Journal of Materials Science and Chemical Engineering
Vol.2 No.5(2014), Article ID:46115,5 pages DOI:10.4236/msce.2014.25003
Stability Evaluation of Surfactant-Grafted Polyacrylamide Used in Oilfields by Evolution of Ammonia
Tingting Jiang1, Baohui Wang1, Haiyu Wang2
1Institute of New Energy Chemistry and Environmental Science, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing, China
2First Oil Plant, Daqing Oilfield Company Limited, Daqing, China
Email: jiangtingting82@163.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 8 April 2014; revised 1 May 2014; accepted 18 May 2014
ABSTRACT
Surfactant-grafted Polyacrylamide (S-PAM) serves as a novel oil displacement agent in oilfield. The analysis on composition of S-PAM shows that due to its instability the harmful ammonia gas may be generated during the operation of S-PAM or high temperature. In this paper, stability of S-PAM was studied by measuring the ammonia release from solid and aqueous S-PAM at different temperatures. The results showed that ammonia release of the solid S-PAM was increased with raising temperature. The ammonia release from S-PAM produced by Haibo Company is more than one from Lianhua in the solid state. The ammonia emissions from Lianhua S-PAM are slightly higher than Haibo one in the solutions prepared by both clean water and oilfield water.
Keywords:Surfactant-Grafted Polyacrylamide, Stability, Ammonia, Oilfield

1. Introduction
The World’s oil output is decreasing with the production of oilfields. The rational utilization of petroleum resources has aroused a great attention of the world. Therefore, the improvement of the oil recovery has become the major subject of oil development. At present, most of Chinese oil fields have encountered the high stage of water cut. The existing flooding technique has been difficult to meet the needs of field [1] [2] .
The Enhanced Oil Recovery (EOR) techniques have widely been used in Oilfields, which mainly include the polymer flooding (PAM), SP flooding (Polymer-Surfactant) and ASP flooding (Alkali-Polymer-Surfactant) [3] . There are varied degrees of problems in the application of the above flooding, such as polymer flooding. It remains the high interfacial tension between the fluid and crude oil, basically no solubilization and emulsification capacity for crude oil, no polymer with the anti-salt, anti-bacterial and anti-oxidation function, and no preparation with sewage. The components of chemicals in Binary or ternary chemical flooding are quite different in the diffusion and migration in the reservoir, exhibited in the “chromatographic effect” while no “synergies effect”, resulting in the low enhanced oil recovery. In addition, the strong base additives also lead to the severe scaling in many facilities during the recovery operation. Therefore, there are some inconveniences to the production [4] [5] . A new type of compound has been required for the above reason. Generally, basic polyacrylamide (PAM), which is simple, long and repeated chain (CH2-CH2-CONH2) polymer with the appropriate stability, is employed for the popular polymer flooding. Recently, Surfactant-grafted Polyacrylamide (Referred to as S-PAM), which combines the advantages of polymer and surfactant, has been developed for the field test. It collects the advantages of enhancing the anti-shearing, reducing the flow ratio, expanding the sweep efficiency, reducing the interfacial tension, improving the solubilization and emulsifying capacity, by multifunction in one.
Based on the structure and chemical properties, the basic PAM is stable at room temperature. The chemical components and stereo structure should be modified in some extensions after the basic PAM is grafted. Due to the modification, the stability will be changed. By the experiments of the basic PAM, the ammonia is eliminated after rearrangement of instability. Therefore, in this paper, stability of the S-PAM was studied by measuring the ammonia release from solid and aqueous S-PAM at different temperatures. It is conducive to the sustainable promotion in the oilfield research and development.
2. Experimental
Two origins of S-PAM were used in this experiment: Daqing Refining &Chemical Company S-PAM (Lianhua type III), which is called Lianhua S-PAM ;Shanghai Haibo company S-PAM (Haibo type III), which is called Haibo S-PAM. The main instrument: AMMONIA METER Z-800.
First of all, ammonia release of two solid S-PAMs was measured at different temperatures respectively. The highest content of ammonia was measured, which was converted to mass of ammonia evolution per gram of solid S-PAM. The ammonia release of S-PAM solutions prepared by clean water and oilfield water were measured during the dissolving time and storage time at room temperature. The highest content of ammonia was converted to mass of ammonia evolution per Liter of S-PAM solution.
3. Results and Discussion
3.1. Stability of Solid S-PAM
Ammonia emissions of two solid S-PAMs were measured at different temperatures respectively. The highest content of ammonia was measured, which was converted to mass of ammonia evolution per gram of solid S-PAM. Figure 1 experimentally shows the data measured at different temperatures.
The experiment results show the following conclusions:
1) With increasing temperature, ammonia emissions of two solid S-PAMs are on the rise.
2) Ammonia emissions of Lianhua S-PAM per gram increases slowly with rising temperature; the amount of Haibo has a slow growth at first, which has a rapid growth when the temperature is higher than 45˚C.
3) Ammonia emissions of Haibo S-PAM per g are higher than Lianhua.
3.2. Stability of S-PAM Solution
3.2.1. Clean Water Prepared S-PAM Solution
The two S-PAM solutions were prepared at a concentration of 1000, 1200 ppm respectively with clean water. Ammonia release was measured during the dissolving time and storage time at room temperature. The highest content of ammonia was converted to mass of ammonia evolution per Liter of S-PAM solution.
Figure 2 and Figure 3 experimentally show the following conclusions:
1) The change in magnitude of ammonia release is larger during the dissolving time, which is smaller at storage time.
2) The ammonia release of Lianhua is higher than Haibo.
3) The ammonia release is larger with higher concentration.
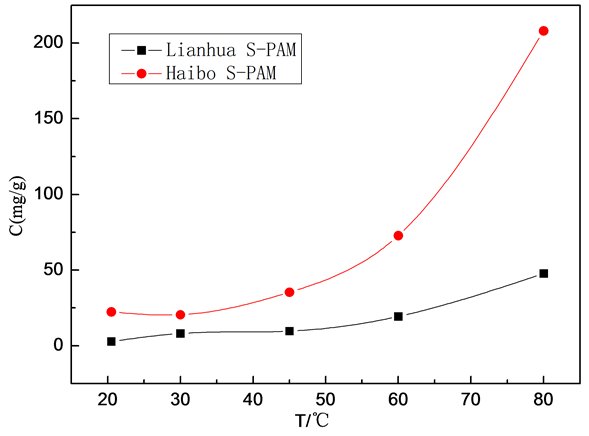
Figure 1. Ammonia emissions of two solid S-PAM at different temperatures.
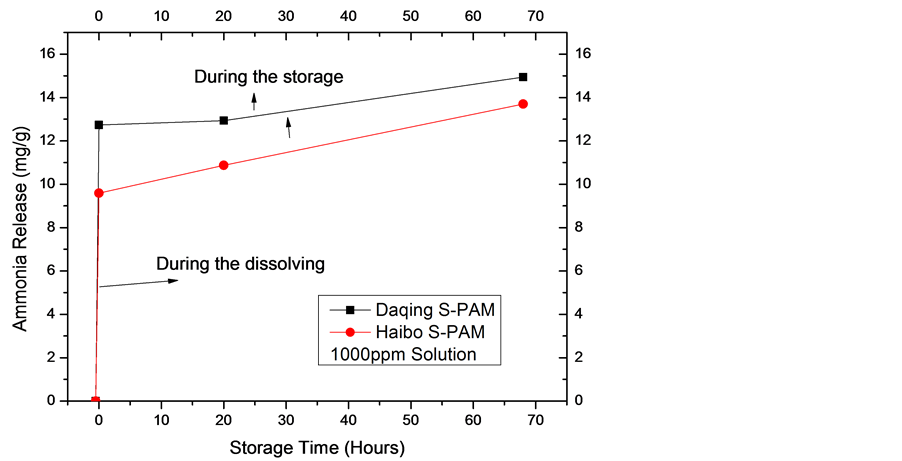
Figure 2. The ammonia release of two S-PAM solutions.
The same law was not showing in solid S-PAM and solution. After analyzing the main reason is that the viscosity of solution Lianhua is greater than Haibo. And ammonia is highly soluble in water, which is more soluble in a solution of low viscosity. Therefore ammonia emission Lianhua is higher than Haibo in monitoring process.
3.2.2. Oilfield Water Prepared S-PAM Solution
Oilfield water and S-PAM mother liquor used in this experiment were retrieved from oilfield. Specific preparation process is to simulate the process of site preparation. Mother liquor (3000 mg/L) was obtained by diluting S-PAM solution (1200 mg/L and 1500 mg/L) using oilfield water. The highest content of ammonia was converted to mass of ammonia evolution per Liter of S-PAM solution.
Figure 4 experimentally shows the following conclusions:
1) The change in magnitude of ammonia release is larger during the dissolving time, which is smaller at storage time.
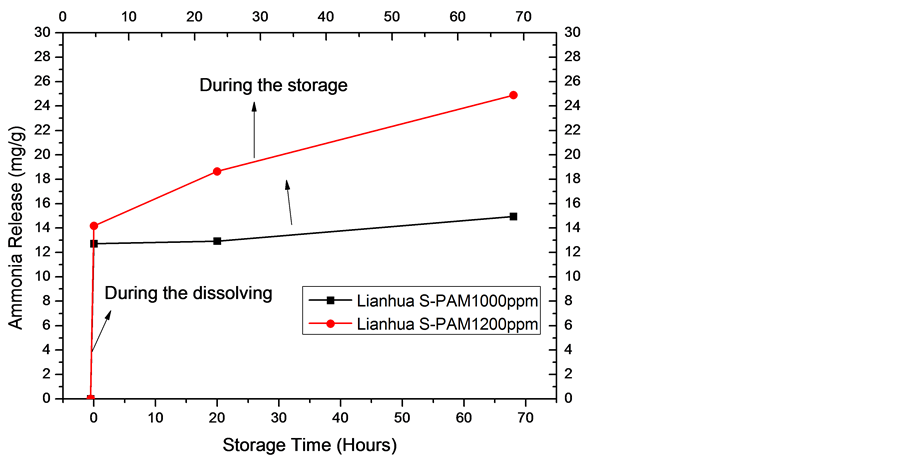
Figure 3. The ammonia release of Lianhua S-PAM solution at different concentrations.
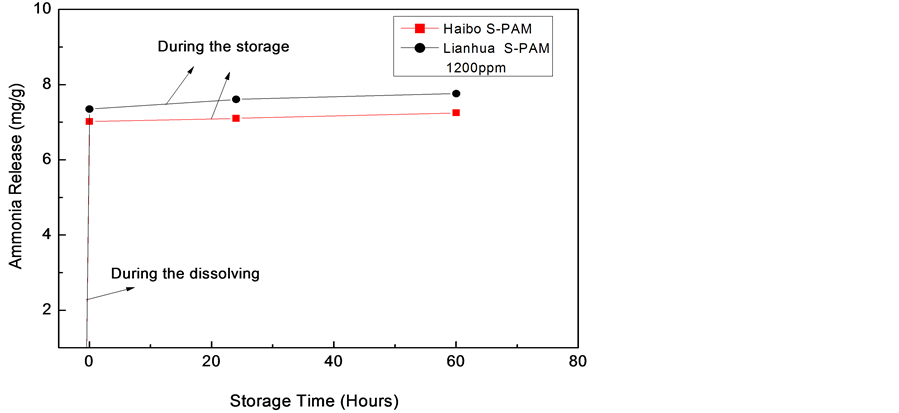
Figure 4. The curve of ammonia emissions of oilfield water prepared S-PAM solution.
2) The ammonia release of Lianhua is higher than Haibo.
In the time range of monitoring, ammonia emissions of Lianhua S-PAM are slightly higher than Haibo S-PAM both in clean water and oilfield water solution.
3.3. Instability Mechanism of S-PAM
Pyrolysis reaction of PAM has been adequately studied under the thermal effect. The solid PAM is thermally stable below 200˚C, which has only slight loss of quality in a relatively short period of time. The mass loss is mainly due to loss of adsorbed water or other volatile impurities. Pendant groups of PAM exploded over 200˚C - 220˚C. Then molecular chain of PAM breaks at higher temperatures. PAM is easily hydrolyzed at lower temperature (40˚C - 60˚C) in alkaline conditions. Acidic hydrolysis of PAM is required high temperatures, which is slower than the alkaline hydrolysis [6] . S-PAM is Surfactant-grafted PAM, the structure and nature of which show some changes after grafted. The ammonia release of solid S-PAM was measured at room temperature to 80˚C. And ammonia release of S-PAM solution was measured at room temperature.
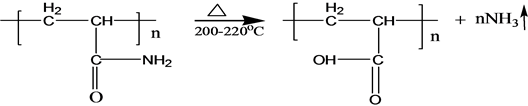
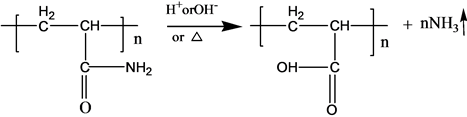
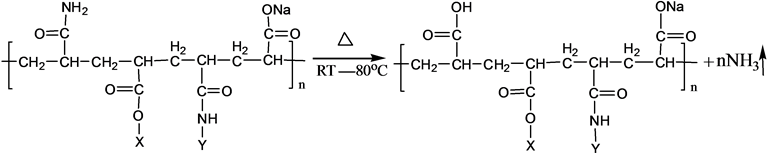
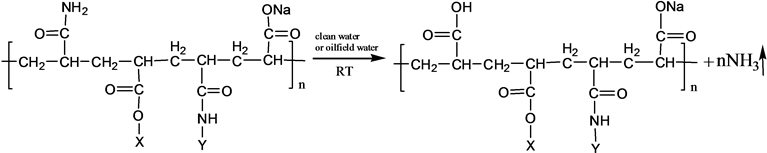
The chemical components and stereo structure should be modified in the some extension after the basic PAM is grafted. The harmful ammonia gas was generated during the operation of S-PAM or high temperature.
1) 4. Conclusions
2) Through the analysis on stability of solid S-PAM, ammonia emissions of solid S-PAM are on the rise with increasing temperature. The ammonia evolutions per gram of Haibo S-PAM are higher than Lianhua.
3) Through the stability analysis of S-PAM solution, the change in magnitude of ammonia release is larger during the dissolving time, which is smaller at storage time. The ammonia evolutions of Lianhua S-PAM are slightly higher than Haibo both in clean water and oilfield water prepared solution in the time range of monitoring.
References
- Hu, B.Z. (1997) Production Engineering of Polymer Flooding. Petroleum Industry Press, Beijing, 70-72 (in Chinese).
- Chen, G. (1997) The Strategic Position of Three Oil Production in the Oil Industry in Development. Energy of China, 9, 17-19 (in Chinese).
- Gang, Q.L. (1998) A Dissertation on Chinese Tertiary Recovery Technology. OGRT, 5, 1-7 (in Chinese).
- Ge, G.Z., Wang, J.Y. and Wang, Y.L. (2001) Polymer Flooding and Related Chemical Flooding Progress. Oilfield Chemistry, 18, 282-284 (in Chinese).
- Moritis, G. (1996) New Technology Improved Economics Boost EOR Hopes. Oil Gas Journal, 15, 39-61.
- Fang, D.B. (2006) Acrylamide Polymers. Chemical Industry Press, Beijing, 58-84 (in Chinese).

