Journal of Materials Science and Chemical Engineering
Vol.2 No.3(2014), Article ID:44107,7 pages DOI:10.4236/msce.2014.23003
Synthesis and Fluorescence Quantum Yield of Gd1−xEuxOOH Crystals
Hiroaki Samata1, Daisuke Itakura1, Shungo Imanaka1, Tadashi C. Ozawa2
1Graduate School of Maritime Sciences, Kobe University, Kobe, Japan
2International Center for Materials Nanoarchitectonics, National Institute for Materials Science, Tsukuba, Japan
Email: samata@maritime.kobe−u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 4 February 2014; revised 4 March 2014; accepted 11 March 2014
Abstract
Eu3+-doped gadolinium oxyhydroxide Gd1−xEuxOOH crystals were synthesized by the flux method. The X-ray diffraction data for the crystals were well refined assuming a monoclinic structure with the P21/m space group. Gd1−xEuxOOH (x ≤ 0.2) crystals showed strong red emission, and the highest fluorescence quantum yield (Φf) was 0.27, obtained for x = 0.10. Φf decreased rapidly as the Eu3+ content x increased above 0.2, owing to concentration quenching. Analysis with a percolation model indicated three-dimensional energy transfer between the Eu3+ ions.
Keywords:Gadolinium Oxyhydroxide; Fluorescence Quantum Yield; Percolation Model

1. Introduction
Some rare-earth compounds have excellent optical properties and hence are widely used in high-performance luminescence devices and as catalyst supports. Trivalent rare-earth ions such as Eu3+, Tb3+, and Tm3+-doped in a suitable host material show strong emission based on electron transition between the 4f orbitals. The luminescence properties depend strongly on the chemical composition and crystal structure of the host material. One of the Gd(III) compounds, gadolinium oxyhydroxide (GdOOH), which has a monoclinic structure with the P21/m space group, can be obtained as a stable phase by the thermal dehydration of hydroxide Gd(OH)3 [1] -[4] . Although this compound can function as a host material for phosphors, there are very few reports on its photoluminescence properties [5] [6] . Fluorescence quantum yield, Φf, which is defined as the ratio of the number of emitted photons to the number of absorbed photons, is a fundamental parameter that decides the photoluminescence efficiency of a compound/phosphor [7] . Hence, evaluation of the activator content dependence of Φf using high-quality single-crystal specimens is important.
In this study, we synthesized Gd1−xEuxOOH crystals and evaluated their luminescence properties, including Φf. In addition, we evaluated the critical Eu3+ concentration in the Gd1−xEuxOOH system by using a percolation model.
2. Experimental
Crystals of Eu3+-doped gadolinium oxyhydroxide, Gd1−xEuxOOH (x = 0, 0.02, 0.04, 0.06, 0.08, 0.1, 0.15, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8, 1), were synthesized by the flux method. Appropriate amounts of Gd2O3 powder (99.9%) and Eu2O3 powder (99.9%) were mixed in an agate mortar for 1 h. Then, the Gd2O3/Eu2O3 mixture was placed in a 20-mL Zr crucible and covered by a layer of a NaOH (15 g)/KOH (5 g) mixture. The crucible was placed in an alumina container, covered with an alumina lid, and heated in an electric furnace at 330˚C for 72 h in ambient atmosphere. Subsequently, the flux was dissolved in large excess of water; the obtained crystals were washed thoroughly in distilled water to remove any residual flux and dried on a hot plate at 80˚C for 1 h.
The chemical composition of the crystals was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES; Seiko Instruments Inc., SPS1700HVR) and an energy-dispersive X-ray spectrometer (EDS; JEOL, EX-23000 BU). The crystal structures were identified by powder X-ray diffraction (XRD; Rigaku, RINT2000). The diffraction data were acquired using CuKα radiation generated at 40 kV and 20 mA, in the 2θ range 10~80˚ at room temperature, and refined by the Rietveld method [8] . Thermogravimetric analysis (TG) and differential thermal analysis (DTA) were carried out in air, at temperatures between 30 and 785˚C at a heating rate of 10˚C /min (Seiko Instruments Inc., TG/DTA6300). The fluorescence excitation and emission spectra were measured at room temperature using a fluorescence spectrophotometer (Hitachi, F-7000). The excitation spectra were corrected for the spectral distribution of the lamp intensity by the Rhodamine B method, and the emission spectra were corrected for the spectral response of the instrument by using a substandard light source. The Φf values were measured using an absolute photoluminescence quantum yield measurement system (Hamamatsu, C9920-02), at room temperature [7] .
3. Results and Discussion
Gd1−xEuxOOH crystallized at the bottom of the crucible. As shown in Figure 1, the as-grown GdOOH crystals were transparent and had a plate-like shape with flat surfaces, which could be attributed to the growth in a liquid phase. The maximum length of the crystal was about 1.2 mm. The external surface of the crystals remained largely unchanged through the entire composition range. No trace of Zr, Na, and K impurities was detected by ICP-OES measurements, although these elements were expected to be incorporated into the crystals because of the use of the Zr crucible and NaOH/KOH mixture. The Gd/Eu ratio in the grown crystals, which was measured by EDS, was in good agreement with the molar ratios of Gd2O3 and Eu2O3, indicating that Gd1−xEuxOOH crystals with different compositions could be obtained by simply changing the molar ratio of the starting materials.
Figure 2 shows the powder XRD profiles of Gd1−xEuxOOH and the result of data refinement by the Rietveld method. The black squares show the experimental data, and the red and blue solid lines show the calculated profile and the difference between the experimental and calculated profiles, respectively. All the diffraction data were well refined assuming a monoclinic structure with the P21/m space group, and no structural change was observed over the entire composition range considered. The lattice parameters for GdOOH (a = 0.4339 nm, b = 0.3717 nm, c = 0.6072 nm, β = 108.79˚) and EuOOH (a = 0.4346 nm, b = 0.3744 nm, c = 0.6107 nm, β = 108.62˚) agreed with those reported in previous studies [9] [10] . When Gd3+ (ionic radius: 0.0935 nm) in GdOOH was replaced by Eu3+ (ionic radius: 0.0947 nm), the lattice constants showed a slight increase, in accordance with Vegard’ law. Gd1−xEuxOOH had low rare-earth site symmetry and a layered structure in which the rare earth layers were sandwiched between an O layer and an OH layer [11] [12] .
The TG curve of the as-grown crystals indicated no apparent weight loss in the temperature range 30~400˚C, indicating that the as-grown crystals did not include Gd(OH)3, which normally decomposes at 270˚C [4] . In the TG profile, the first large weight loss was observed at 445˚C, with a corresponding endothermic effect, attributable to the removal of water molecules from GdOOH crystals. The experimental weight loss of 4.5% agreed well with the theoretical value of 4.7% calculated assuming the following thermal decomposition: 2GdOOH → Gd2O3 + H2O. The XRD profiles of the specimens subjected to TG/DTA measurements showed a single phase, Gd2O3. These results indicated that the as-grown crystals comprised a single phase of GdOOH and did not contain Gd2O3 and Gd(OH)3 at all.
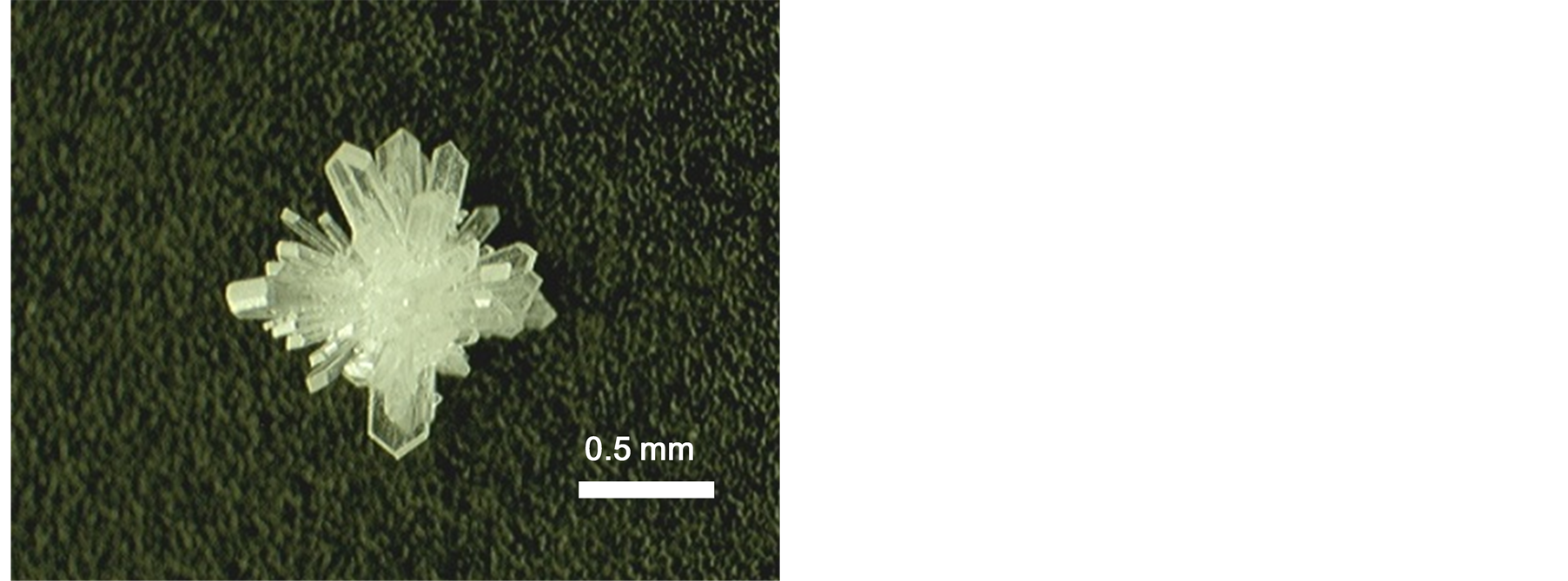
Figure 1. Photograph of as-grown GdOOH crystals synthesized at 330˚C for 72 h.
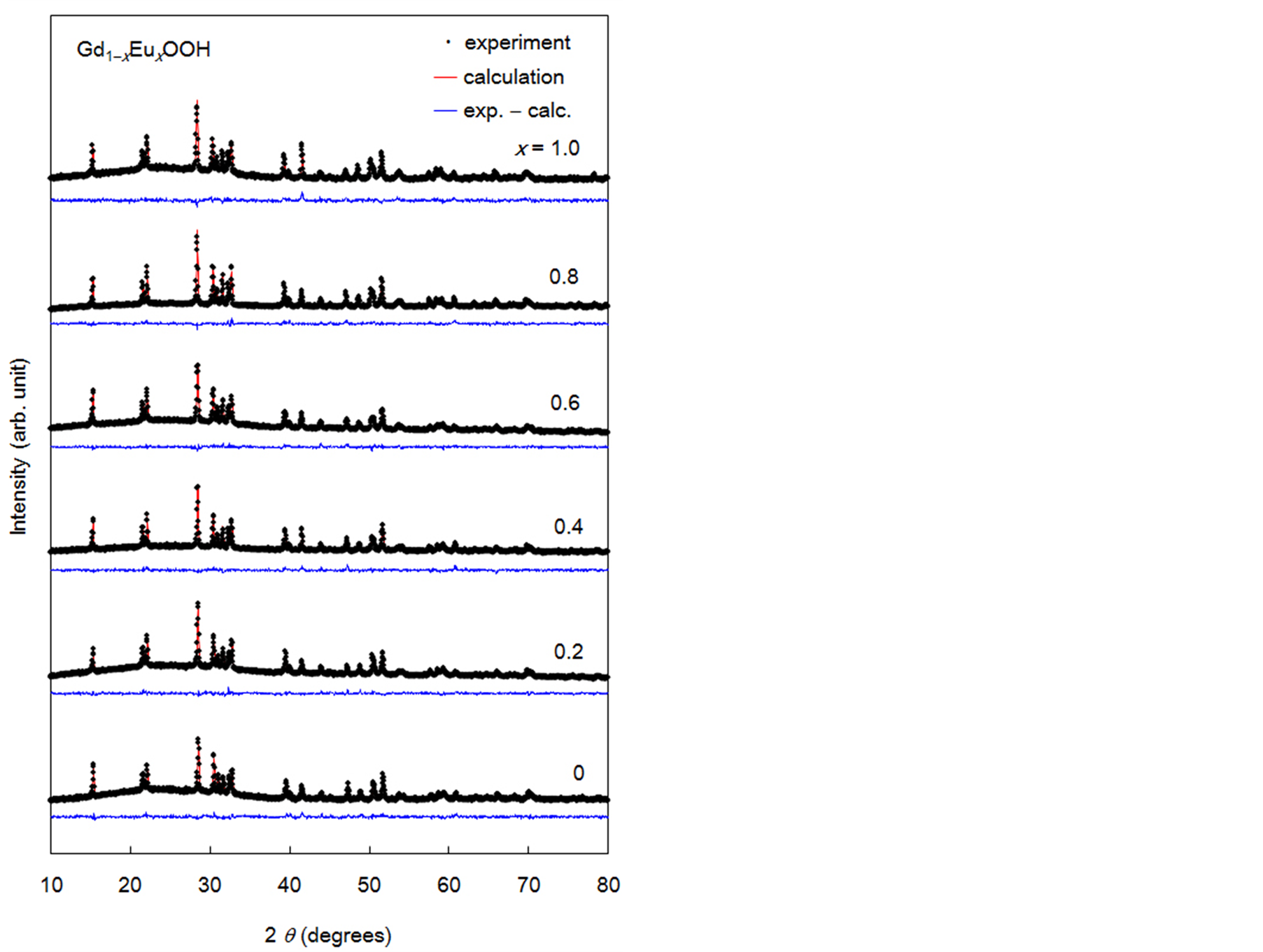
Figure 2. Powder X-ray diffraction profiles of Gd1−xEuxOOH crystals and results of data refinement by the Rietveld method.
Figure 3 shows the excitation spectra of the Gd1−xEuxOOH crystals; spectral measurements were performed at 617 nm, which is the maximum emission wavelength of Eu3+ in this system (5D0→7F2 transition). The excitation spectra showed sharp peaks between 300 nm and 550 nm, which were attributed to the direct intraconfigurational 4f-4f transitions from the 7F0 and 7F1 levels of Eu3+. The intensity of these peaks increased as the Eu3+ content increased up to x = 0.1. The wide peak between 200 nm and 300 nm, which was attributed to the overlapping of
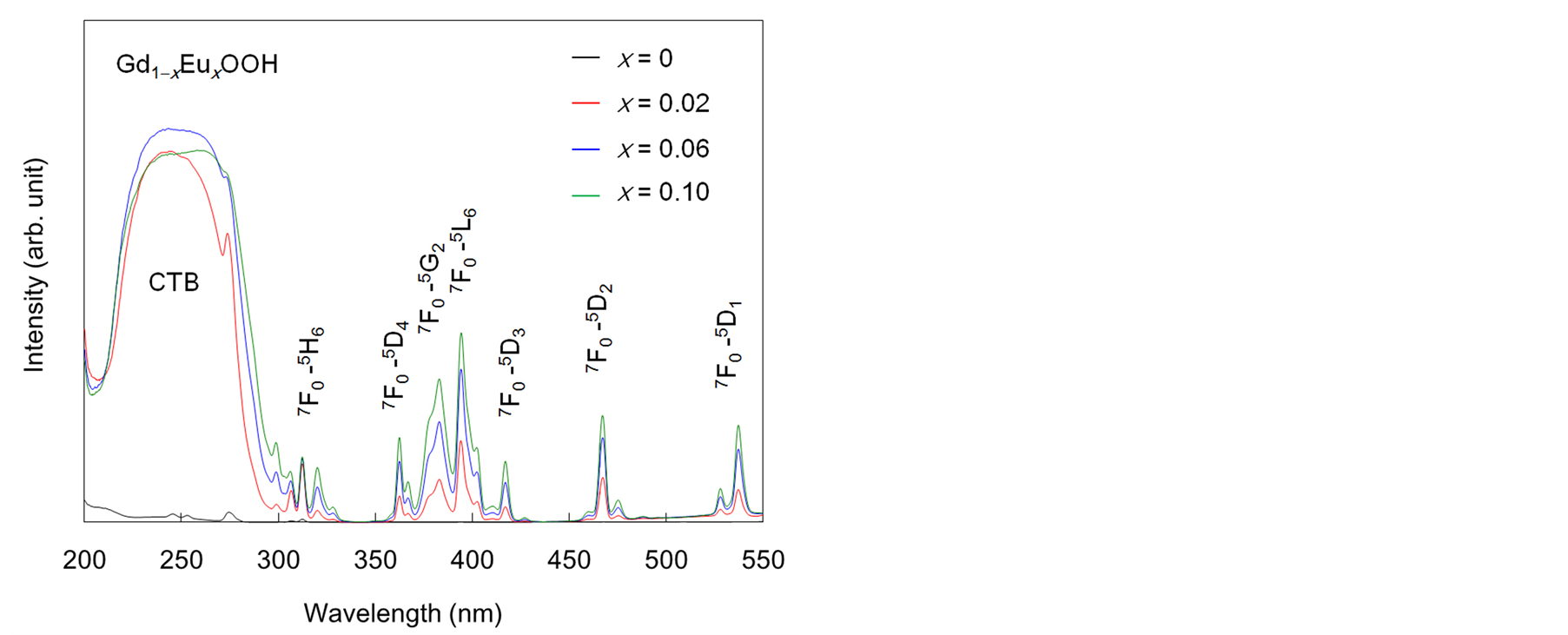
Figure 3. Excitation spectra of Gd1−xEuxOOH crystals.
the O2− to Eu3+ charge transfer band (CTB) and the band due to energy transfer from Gd3+ to Eu3+ [13] , indicated energy transfer from the oxyhydroxide host to Eu3+. The intensity of the sharp peaks appearing at wavelengths longer than 300 nm changed with the Eu3+ content, but that of the wide peak at wavelengths below 300 nm did not change much.
Figure 4 shows the emission spectrum of the Gd0.9Eu0.1OOH crystals under 243 nm illumination. Strong red luminescence from Eu3+ was observed in the visible range. These peaks could be attributed to the transition from the lowest exited level 5D0 to the 7F0−4 levels of the ground term. The most intense transition was 5D0→7F2, the allowed electric dipole transition, at 617 nm. The other electric dipole transition, 5D0→7F4, was observed near 710 nm. In addition to electric dipole transitions, a strong magnetic dipole transition, 5D0→7F1, was observed near 595 nm. A 5D0→7F0 transition, which is normally forbidden by the free-ion selection rules, occurred at around 580 nm, as the selection rules are broken by the low symmetry of the rare-earth sites.
Figure 5 shows the Eu3+ content dependence of Φf for the Gd1−xEuxOOH crystals under excitation at 243, 258, and 394 nm (Eu3+: 7F0→5L6), with the former two wavelengths being close to the peak maxima in the excitation spectra. The highest Φf of 0.27 was obtained when the Eu3+ content was around 0.1. The decrease in Φf for x < 0.1 was attributed to the small amount of Eu3+, which is the photoactivator, in the system. At all excitation wavelengths, Φf decreased rapidly as the Eu3+ content increased beyond 0.2, because of concentration quenching caused by the transfer of excitation energy between the Eu3+ ions.
The critical Eu3+ concentration was evaluated by using the percolation model, which has been used to explain energy transfer in the solid state [14] -[16] . In this study, the aforesaid model was applied to the system under the following assumptions: energy transfer between the Eu3+ ions occurs only among the neighbor sites in the rare-earth sublattice. When the Eu3+ concentration was below the critical value, the Eu3+ ions showed emission individually, and the Φf values were high. When the Eu3+ concentration exceeded the critical value Pc, Φf decreased rapidly because of energy transfer from the percolating cluster to the killer center, which consists of vacancies and impurities. V. A. Vyssotsky et al. suggested the following expression for calculating the critical concentration in the percolation model:
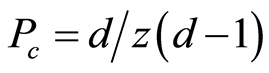 (1)
(1)
where Pc, z, and d are the critical concentration, number of neighbor sites, and number of dimensions, respectively [16] . When energy transfer between the Eu3+ ions occurs only in the inner layers of the rare-earth sublattice, the number of dimensions is considered to be 2 (pseudo-two-dimensional energy transfer). When energy transfer occurs in the interlayers, the number of dimensions is considered to be 3 (three-dimensional energy transfer). Figure 6 shows the percolation model and the Pc value calculated using Equation (1). In this figure, only the rare-earth sublattice is depicted, and the Eu3+ ions between which energy transfer occurs are bonded by gray cylinders. Energy transfer between the Eu3+ ions is assumed to occur within 0.363 nm for (a), 0.373 nm for (b), 0.404 nm for (c), and 0.435 nm for (d). The experimental result agrees well with the calculated value of Pc (0.19) estimated for Eu3+-Eu3+ interactions within 0.404 nm (c). In this case, the Eu3+ ions have eight neighbors
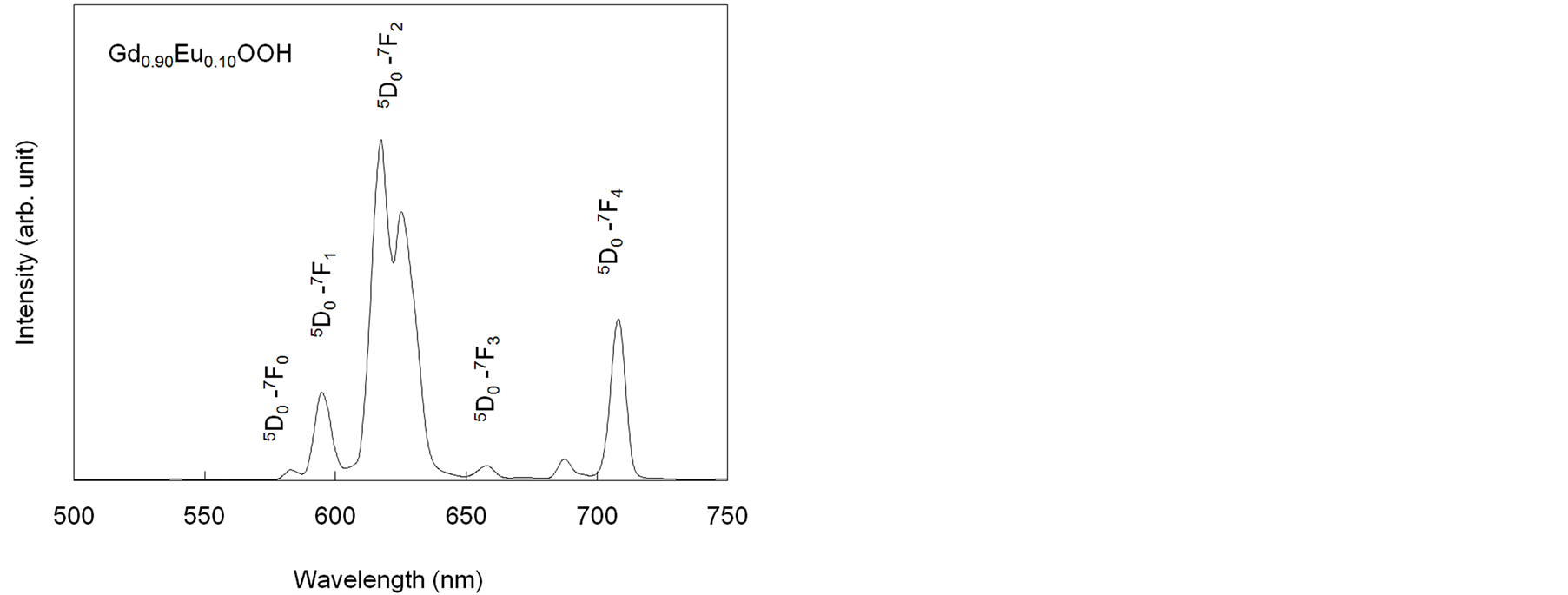
Figure 4. Emission spectrum of Gd0.9Eu0.1OOH crystals.
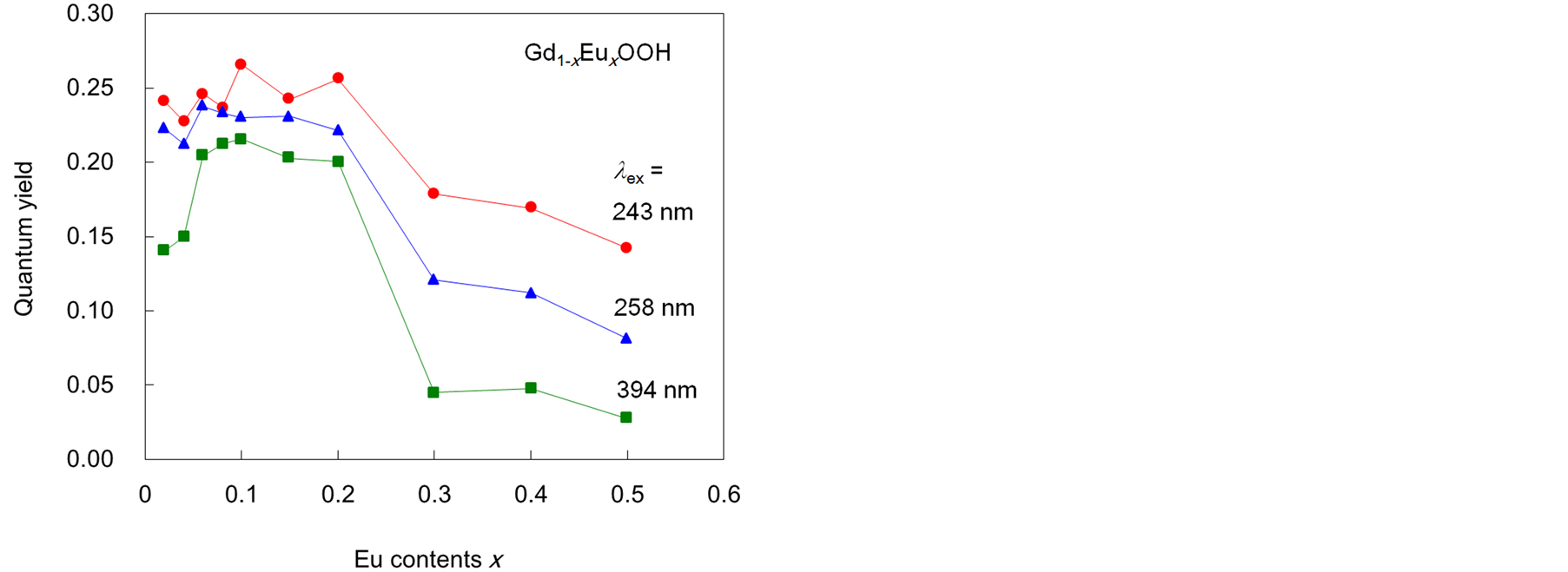
Figure 5. Eu3+ content dependence of the fluorescence quantum yield Φf of Gd1−xEuxOOH crystals.
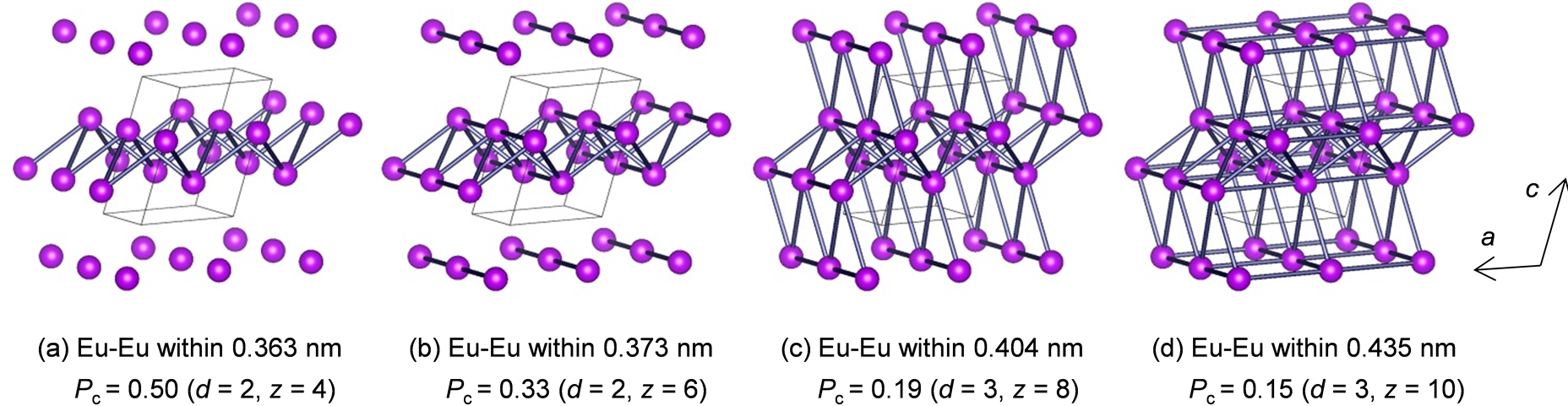
Figure 6. Percolation model of the Gd1−xEuxOOH system.
with three-dimensional energy transfer. This result indicates three-dimensional energy transfer occurs between the Eu3+ ions in this system.
To improve the luminescence properties of the crystals, it is necessary to increase the critical concentration of Eu3+. In general, compounds with a low-dimensional arrangement of rare-earth ions show a high critical concentration. On the basis of the results obtained using the percolation model in this study, we state that two-dimensional models have a higher critical concentration (0.50 and 0.33) than do three-dimensional models. When the increase in the distance between the rare-earth layers restrains interlayer energy transfer, the critical concentration of Eu3+ in this system can increase. Therefore, excellent luminescence properties can be obtained by co-doping larger ions in the rare-earth sublattice with a high level of Eu3+ doping. The high alkali durability of the Gd1−xEuxOOH crystals is confirmed by the fact that they are stable in NaOH/KOH solution at 330˚C.
4. Conclusion
Gd1−xEuxOOH crystals with a maximum length of 1.2 mm were synthesized by the flux method. The Gd1−xEuxOOH (x ≤ 0.2) crystals showed strong red emission, with the maximum Φf of 0.27 for x = 0.10. The critical Eu3+ content was 0.2, because Φf decreased rapidly beyond this level owing to concentration quenching. This critical content was the closest to that estimated by a percolation model for Eu3+-Eu3+ interactions within 0.404 nm, wherein Eu3+ had eight neighbors, indicating three-dimensional energy transfer between the Eu3+ ions in the rare-earth sublattice. The synthesized crystals showed excellent alkali durability and hence may be used as a high-efficiency host material in unique photomedical applications such as photochemical internalization and photodynamic therapy.
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 21560696, 24560827.
References
- Klevtsov, P.V. and Sheina, L.P. (1965) Hydrothermal Synthesis and Crystal Structure of Rare-Earth Hydroxides. Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy, 1, 912.
- Klevtsov, P.V. and Sheina, L.P. (1965) Thermographic and X-ray Studies of Crystalline Hydroxides of the Rare Earth Elements. Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy, 1, 2219.
- Gondrand, M. and Christensen, A.N. (1971) Hydrothermal and High Pressure Preparation of Some Rare Earth Trihydroxides and Some Rare Earth Oxide Hydroxides. Materials Research Bulletin, 6, 239-246. http://dx.doi.org/10.1016/0025-5408(71)90036-5
- Yamamoto, O., Takeda, Y., Kanno, R. and Fushimi, M. (1985) Thermal Decomposition and Electrical Conductivity of M(OH)3 and MOOH (M=Y, Lanthanide). Solid State Ionics, 17, 107-114. http://dx.doi.org/10.1016/0167-2738(85)90057-8
- Hölsä, J., Leskelä, T. and Leskelä, M. (1985) Luminescence Properties of Europium(3+)-Doped Rare-Earth Oxyhydroxides. Inorganic Chemistry, 24, 1539-1542. http://dx.doi.org/10.1021/ic00204a026
- Hölsä, J. (1990) Simulation of Crystal Field Effect in Monoclinic Rare Earth Oxyhydroxides Doped with Trivalent Europium. The Journal of Physical Chemistry, 94, 4835-4838. http://dx.doi.org/10.1021/j100375a016
- Suzuki, K., Kobayashi, A., Kaneko, S., Takehira, K., Yoshihara, T., Ishida, H., Shiina, Y., Oishic, S. and Tobita, S. (2009) Reevaluation of Absolute Luminescence Quantum Yields of Standard Solutions Using a Spectrometer with an Integrating Sphere and a Back-Thinned CCD Detector. Physical Chemistry Chemical Physics, 11, 9850-9860. http://dx.doi.org/10.1039/b912178a
- Izumi, F. and Ikeda, T. (2000) A Rietveld-Analysis Programm RIETAN-98 and Its Applications to Zeolites. Materials Science Forum, 321-324, 198-205. http://dx.doi.org/10.4028/www.scientific.net/MSF.321-324.198
- Chang, C. and Mao, D. (2006) Thermal Dehydration Kinetics of a Rare Earth Hydroxide, Gd(OH)3. International Journal of Chemical Kinetics, 39, 75-81. http://dx.doi.org/10.1002/kin.20221
- Bärnighausen, V. H. (1965) Die Elementarzelle und Raumgruppe von EuOOH. Acta Crystallographica, 19, 1047.
- Samata, H., Kimura, D., Mizusaki, S., Nagata, Y., Ozawa, T.C. and Sato, A. (2009) Synthesis and Characterization of Neodymium Oxyhydroxide Crystals. Journal of Alloys and Compounds, 468, 566-570. http://dx.doi.org/10.1016/j.jallcom.2008.01.056
- Samata, H., Kimura, D., Saeki, Y., Nagata, Y. and Ozawa, T.C. (2007) Synthesis of Lanthanum Oxyhydroxide Single Crystals Using an Electrochemical Method. Journal of Crystal Growth, 304, 448-451. http://dx.doi.org/10.1016/j.jcrysgro.2007.03.025
- Yang, J., Li, C., Cheng, Z., Zhang, X., Quan, Z., Zhang, C. and Lin, J. (2007) Size-Tailored Synthesis and Luminescent Properties of One-Dimensional Gd2O3:Eu3+ Nanorods and Microrods. The Journal of Physical Chemistry C, 111, 18148-18154. http://dx.doi.org/10.1021/jp0767112
- Vyssotsky, V.A., Gordon, S.B., Frisch, H.L. and Hammersley, J.M. (1961) Critical Percolation Probabilities (Bond Problem). Physical Review, 123, 1566. http://dx.doi.org/10.1103/PhysRev.123.1566
- Keyes, K. and Pratt, S. (1979) The Relation of Percolation to Energy Migration Dynamics. Chemical Physics Letters, 65, 100-104. http://dx.doi.org/10.1016/0009-2614(79)80136-0
- Honma, T., Toda, K., Ye, Z.G. and Sato, M. (1998) Concentration Quenching of the Eu3+-Activated Luminescence in Some Layered Perovskites with Two-Dimensional Arrangement. The Journal of Physical Chemistry of Solids, 59, 1187-1193. http://dx.doi.org/10.1016/S0022-3697(98)00056-0

