. There Is a Demarcation between the Catalyst and the Inhibitor or Poison
For the catalyzed gasification reaction of carbon, the demarcation is the ENV of carbon or the position of carbon at the Periodic Table of Elements. Any substances or materials that is less ENV than carbon, which can contribute electrons to carbon, is a catalyst. The carbon ENV is 2.55 (Pauling). Hence, it is very natural that these elements or compounds that the ENVs are less than 2.55 should be catalyst. Conversely, those elements or compounds that the ENVs are greater than 2.55 must be an inhibitor. Carbon is naturally the demarcation.
In the Periodic Table of Elements, the ENVs of elements increase gradually from the right to the left in a given period; and decrease from top to bottom in a given group. Therefore, all elements that are located at the right and the bottom of carbon should be catalysts, such as alkali metal, alkaline earth metal, transition metals, and noble metals; while those that are located at the left of carbon should be inhibitors, such as F, Cl, O, N etc.
It is worth noticing that the size arrangement of ENVs of transition elements is different from the main group elements. According Pauling’s marked values, the ENVs of Fe, Co, Ni, Cu, Mn, Cr and Ti which are located above the Pt group at the Periodic Table are less than that of Pt group elements, on contrary to main group elements; for this reason, Fe, Co, Ni and Cu etc. have became a promoter for noble metal reactants.
As for Fe based ammonia synthesis catalyst, the demarcation between promoter and poison is the ENV of Fe or the position of Fe at the Periodic Table. Fe’s ENV is χFe = 1.83, hence, the elements or compounds that the ENVs are less than 1.83 should be a catalyst, and those elements that the ENVs are higher than 1.83 should be a poison. The right elements of Fe should be a catalyst such as Alkali, Alkaline earth metal, and the left elements of Fe should be a poison such as C, N, S, N, O, and F etc. Co, Ni, and Cu have become a poison. Fe is naturally the demarcation.
As for the noble metal catalyst, the demarcation is the ENVs of noble metal or their position at the Periodic Table. Alkali and Alkaline-earth metals is still the promoter, and the F, Cl, O, N, S, and C etc. is still the poisons, while the Fe, Co, Ni, Cu just became a promoter.
The most powerful proof about demarcation is Both Cu and Ni. At the Periodic Table, the Ni, Cu are located between Fe and C. Based on ECDAM, they should be catalysts for gasification reaction of carbon, while they just are a poison for the iron base ammonia synthesis catalyst. Long-term production practice has proved that the Cu, Ni is a poison for Fe base ammonia synthesis catalyst, while they just are catalysts for gasification reaction of carbon based on many experiments.
Table 2 is some elements ENVs (Pauling).
When it comes to Cobalt, it is located between Fe and C; it should be a poison on the Fe reactant, although its poison is small. Due to the Co is a red hard element, it can hinder the iron grain growing at high temperature, and therefore keeps the Fe reactant to possess high specific surface, and therefore increases the raction activity of Fe. Similar to alumina on the Fe reactant, the advantage of physical properties has covered up the disadvantage of chemistry properties. If the ENVs of iron and cobalt is only compared, the cobalt should be a poison.
3.2. The Relative Activities of Catalyst Depend on Its ENVs
Figure 1 [6] shows the relationship between the ENVs difference and catalytic activity.
In Figure 1, Δχcm-ip = χcm − χip
χcm―ENV of carbon or metal catalyst
Table 2. Some elements electronegativities value (Pauling).
From: http://zh.Wikipedia.2012
Figure 1. Relationship between electronegativity differences and catalytic activities.
χip―ENV of inhibitor, poison, promoter, catalyst or support.
From Figure 1, when χcm = χip, Δχcm-ip = 0, in that case the substance added to carbon is neither catalyst nor poison. Therefore, the “0” point is naturally the demarcation between the catalyst and the inhibitor or poison.
When χcm > χip, Δχcm-ip > 0, if these substances are added to carbon, they must be catalysts. The larger the positive differences +Δχcm-ip is, the higher the activity will be. Conversely, the larger the negative differences −Δχcm-ip is, the higher the poisoning ability will be. For example: Fe, Co, Ni, and Cu, their difference values Δχc-pi are: 0.72, 0.67, 0.63, 0.65, so that they are all active catalyst; their relative activities are Fe > Co > Cu > Ni. As for Cl, O, N, S, the Δχcm-ip are: −0.61, −0.89, −0.49, −0.03, they are all a poison, the relative poisoning abilities are O > Cl > N > S.
According to trends of the ENV size of elements at the Periodic Table, the sequence of relative catalytic activity of elements should be alkali-metals > alkaline-earth metals > transition metals > noble metal on the catalyzed gasification reaction of carbon. As for the alkali-metals, the order of relative activity should be Cs > Rb > K > Na > Li. As for alkaline-earth metals, the order should be Ba > Sr > Ca > Mg > Be. As for transition metals, the order should be Fe > Co > Cu > Ni.
As for Fe catalyst, ΔχFe-pi = 1.83 − χpi, all substances with ENVs less than 1.83 should be promoters, while those substances with ENVs higher than 1.83 should be poison. Alkali metal, alkaline-earth metal are still promoter; F, Cl, O, N are still poison; but the Co, Ni, Cu, C have became a poison.
As for noble metal catalysts, Δχ = 2.28 − χip, all substances with ENVs less than 2.28 should be promoter such as Fe, Co, Ni and Cu; While those substances with ENVs higher than 2.28 should be poison. Alkali metal, alkaline-earth metal are still promoter; F, Cl, O, N are still poison.
It is important to notice that the ENV of Al2O3 is about 2.5. Therefore; Al2O3 is poison on the Fe, Pt, Ru catalyst. However
; , it showed that the poisoning ability of Al2O3 on Fe catalyst will be far higher than that on noble metal catalyst. The ENV of Carbon is 2.55; hence, it is also a poison on the Fe, Pt, and Ru catalyst. Therefore, it is not suitable to use alumina or active carbon as the promoter of iron based ammonia synthesis catalyst or the support of noble metal catalyst such as fuel cells platinum catalyst.
In 1985, Aika et al. [7] has studied the effect of different metallic oxide supports on the activity of Ru. Figure 2 shows the relationship between the relative catalytic activities Of the Ru and the ENVs of the support. From Figure 2, we can get the following conclusion:
1) The catalytic activity of Ru is in inverse relation to the ENVs of supports. The lower the ENVs of support are, the higher the catalytic activity will be. Activity sequence of supported catalyst without alkali promoter is as follows: Ru/CaO > Ru/MgO > Ru/BeO > Raney Ru > Ru/AlO > Ru powder > Ru/AC.
2) The different chemical states of the support have appeared different catalytic activity of the Ru.
3) Al2 O3 and active carbon are a poison on the Ru catalyst due to the catalytic activity of Ru with Al2O3 or AC as support are less than the activity of Ru with Ru powder as support.
The three results mentioned above are completely accordance with the estimate of ECDAM or EODRM.
In 1975, Mckee et al. [8] have measured the effects of alkali-metal carbonates on the catalyzed gasification of graphite powder in CO2 by weight-loss measurement. The test results are presented in Figure 3. The relative catalytic activities decrease in the following order Li2CO3 > Cs2CO3 > Rb2CO3 > K2CO3 > Na2CO3; with the highest activity being exhibited by the Li2CO3.
Mckee [9] has also studied the catalytic effects of alkaline-earth metal carbonates on the gasification reaction of pure graphite in CO2 between 700˚C and 1000˚C, and he proved that the order of relative catalytic activity is BaCO3 > SrCO3 > CaCO3 > MgCO3.
Figure 2. Relationship between ENV of support materials and activity of Ru in Ru base ammonia synthesis catalyst (580˚K, N2 + H2 = 80 Kpa) From [7] .
Figure 3. Effect of alkali-metal carbonates on the gasification of graphite powder in CO2. Weight changes vs. temperature. Heating rate = 100c. gas rate = 400 ml・min−1.
In Fisher synthesis ammonia reaction, the catalytic activities of Fe increase if the promoter of alkali-metal is added to it. The activities of the promoter decrease in the following order Rb > Cs > K > Na > Li [10] . Li and Na have almost no activity.
As mentioned above, the order of activities of alkali-metal salts and alkaline-earth metal carbonates as catalyst or promoter is consistent with judgment of ECDAM or EODRM except Li2CO
In 1976, Kayembe and Pulsifer [11] have studied the catalytic effect of various salts on the kinetics of gasification of coal chars by steam between 600˚C and 850˚C at atmospheric pressure. Table 3 shows that the observed order of activities is K2CO3 > Na2CO3 > Li2CO3 > KCl > NaCl > CuO, Fe2O3 and CaO are totally ineffective.
The order of relative catalytic activity of various salts with different cation and anion coincides well with the prediction of ECDAM.
Walker et al. [12] have studied the relative catalytic activities of Fe, Co, and Ni on the catalyzed gasification reaction of graphite with CO2 at 807˚C - 1030˚C by magnetic susceptibility. The test results show that all Fe, Co, and Ni are very active catalysts, and the order of relative activities is Fe > Co > Ni. According to ECDAM or EODRM, it is inevitable.
In 1970s, Jin etc. [13] has studied the effect of Fe, Co, Ni, Cu, Ag and SiO2 on the reducing rate of ferric oxide by coke powder as reducer for checking ECDAM or EODRM proposed by author. The experimental results can be described as kill two birds with one stone. On the one hand, It shows that the
Table 3. Effects of catalysts on the steam gasification of coal char.
Source: From Ref. [12] .
CRMM or OTT is not credible. Because there is a large amount of reducer carbon in the reaction tank, Fe, Co, Ni, Cu, Ag and so on are unlikely to have the oxidation-reduction cyclic reaction, and, on the other hand, it shows that the ECDAM or EODRM is credible. Because of the experimental results on the relative active of Fe, Cu, Ni, Co, Ag and SiO2 poison were basically consistent with ECDAM or EODRM’s judgment.
In 1980s, Hong et al. [14] have studied the effects of K2CO3, W, S and SiO2 on the rate of carbon dissolving into austenite Fe in the sintering process of iron-graphite mixture compact. The micro-metallographic pictures obtained after sintering shows that the K2CO3 and W are very active catalyst, while the S and SiO2 are poison, and that the poisoning ability of SiO2 seems slightly higher than that of the S.
Du and Yang [15] found that the Al2O3 and B2O3 appeared the negative catalysis on the carbon gasification. While poisoning ability of the B2O3 is more than that of the Al2O3. According to ECDAM or EODRM, it is inevitable.
It is well known that all oxidation inhibitors of carbonaceous material are completely composed from those elements that have higher ENVs than that of the carbon such as F, Cl, O, N, P. Their molecular ENVs such as CO2, CCl4, CCl
3.3. The Activity of the Catalyst Depends upon Its Chemical State
The catalyst chemical state is very important and very complex as well. Different chemical states will have different ENVs, in consequence it will appears different catalytic activity. For example:
1) Iron catalyst
Iron has three chemical states, namely Fe, FeO (wüstite), and Fe2O3 (Fe3O4 is a solid-solution of FeO + Fe2O3). Based on the electronegativity equilibrium principle, the molecule ENVs of FeO and Fe2O3 can be estimated by taking the geometric means of ENVs of all atoms before combination; therefrom, the resulted values are χFeO = 2.51, . When Comparing with carbon (χc = 2.55), ; ; , it can concluded that the Fe should be an active catalyst, and FeO must be inactive or small active, while Fe2O3 must be a poison for the carbon gasification reaction.
King and Joes [16] studied the activation and deactivation cycles of Fe catalyst during reaction of coke with CO2 at 950˚C. Figure 4 shows reactivation in one atm. of H2 at 950˚C. Activation is achieved by using H2.
Tayor and Neville [17] proved also that the iron oxide is entirely ineffective at low temperature.
Rakszawski et al. [18] concluded that for Fe to be an effective catalyst in the C-CO2 reaction, it must be free of dissolved carbon. It is shown that the dissolved carbon is also a poison on the Fe synthesis ammonia catalyst.
2) salts catalyst
・ The salts such as carbonates are commonly used as catalysts. In general, the alkali metal carbonates have more active than other salts such as sulfates, nitrate or halides. According to ECDAM, this is inevitable as well, due to the molecule ENVs of carbonates are less than that of sulfate, nitrate and halides. For various salts, the relative catalytic activities depend upon the cation and anion by which the compound has formed. As to a group of compounds, if the anion in the compound is the same, then molecule ENVs will decrease with the decrease of ENVs of cation group, while the relative catalytic activity will increase. For example, in the following chloride: χLiCl = 2.13, χNaCl = 1.93, χKCl = 1.77, χRbCl = 1.74 [19] . We can judge that their relative activities should be increase gradually in the following order: LiCl < NaCl < KCl < RbCl. For alkali metal carbonates, the relative activities should be Cs2CO3 > Rb2CO3 > K2CO3 > Na2CO3 > Li2CO3. The activity of LiC2O3 is the smallest. For alkaline-earth metal carbonates, the relative activities should be: BaCO3 > SrCO3 > CaCO3 > MgCO3 > BeCO3.
Figure 4. Activation and deactivation cycles of Fe catalyst during reaction of coke with CO2 activation is achieved using H2. Source: from [16] .
If the cation of a group of salts is the same, the molecule ENVs will be increase with the increase of ENVs of anion, and the relative activity will be gradually decrease. For example: χkF = 2.02, χKCl = 1.77, χKBr = 1.70, χKI = 1.62 [19] , the relative activities should be KI > KBr > KCl > KF.
Table 4 shows also that:
1) Different supports appeared different catalytic activities. Catalytic activities of Al2O3 as support are less than that of Ru with MgO as support.
2) Different promoters appeared different catalytic activities. Catalytic activities of Ru with Cs2CO3 as promoter are higher than that of the CsNO3 as promoter at the same support.
The two test results mentioned above are completely consistent with ECDAM.
As has mentioned before, the catalytic activity of Li2CO
We can also cite many examples to illustrate the effect of different chemical state on the catalytic activity, due to space limitation, no more detailed description.
The three main arguments mentioned above have been verified and are credible, but when examining the actual production situation, it is finding many
Table 4. The effect of several supports and promoters on the Ru catalytic activity from [20] .
problems, for example;
The biggest problem of iron-based ammonia synthesis catalyst is that the alumina is a poison on the iron catalyst. The alumina appears all a poison on the C, Ru, Fe, but the poisoning on Fe catalyst is biggest. Due to , , . The ENV values of alumina and carbon found from the data are all 2.55, but the ENV of the alumina should be greater than the ENV of the carbon, namely, , due to according to the experimental results that alumina is poison to carbon. As for high energy consumption in Synthetic ammonia production, the authors believe that may be caused by alumina poisoning.
The biggest problem in the automotive exhaust gas purification catalyst is that the support material Cordierite (2MgO-2Al2O3-5SiO2) is an acidic materials, it is a poison on the noble metal catalyst, to use it as the support material will consume more noble metals. Comparing with metal roll film honeycomb, with the ceramics honeycomb as support, global consumption of noble metals is more than 100 tons. It is gratifying that the ceramic honeycomb support has been replaced by a metal roll honeycomb support, and the second support alumina has been replaced by rare earth oxides, and this development is in good agreement with ECDAM’s judgment.
Newly developed ruthenium-based ammonia synthesis catalyst, active carbon obtained after high temperature graphitization is used as Su support, it is clearly not appropriate. Because carbon is poison to ruthenium. If using it to do the support, it must consume more noble metal ruthenium. On fuel cell Platinum catalyst, to use the graphite or carbon black as a support, it is also not appropriate. At present, it’s going in the direction of C60, but whether it is appropriate or not. On for diesel vehicle exhaust soot filter, using acidic cordierite ceramic materials, it’s obviously not appropriate. The regeneration of the filter will be slow. So, using ECDAM or EODRM to check the current production, we will find a lot of unreasonable places.
4. Conclusions
For more than 50 years, the author has repeatedly considered and examined the credibility of the ECDAM or EODRM. Therefore we believe that the derivation of the theory is very natural, no unreasonable inference was found.
Three main arguments have been experimentally validated by author, especially the experimental results of other’s scholar’s. In view of the ECDAM or EODRM proposed by author can satisfactorily account for many experimental results, including catalysis and poisoning, the arrangement of activity size of various catalysts, support material selecting. It has not yet found with a big contradiction. Therefore, the author believes that the ECDAM or EODRM is credible
It is well known that there are many allotrope such as αFe・γFe; αAl2O3, γAl2O3; graphite, diamond, grapheme and so on. The essence of allotropicity is that there are different electronic orbital inside crystals. The core idea or advance thinking of the ECDAM or EODRM is the repeated deformation-recovery of electron orbital in the carbon or metallic catalyst crystal or the cyclic donate-accept of electron between the carbon-catalyst or Fe-promoter caused due to chemical reaction. The mechanism of cyclic catalysis is completely different from CRM. It has no chemical reaction, no crystal restructuring. According to this idea, the catalysis phenomenon should be physical rather than chemical or complete chemical, at least for heterogeneous catalysis. Perhaps this theory can be used to explain photocatalysis and electrocatalysis, although author is ignorant about the area of electrocatalysis and photocatalysis.
As knowledge is limited, the inappropriate ideas are inevitable; the different idea is welcome.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Jin, J.M. and Bao, W.F. (2018) Study on the Mechanism of Heterogeneous Catalysis (4)―Electron Cyclic Donate-Accept Catalysis Mechanism- ECDAM or Electron Orbital Deformation-Reversion Cyclic Catalysis Mechanism-EODRM. Journal of Materials Science and Chemical Engineering, 6, 15-30. https://doi.org/10.4236/msce.2018.68003
References
- 1. Jin, J.M. and Bao, W.F. (2017) Study on the Mechanism of Heterogeneous Catalysis (1) The Measurement of Equilibrium Pressure of Reaction BaCO3 + C = BaO + 2CO, the Chemical States of Fe in Reaction Tank, and Chemical Reaction Model Catalytic Cycle Mechanism (CRMM). Journal of Materials Science and Chemical Engineering, 5, 90-100. https://doi.org/10.4236/msce.2017.57010
- 2. Jin, J.M. and Bao, W.F. (2018) Study on the Mechanism of Hetrogeneous Catalysis (2) The Relative Catalytic Activities of Fe, Co, Ni, Cu, Ag and SiO2 in the Carbon Catalyzed Gasification Reaction. Journal of Materials science and Chemical Engineering, 6, 191-201. https://doi.org/10.4236/msce.2018.64018
- 3. Jin, J.M., Hong, T. and Wu, J.Q. (2017) Study on the Mechanism of Heterogenrous Catalysis (3) The Catalysis of W, Mo, S and SiO2 on Carbon Dissolving Mechanism of Carbon Dissolving in the Iron-Graphite Compact. Journal of Materials Science and Chemical Engineering, 5, 77-86. https://doi.org/10.4236/msce.2017.511007
- 4. Jin, J.M. (1977) Iron Production for 10 Years. East China P/M Conference Data. Xiaoshan, China.
- 5. Yesin, O.A. and Geld, F. (1962) Physical Chemistry of High Temperature Metallurgical Process. Part 1. Metallurgy Press, Sveldrovsk, 123.
- 6. Jin, J.M., Bao, W.F., Wu, J.Q. and Zhang, D.M. (2007) Support Material and Noble Metal Catalyst. Materials Review, 2, 45-50.
- 7. Aika, K., Ohya, A., Ozaki, A., Inoue, Y. and Yasumoli, I. (1985) Support and Promoter Effects of Ruthenium Catalyst. Journal of Catalysis, 92, 305-311. https://doi.org/10.1016/0021-9517(85)90265-9
- 8. McKee, D.W. and Chatterji, D. (1975) The Catalytic Behavior of Alkali Metal Carbonates and Oxides in Graphite Oxidation Reaction. Carbon, 13, 381-390. https://doi.org/10.1016/0008-6223(75)90006-8
- 9. McKee, D.W. (1980) Catalytic Effects of Alkaline Earth Carbonates in the Carbon-Carbon Dioxide Reaction. Fuel, 59, 308-314. https://doi.org/10.1016/0016-2361(80)90215-X
- 10. Osaki, A. (1982) Catalyst Handbook. Chemical Industry Press, Bejing, 964.
- 11. Kayembe, N. and Pulsifer, A.H. (1976) Kinetics and Catalysis of the Reaction of Coal Char and Steam. Fuel, 55, 211-216. https://doi.org/10.1016/0016-2361(76)90090-9
- 12. Walker Jr., P.L., Shelef, M. and Anderson, R.A. (1968) Catalysis of Carbon Gasification. In: Walker Jr., P.L., Ed., Chemistry and Physics of Carbon, Vol. 4, Marcel Dekker, New York, 287-383.
- 13. Jin, J.M., Jian, G.Y., Reng, J.Y. and Zhu, C.Z. (1982) The Catalysis and Poison Mechanism of Mineral Impurities in Carbon on the Process of Carbon Reduction of Iron Oxide-ECDAT Validation. Powder Metallurgy Conference Data.
- 14. Hong, T., Wu, J.Q. and Jin, J.M. (1993) Study on the Mechanism of Carbon Dissolution and the Factors Affecting the Rate of Carbon Dissolution during Fe-C Alloy Sintering. Master’s Thesis, Shanghai Research institute of Materials, Shanghai.
- 15. Du, H.G. and Yang, J.H. (2002) Catalysis of Metallurgical Coke Solution Reaction by Minerals. Iron Making, 21-22, 4-24.
- 16. King, J.G. and Jones, J.H. (1931) The Reactivity of Coke. Journal of the Institute Fuel, 5, 39-55.
- 17. Taylor, H.S. and Neville, H.A. (1921) Catalysis in the Interaction of Carbon with Steam and with Carbon Dioxide. Journal of the American Chemical Society, 43, 2055-2071. https://doi.org/10.1021/ja01442a009
- 18. Rakszawski, J.F., Rusinko Jr., F. and Walker Jr., P.L. (1962) Penn State, 1961. Vol. 2, Pergamon Press, New York, 243-250.
- 19. Zhang, G.Y. (1978) Shanghai Library Holding.
- 20. Lin, H. (2000) Recent Advances in Ruthenium-Based Low-Temperature and-Pressure Ammonia Synthesis Catalyst. Industrial Catalysis, 8, 3-11.






