Journal of Materials Science and Chemical Engineering
Vol.03 No.12(2015), Article ID:62344,8 pages
10.4236/msce.2015.312013
First Principles DFT Study of Hydrogen Storage on Graphene with La Decoration
Yuanyuan Li, Yiming Mi*, Gaili Sun
School of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai, China

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 November 2015; accepted 26 December 2015; published 29 December 2015
ABSTRACT
The properties of hydrogen storage on graphene with La decoration are investigated using a first- principles plane-wave pseudopotential method based on the density functional theory in this paper. The clustering problem of La decorated graphene is considered and B doping can solve it effectively in theory. We obtain the stable geometrical configuration of the modified system and the properties of hydrogen storage are excellent. It can absorb up to 6 H2 molecules with an average adsorption energy range of −0.529 to −0.655 eV/H2, which meets the ideal range between the physisorbed and chemisorbed states for hydrogen storage. Furthermore, it is proved that the existence of La atom alters the charge distribution of H2 molecules and graphene sheet based on the calculation and analysis about the electronic density of states and charge density difference of the modified system. La atom interacts with hydrogen molecules through Kubas interaction. Thereby, it improves the performance of graphene sheet for hydrogen storage. The modified system exhibits the excellent potential to become one of the most suitable candidates for hydrogen storage medium at near ambient conditions with molecule state.
Keywords:
Graphene, La Decoration, First-Principles, Hydrogen Storage

1. Introduction
Due to the high energy density and clean fuel source, hydrogen as a promising alternative energy has attracted great attention all over the world [1] [2] . However, there is a major challenge to find an excellent hydrogen storage medium under normal conditions [3] [4] . The desirable system for storing hydrogen should have high gravimetric and volumetric densities at near room temperature and ambient pressure [5] . Moreover, the US Department of Energy (DOE) has set a target for the efficient materials to store hydrogen, which requires gravimetric and volumetric densities of 7.5 wt% and 70 g/L, respectively, an operating temperature between −40˚C and 60˚C and the safe, durable (1500 operational cycle life) storage system [6] [7] . In addition, the ideal adsorption energy should be in the range of −0.2 - −0.7 eV/H2 [8] . This range of energy is intermediate between the physisorbed and chemisorbed states. It is ideal for hydrogen storage under ambient pressure and temperature. To achieve this, a variety of materials about hydrogen storage have been investigated by several theoretical and experimental studies. The development of metal hydride starts early [9] [10] and other materials begin to be developed rapidly. But it is still an open-ended problem to find suitable novel materials for hydrogen storage which can meet the requirements.
In recent years, carbon nanomaterials have been widely studied for hydrogen storage [11] - [13] . Due to the light weight, high specific surface and unique properties, graphene has become a promising material to store hydrogen [14] . However, similar to other carbon nanomaterials, the interaction between graphene and H2 is through weak van der Waals forces with low adsorption energy ~−0.1 eV/H2 at ambient conditions [15] - [17] . Thus, it is meaningless for using pristine graphene to store hydrogen. Recently, to improve the binding energy between graphene and H2 molecules, many studies have been devoted to modifying the surface of graphene with transition metals (TMs) [18] - [21] . The TM atom interacts with H2 molecules through Kubas [22] interactions due to the empty d orbitals. It increases the binding ability of hydrogen with graphene. However, because of the high cohesive energy, TMs tend to form cluster on the surface of the carbon nanostructures easily [23] [24] . My understanding is that, typically, TM-TM is much stronger than grapheme-TM. Therefore, it is difficult to achieve uniformly coated monolayer of TM atoms experimentally. This is unfavorable for the capacity of hydrogen storage.
Numerous studies have shown that, boron (B) doped carbon nanomaterials can prevent the TMs clustering availably. B acts as a strong charge acceptor and consequently enhances the adsorption energy of foreign atoms on the surface of the carbon nanostructures due to its empty p orbital [25] . Nachimuthu et al. [26] investigated that TM atoms such as the Ni, Pd and Co atoms are suitable for decorating B-doped graphene surface, which can be adsorbed stably on the surface of graphene. Besides, B can be easily incorporated substitutionally in the hexagonal structure of graphene and change the electronic structure of graphene effectively to prevent the clustering of foreign atoms. It has been proved that B doped graphene has been successfully synthesized experimentally [27] [28] .
Because of the chemical activity and small cohesive energy, the La-Ni alloy has been used as hydrogen storage materials; La has great potential in the aspect of storing hydrogen. We focus primarily on the stable geometrical configuration of graphene with La decoration, the adsorption of hydrogen and the effects of La for the process of hydrogen storage using a first-principles plane-wave pseudopotential simulations based on the density functional theory in this paper.
2. Simulation Details
All the density-functional theory (DFT) calculations are performed using a plane-wave basis set with the projector augmented plane wave (PAW) method as implemented in the Vienna ab initio simulation package (VASP) [29] - [31] . We select the local-density approximation (LDA) as the exchange and correlation potential for our work. Although no DFT functional describes accurately all the characteristics of molecular interactions, especially van der Waals (vdW) interactions [32] - [34] , the prediction of the physisorption energies of H2 on the surface of carbon nanomaterials as based on the LDA are in good agreement with experiments [35] - [37] . More than that, the overestimate of the binding energy by LDA is almost compensated by the ignored van der Waals interactions [38] [39] . The electron wave functions are expanded by plane waves with a kinetic energy cutoff of 450 eV to attain the required convergence. All of the self-consistent loops are iterated until the total energy difference of the systems between the adjacent iterating steps is less than 10−7 eV. The Brillouin zone is sampled by 5 × 5 × 1 mesh points in k-space based on Monkhorst-Pack scheme [40] . The effective range of the kinetic energy cutoff and the validity of the mesh density used in this calculation are determined by a convergence test using the theoretically estimated lattice constants of the pristine graphene, 2.46 Å. To avoid the interactions of adjacent slabs, the vacuum space of 20 Å is introduced for 4 × 4 supercell which contains 32 carbon atoms.
3. Results and Discussions
3.1. The Decoration of La
Initially, we assume that La atoms distributed uniformly on graphene sheet and study the adsorption behavior of La on pristine graphene. The adsorption energy of La on graphene is defined as
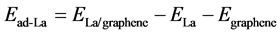 (1)
(1)
where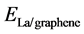 ,
, 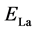 , and
, and 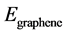 are the total energies of the La-decorated graphene sheet, an isolated La atom, and pristine graphene sheet, respectively. Similarto other rare earth metal atoms attaching on carbon nanomaterials [41] [42] , the favorite adsorption site for an isolated La atom on the surface of graphene is the center of a hexagonal ring. Meanwhile, the adsorption energy of La on grapheme
are the total energies of the La-decorated graphene sheet, an isolated La atom, and pristine graphene sheet, respectively. Similarto other rare earth metal atoms attaching on carbon nanomaterials [41] [42] , the favorite adsorption site for an isolated La atom on the surface of graphene is the center of a hexagonal ring. Meanwhile, the adsorption energy of La on grapheme 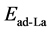 is −2.99 eV which is calculated by Formula (1). However, the cohesive energy of bulk solid phase La (−4.47 eV/atom) is higher than the calculated adsorption energy of La atom on pristine graphene. It indicates that La atoms tend to cluster.
is −2.99 eV which is calculated by Formula (1). However, the cohesive energy of bulk solid phase La (−4.47 eV/atom) is higher than the calculated adsorption energy of La atom on pristine graphene. It indicates that La atoms tend to cluster.
In order to have a stable and uniform decoration of individual La atoms on graphene, we introduce B atom to 4 × 4 × 1 graphene supercell. The adsorption energy of La on B-doped graphene is calculated by
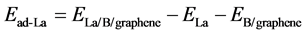 (2)
(2)
where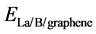 ,
, 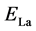 , and
, and  are the total energies of the La decorated B doped graphene sheet, an isolated La atom, and B doped graphene sheet, respectively.
are the total energies of the La decorated B doped graphene sheet, an isolated La atom, and B doped graphene sheet, respectively.
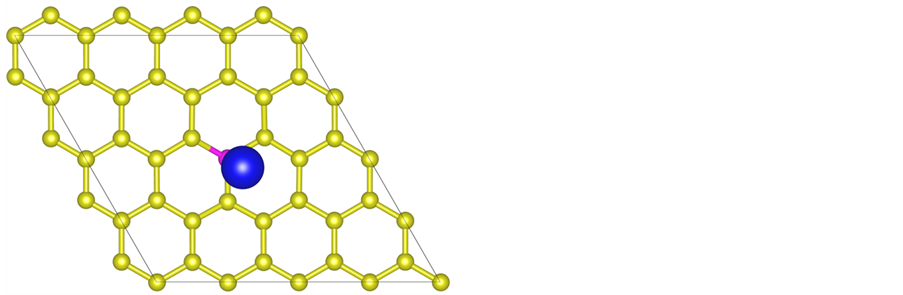
We obtain the stable configuration after the modified system is fully relaxed. As shown in Figure 1, the La atom tends to stay near the top site above the B atom and the distance between La and B atom is 2.43 Å. Simulation results show that the adsorption energy of La atom on B-doped graphene sheet is −4.59 eV/atom which is higher than the cohesive energy of bulk La (−4.47 eV/atom). It turns that B doping can prevent the cluster of La atoms on graphene sheet successfully in theory.
3.2. The Adsorption of Hydrogen on La/B/Graphene
To explore the performance of the active structure La/B/Graphene for hydrogen storage, we investigate the behavior of H2 molecules adsorbed on La-decorated graphene. The finally optimized configurations after full relaxation of all H2 molecules are shown in Figures 2(a)-(f). The Figures 2(a)-(f) show that H2 molecules always take the sites near La atoms and the vertical distance between the first H2 molecule and graphene sheet is 2.50 Å, other H2 molecules have similar distance with graphene. It should be noticed that the average bond length dH?H is about 0.85 Å, which is elongated compared to the length 0.74Å in gas-phase H2 molecules. From all configurations we can also find that there is slight distortion especially in-plane distortion to the graphene as displayed in the side view of Figure 2. The similar phenomenon can be observed from the adsorption of other 3d-transi- tion metals which induces lattice distortion in the graphene layer [43] .
To examine the stability that H2 molecules adsorbed on the modified graphene, we calculate the average adsorption energy of H2 molecules by Formula (3)
Figure 1. The stable structure of La decorated graphene sheet, where yellow, rose and gray balls stand for C atom, B atom and La atom, respectively.
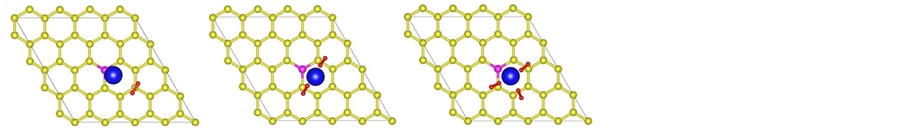
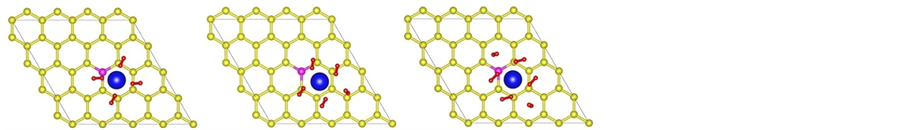
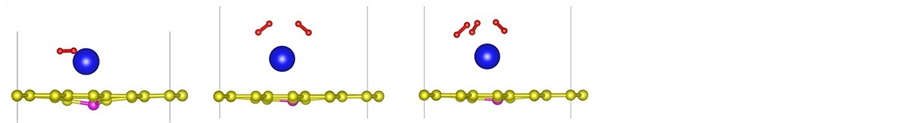
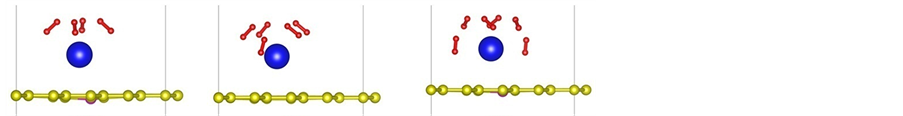
Figure 2. (a)-(f) depict the optimized structures of H2 molecules adsorption on La/B/grapheme (I: top view, II: side view. Red balls stand for H atom).
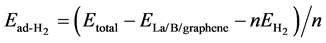 (3)
(3)
where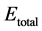
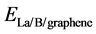
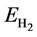
3.3. The Influence of La on Hydrogen Storage
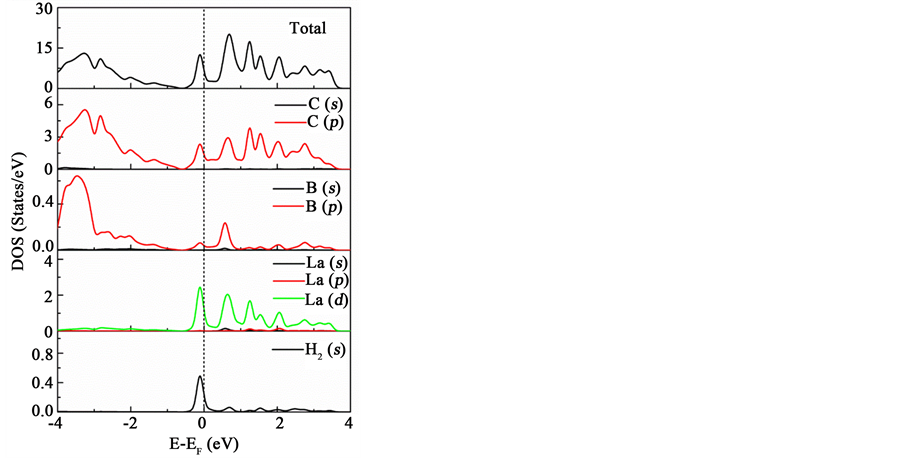
In order to get a better understanding about the influence of La atom on the process of hydrogen storage, we analyze the interaction between La atom with graphene and H2 molecules. B atom can accept charge from C atoms and enhance the adsorption energy of La atom on the surface of graphene due to its empty p orbitals. Then, the La atom can be adsorbed stably. Furthermore, we analyze the total and partial density of states for the system 1H2/La/B/Graphene as displayed in Figure 3. It clearly shows that there are several overlaps of main peaks of s orbitals of H2 molecule and the d orbitals of La atom at around −0.1 eV, indicating strong binding and hybridizing between orbitals of H2 molecule and La. It can be inferred that there is a small charge transfer from the
Figure 3. The total and partial density of states of 1H2/La/B/graphene system.
Table 1. The average adsorption energy of H2 molecules and the distances between La atom and graphene sheet.
σ-bonding orbitals of H2 molecule to the empty d orbitals of La atom. While the back donation from the filled states of d to the σ*-antibonding orbitals of H2 molecule enhances the interaction. It is a typical Kubas interaction [44] [45] . This charge transfer is responsible for the elongation of H-H bond length in H2 molecules and improves the adsorption ability between La atom and graphene sheet.
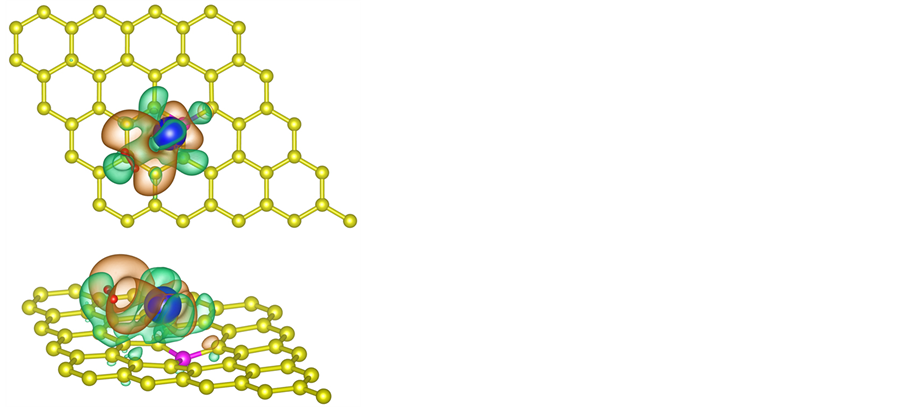
Meanwhile, to illustrate the electronic distribution of system, we calculate the charge density difference of the system caused by one H2 molecule which is determined by the following function
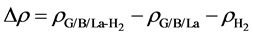
where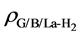
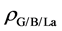
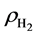
the sheet and isolated H2 molecule, respectively. The corresponding distribution of charge density difference is shown in Figure 4. It clearly shows that on the surface of sheet, the electron density increases in the region between H2 molecule and La atom and this causes the electron density of surrounding C atoms to decrease. This means that H2 molecule and La atom interact with each other, and there is also interaction between graphene sheet and La. The results are consistent with the analysis of the total and partial density of states. In conclusion, La atom plays an important role in the process of hydrogen storage.
4. Conclusion
We have performed first-principles electronic structure calculations to study the properties of hydrogen storage on graphene with La decoration. A stable and uniform decoration of individual La atom on graphene can be ob-
Figure 4. The electron charge density difference for 1H2/La/B/graphene system, where orange and green contours stand for high and low electron density region, respectively.
tained upon substitutional B doping. We find the stable geometry configurations of H2 molecules absorbed on graphene sheet with La decoration. The modified system can absorb 6H2 molecules with the adsorption energy rang from −0.529 to −0.655 eV/H2 which meets the ideal adsorption energy range for H2 molecules to be recycled at near ambient conditions. The calculation and analysis of electronic structure demonstrate that La atom interacts with H2 molecules and graphene through the charge-transfer. La atom becomes a bridge linking H2 molecules and graphene and improves the adsorption capacity of graphene sheet for hydrogen storage. Thereby, the modified system displays the outstanding potential to become one of suitably promising candidate materials for hydrogen storage.
Acknowledgements
This work was supported by Innovation Program of Shanghai Municipal Education Commission, China (10YZ172) and Subjects Construction Program of Shanghai University of Engineering Science, China (2012gp43).
Cite this paper
YuanyuanLi,YimingMi,GailiSun, (2015) First Principles DFT Study of Hydrogen Storage on Graphene with La Decoration. Journal of Materials Science and Chemical Engineering,03,87-94. doi: 10.4236/msce.2015.312013
References
- 1. Schlapbach, L. and Züttel, A. (2001) Hydrogen-Storage Materials for Mobile Applications. Nature, 414, 353-358.
http://dx.doi.org/10.1038/35104634 - 2. Tollefson, J. (2010) Hydrogen Vehicles: Fuel of the Future. Nature, 464, 1262-1264.
http://dx.doi.org/10.1038/4641262a - 3. Marban, G. and Vales-Solis, T. (2007) Towards the Hydrogen Economy? International Journal of Hydrogen Energy, 32, 1625-1637.
http://dx.doi.org/10.1016/j.ijhydene.2006.12.017 - 4. Miwa, R.H., Martins, T.B. and Fazzio, A. (2008) Hydrogen Adsorption on Boron Doped Graphene: An ab initio Study. Nanotechnology, 19, Article ID: 155708.
http://dx.doi.org/10.1088/0957-4484/19/15/155708 - 5. Jena, P. (2011) Materials for Hydrogen Storage: Past, Present, and Future. Journal of Physical Chemistry Letters, 2, 206-211.
http://dx.doi.org/10.1021/jz1015372 - 6. US Department of Energy’s Energy Efficiency and Renewable Energy Website.
http://www.hydrogen.energy.gov/annual_progress14_storage.html#c - 7. Yoon, M., Yang, S., Hicke, C., Wang, E., Geohegan, D. and Zhang, Z. (2008) Calcium as the Superior Coating Metal in Functionalization of Carbon Fullerenes for High-Capacity Hydrogen Storage. Physical Review Letters, 100, Article ID: 206806.
http://dx.doi.org/10.1103/physrevlett.100.206806 - 8. Ao, Z., Dou, S., Xu, Z., Jiang, Q. and Wang, G. (2014) Hydrogen Storage in Porous Graphene with Al Decoration. International Journal of Hydrogen Energy, 39, 16244-16251.
http://dx.doi.org/10.1016/j.ijhydene.2014.01.044 - 9. Schuth, F., Bogdanovic, B. and Felderhoff, M. (2004) Light Metal Hydrides and Complex Hydrides for Hydrogen Storage. Chemical Communications, 20, 2249-2258.
http://dx.doi.org/10.1039/b406522k - 10. Fukuzumi, S. and Suenobu, T. (2013) Hydrogen Storage and Evolution Catalysed by Metal Hydride Complexes. Dalton Transactions, 42, 18-28.
http://dx.doi.org/10.1039/C2DT31823G - 11. Yildirim, T. and Ciraci, S. (2005) Titanium-Decorated Carbon Nanotubes as a Potential High-Capacity Hydrogen Storage Medium. Physical Review Letters, 94, Article ID: 175501.
http://dx.doi.org/10.1103/PhysRevLett.94.175501 - 12. Shalabi, A.S., Taha, H.O., Soliman, K.A. and Abeld, A.S. (2014) Hydrogen Storage Reactions on Titanium Decorated Carbon Nanocones Theoretical Study. Journal of Power Sources, 271, 32-41.
http://dx.doi.org/10.1016/j.jpowsour.2014.07.158 - 13. Ataca, C., Aktürk, E. and Ciraci, S. (2009) Hydrogen Storage of Calcium Atoms Adsorbed on Graphene: First-Principles Plane Wave Calculations. Physical Review B, 79, Article ID: 041406.
http://dx.doi.org/10.1103/PhysRevB.79.041406 - 14. Stankovich, S., Dikin, D.A., Dommett, G.H.B, Kohlhaas, K.M., Zimney, E.J., Stach, E.A., Piner, R.D., Nguyen, S.T. and Ruoff, R.S. (2006) Graphene-Based Composite Materials. Nature, 442, 282-286.
http://dx.doi.org/10.1038/nature04969 - 15. Panella, B., Hirscher, M. and Roth, S. (2005) Hydrogen Adsorption in Different Carbon Nanostructures. Carbon, 43, 2209-2214.
http://dx.doi.org/10.1016/j.carbon.2005.03.037 - 16. Ritschel, M., Uhlemann, M., Gutfleisch, O., Leonhardt, A., Graff, A., Taäschner, C. and Fink, J. (2002) Hydrogen Storage in Different Carbon Nanostructures. Applied Physics Letters, 80, 2985-2987.
http://dx.doi.org/10.1063/1.1469680 - 17. Patchkovskii, S., Tse, J.S., Yurchenko, S.N., Zhechkov, L., Heine, T. and Seifert, G. (2005) Graphene Nanostructures as Tunable Storage Media for Molecular Hydrogen. Proceedings of the National Academy of Sciences of the United States of America, 102, 10439-10444.
http://dx.doi.org/10.1073/pnas.0501030102 - 18. Bhattacharya, A., Bhattacharya, S., Majumder, C. and Das, G. (2010) Transition-Metal Decoration Enhanced Room-Temperature Hydrogen Storage in a Defect-Modulated Graphene Sheet. Journal of Physical Chemistry C, 114, 10297-10301.
http://dx.doi.org/10.1021/jp100230c - 19. Chu, S., Hu, L., Hu, X., Yang, M. and Deng, J. (2011) Titanium-Embedded Graphene as High-Capacity Hydrogen-Storage Media. International Journal of Hydrogen Energy, 36, 12324-12328.
http://dx.doi.org/10.1016/j.ijhydene.2011.07.015 - 20. Gaboardi, M., Bliersbach, A., Bertoni, G., Aramini, M., Vlahopoulou, G., Pontiroli, D., Mauron, P., Magnani, G., Salviati, G. and Züttel, A. (2014) Decoration of Graphene with Nickel Nanoparticles: Study of the Interaction with Hydrogen. Journal of Materials Chemistry A, 2, 1039-1046.
http://dx.doi.org/10.1039/C3TA14127F - 21. López-Corral, I., Germán, E.A., Juan, A., Volpe, M.A.A. and Brizuela, G.P. (2011) DFT Study of Hydrogen Adsorption on Palladium Decorated Graphene. Journal of Physical Chemistry C, 115, 4315-4323.
http://dx.doi.org/10.1021/jp110067w - 22. Kubas, G.J. (1988) Molecular Hydrogen Complexes: Coordination of a σ Bond to Transition Metals. Accounts of Chemical Research, 21, 120-128.
http://dx.doi.org/10.1021/ar00147a005 - 23. Durgun, E., Ciraci, S., Zhou, W. and Yildirim, T. (2006) Transition-Metal-Ethylene Complexes as High-Capacity Hydrogen-Storage Media. Physical Review Letters, 97, Article ID: 226102.
http://dx.doi.org/10.1103/PhysRevLett.97.226102 - 24. Sun, Q., Wang, Q., Jena, P. and Kawazoe, Y. (2005) Clustering of Ti on a C60 Surface and Its Effect on Hydrogen Storage. Journal of the American Chemical Society, 127, 14582-14583.
http://dx.doi.org/10.1021/ja0550125 - 25. Beheshti, E., Nojeh, A. and Servati, P. (2011) A First-Principles Study of Calcium-Decorated, Boron-Doped Graphene for High Capacity Hydrogen Storage. Carbon, 49, 1561-1567.
http://dx.doi.org/10.1016/j.carbon.2010.12.023 - 26. Nachimuthu, S., Lai, P.J. and Jiang, J.C. (2014) Efficient Hydrogen Storage in Boron Doped Graphene Decorated by Transition Metals—A First-Principles Study. Carbon, 73, 132-140.
http://dx.doi.org/10.1016/j.carbon.2014.02.048 - 27. Shirasaki, T., Derré, A., Ménétrier, M., Tressaud, A. and Flandrois, S. (2000) Synthesis and Characterization of Boron-Substituted Carbons. Carbon, 38, 1461-1467.
http://dx.doi.org/10.1016/S0008-6223(99)00279-1 - 28. Chung, T.M., Jeong, Y., Chen, Q., Kleinhammes, A. and Wu, Y. (2008) Synthesis of Microporous Boron-Substituted Carbon (B/C) Materials Using Polymeric Precursors for Hydrogen Physisorption. Journal of the American Chemical Society, 130, 6668-6669.
http://dx.doi.org/10.1021/ja800071y - 29. Kresse, G. and Hafner, J. (1993) Ab Initio Molecular Dynamics for Open-Shell Transition Metals. Physical Review B, 48, 13115-13118.
http://dx.doi.org/10.1103/PhysRevB.48.13115 - 30. Kresse, G. and Furthmüller, J. (1996) Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Computational Materials Science, 6, 15-50.
http://dx.doi.org/10.1016/0927-0256(96)00008-0 - 31. Kresse, G. and Furthmüller, J. (1996) Efficient Iterative Schemes for Ab initio Total-Energy Calculations Using a Plane-Wave Basis Set. Physical Review B, 54, 11169-11186.
http://dx.doi.org/10.1103/PhysRevB.54.11169 - 32. Cohen, A.J., Mori-Sánchez, P. and Yang, W. (2008) Insights into Current Limitations of Density Functional Theory. Science, 321, 792-794.
http://dx.doi.org/10.1126/science.1158722 - 33. Khantha, M., Cordero, N., Molina, L., Alonso, J. and Girifalco, L. (2004) Interaction of Lithium with Graphene: An Ab initio Study. Physical Review B, 70, Article ID: 125422.
http://dx.doi.org/10.1103/PhysRevB.70.125422 - 34. Lee, K., Murray, é.D., Kong, L., Lundqvist, B.I. and Langreth, D.C. (2010) Higher-Accuracy van der Waals Density Functional. Physical Review B, 82, Article ID: 081101.
http://dx.doi.org/10.1103/PhysRevB.82.081101 - 35. Perdew, J.P. and Zunger, A. (1981) Self-Interaction Correction to Density-Functional Approximations for Many-Electron Systems. Physical Review B, 23, 5048-5079.
http://dx.doi.org/10.1103/PhysRevB.23.5048 - 36. Okamoto, Y. and Miyamoto, Y. (2001) Ab initio Investigation of Physisorption of Molecular Hydrogen on Planar and Curved Graphenes. Journal of Physical Chemistry B, 105, 3470-3474.
http://dx.doi.org/10.1021/jp003435h - 37. Ao, Z., Jiang, Q., Zhang, R., Tan, T. and Li, S. (2009) Al Doped Graphene: A Promising Material for Hydrogen Storage at Room Temperature. Journal of Applied Physics, 105, Article ID: 074307.
http://dx.doi.org/10.1063/1.3103327 - 38. Leenaerts, O., Partoens, B. and Peeters, F. (2008) Adsorption of H2O, NH3, CO, NO2, and NO on Graphene: A First-Principles Study. Physical Review B, 77, Article ID: 125416.
http://dx.doi.org/10.1103/PhysRevB.77.125416 - 39. Cabria, I., López, M. and Alonso, J. (2008) Hydrogen Storage in Pure and Li-Doped Carbon Nanopores: Combined Effects of Concavity and Doping. Journal of Chemical Physics, 128, Article ID: 144704.
http://dx.doi.org/10.1063/1.2900964 - 40. Monkhorst, H.J. and Pack, J.D. (1976) Special Points for Brillouin-Zone Integrations. Physical Review B, 13, 5188-5192.
http://dx.doi.org/10.1103/PhysRevB.13.5188 - 41. Chakraborty, B., Modak, P. and Banerjee, S. (2012) Hydrogen Storage in Yttrium-Decorated Single Walled Carbon Nanotube. Journal of Physical Chemistry C, 116, 22502-22508.
http://dx.doi.org/10.1021/jp3036296 - 42. Fair, K.M., Cui, X.Y. and Li, L. (2013) Hydrogen Adsorption Capacity of Adatoms on Double Carbon Vacancies of Graphene: A Trend Study from First Principles. Physical Review B, 87, Article ID: 014102.
http://dx.doi.org/10.1103/PhysRevB.87.014102 - 43. Liu, X., Wang, C.Z., Yao, Y.X., Lu, W.C., Hupalo, M., Tringides, M.C. and Ho, K.M. (2011) Bonding and Charge Transfer by Metal Adatom Adsorption on Graphene. Physical Review B, 83, Article ID: 235411.
http://dx.doi.org/10.1103/PhysRevB.83.235411 - 44. Froudakis, G.E. (2001) Why Alkali-Metal-Doped Carbon Nanotubes Possess High Hydrogen Uptake. Nano Letters, 1, 531-533.
http://dx.doi.org/10.1021/nl0155983 - 45. Kubas, G.J. (2007) Fundamentals of H2 Binding and Reactivity on Transition Metals Underlying Hydrogenase Function and H2 Production and Storage. Chemical Reviews, 107, 4152-4205.
http://dx.doi.org/10.1021/cr050197j
NOTES
*Corresponding author.