Open Journal of Marine Science
Vol.3 No.3(2013), Article ID:34580,6 pages DOI:10.4236/ojms.2013.33016
A Robust and Economical Underwater Stereo Video System to Observe Antarctic Krill (Euphausia superba)
1Centre for Marine Futures, Oceans Institute, The University of Western Australia, Crawley, Australia
2Australian Antarctic Division, Channel Highway, Kingston, Australia
3Department of Biological Sciences, Faculty of Science, Macquarie University, Sydney, Australia
Email: *tom.letessier@uwa.edu.au
Copyright © 2013 Tom B. Letessier et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 22, 2013; revised May 23, 2013; accepted June 4, 2013
Keywords: Behaviour; Orientation; Size; Aquaria; Photogrammetry; GoPro
ABSTRACT
In situ characterization of krill morphometry, behaviour and orientation is not yet routinely feasible, yet is critical to understanding swarm characteristics. A first step is to measure individual and aggregation behaviour. We report on successful use of a robust, low-cost underwater stereo video camera system to observe live Antarctic krill (Euphausia superba) in aquaria. The application of photogrammetry techniques allows animal length, orientation and three-dimensional position to be calculated from stereo video camera observations. Initially, we tested the efficacy of the stereo system by observing synthetic targets of known length and orientation to obtain estimates of measurement error. We found that on average the stereo camera system underestimated length by 0.6 mm and vertical tilt angle by +0.34˚ (head up), but that photogrammetric measurements of 100 randomly selected krill lengths were not significantly different from measurements of 100 randomly caught krill measured physically. During our investigation, we analysed three krill behavioural metrics: swimming speed, tortuosity, and vertical orientation under three behavioural states (undisturbed, feeding, and escape). We found that swim speed and tortuosity significantly increased when animals were feeding or exhibiting an escape response, but vertical orientation was not significantly different across states. Our investigation demonstrates that low-cost stereo video cameras can produce precise measurements that can be used for monitoring krill behaviour and population structure.
1. Introduction
Stereo cameras are increasingly utilized to conduct insitu observations of fish in demersal and mid-water systems for non-extractive monitoring purposes [1,2]. In contrast to single camera systems, stereo systems allow measurements in multidimensional space, such as the length and position of the animal, to be computed. For example, Watson et al. [3] used stereo cameras to monitor population structure and in particular fish size, inside and outside marine protected areas. While cameras have recently been used to monitor the behaviour of Antarctic krill (Euphausia superba; hereafter krill), underwater stereo systems have been large and unsuitable for use in aquaria [4] or in the field with one exception [5]. Stereophotography is likely to have widespread application in schooling animals such as krill, providing important information on behaviour and size. Krill are obligate schoolers and exhibit a variety of schooling behaviours; [6] resulting in a wide range of school shapes, e.g. [7]. Observations of krill in the wild have provided important insights into these and other non-schooling behaviours. For example, Kawaguchi et al. [8] deployed a video camera to a depth of 860 m and successfully monitored krill mating behavior, while Schmidt and colleagues analysed the results of single camera systems deployed to investigate krill depth distribution [9].
A major limitation of extant systems is that they are mono, making length orientation and positional measurements difficult, and that they are large, thus making them expensive and logistically difficult to deploy in the field. Many field-based investigations of krill take place in the top 200 m of the water column within the diving range of air-breathing krill predators such as whales, seals, and seabirds and thus within the sampling range of hull mounted acoustic instruments. A light-weight, robust, economical stereo camera unit, with modest depth rating (<200 - 50 m) could therefore have widespread utility for sampling behavior of krill swarms, even if restricted to the photic zone.
Applications of stereo systems to krill are likely to be twofold. First, krill biomass is commonly estimated using acoustic surveys [10]. In order to calculate biomass, these surveys rely on acoustic target strength models (see [11] for a recent krill target strength model adopted for krill biomass surveys) that quantify the amount of sound scattered by an organism. The target strength models are used to identify krill from the acoustic returns and scale acoustic energy to biomass. Important parameters in krill target strength models include krill length and vertical orientation [11]. Typically krill lengths are measured from animals caught with nets, e.g. [12] and krill orientation is inferred, not observed [13-15] for in situ measurement of orientation. Stereo cameras afford the opportunity to observe both krill length and orientation and thus potentially improve the accuracy of in situ krill biomass estimates.
Second, stereo systems potentially can provide important insights into krill behavior under different behavior states. Published field photographic measures of krill have so far been limited to mono systems, with one notable exception [5], and stereo cameras have been mounted in air vertically above aquaria to successfully monitor krill behaviour e.g. [4]. Kawaguchi et al. [4] showed that stereo systems could successfully extract quantitative metrics, such as krill speed. However their system was limited to behaviour visible from the surface and so of limited utility in situ.
In this study we assess the capability of an underwater stereo video system (USVS) submerged in an aquarium to observe and monitor krill. Our research objectives were two-fold: 1) assess the potential contribution of the USCS to krill biomass estimates and 2) extract krill behaviour metrics to determine behaviour state.
2. Methods and Results
We conducted a series of krill observations using stereo cameras in an aquarium with dimensions 1.0 m deep with a 1.860 m internal diameter (Figure 1, and see Kawaguchi et al., [4] for a full description of the aquarium). Water temperature was between −1.0˚C and +1.0˚C. The USVS was comprised of two GoPro HDHero2 cameras encased in GoPro flat port underwater housings (rated to 60 m), separated by a 0.8 m baseline with an inward convergence angle of four degrees, providing an optimal field of view at a range of 1 to 5 m distance (see [16,17] for calibration procedure). Each unit, including cameras costs approximately $ 1000 US. The battery and extension allows recording for approximately 3 hours.
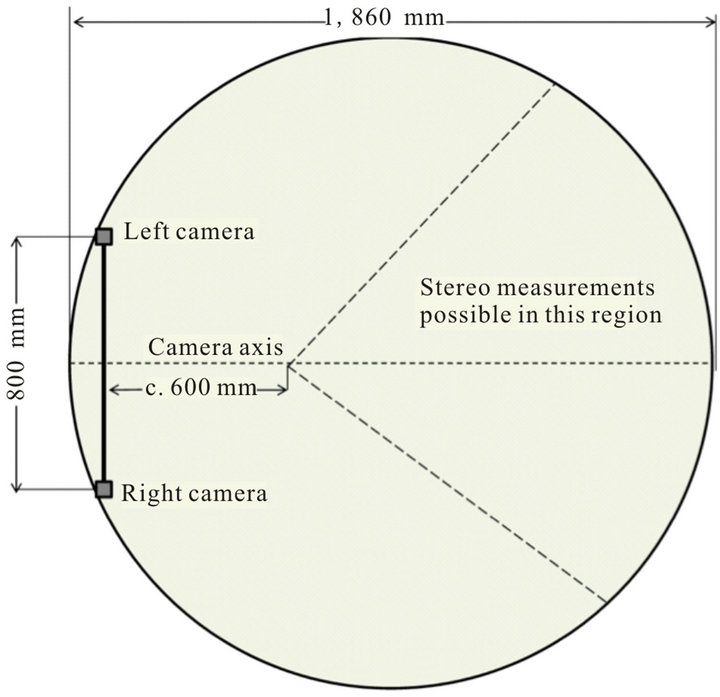
Figure 1. Schematic plan view of the stereo camera rig set up and the aquarium. Stereo camera measurements were possible in the region starting 600 mm to the right of the camera axis, enclosed by dashed lines, and extending to the aquarium wall.
Maximum camera deployment time was 2.5 hr and the USVS observations were made over a range of surface light levels from 60 to 178 lx. The USVS imagery was converted from the as-recorded MPEG-4 part 14 to AVI using Xilisoft Video Converter and stereo image processing was conducted using EventMeasure software [18]. Individual metrics (size, orientation, and location) were manually derived from digitally captured images in the computer program PhotoMeasure [18]. PhotoMeasure allows the two stereo video record files to be synchronized. Metrics were captured by selecting two points— anterior edge of the eye to the tip of the telson—in each video. The software then calculated the metrics from the offset between the pair of points in each video.
We used synthetic targets of known lengths (20, 30, 40 and 50 mm) and vertical orientation (0˚, 30˚, 45˚, and 60˚) to test stereo camera system accuracy. We found vertical orientation measurements to have a mean difference of +0.34˚ (krill head up) with a range of −0.2˚ deg to +1.5˚ and a mean length measurement difference of −0.6 mm with a range of −2.1 to + 2.5 mm.
2.1. Krill Size
We selected 100 krill at random from the USVS imagery, and measured their length (from anterior edge of the eye to the tip of the telson) and vertical orientation. Random sampling was achieved by selecting a single frame (synchronised between cameras using the EventMeasure software) and selecting krill nearest to a randomly selected X, Y pixel coordinate. The wrapped normal distribution [19] was used to describe krill orientation. Maximum likelihood (ML) estimates of vertical orientation (where 0˚ is horizontal) were mean = 23.5˚, angular standard deviation = 0.64˚ (Figure 2(a)). ML estimates were obtained using the circular package [20] in R v2.5.2, [21].
To validate the USVS derived krill length frequency distribution we also randomly removed 100 krill from the aquarium and measured individual krill total length in the laboratory as from anterior edge of the eye to the tip of the telson (“AT” of Morris et al. [22]; grey histogram, Figure 2(b)). The mean length bias was applied to the camera length observations resulting in a set of corrected observations of mean length = 37.0 mm and standard deviation = 4.1 mm. The laboratory-based mean krill length was = 37.7 mm and standard deviation = 3.7 mm (Fig. 2B). These length means were not significantly different (t = −1.26; p-value = 0.21; Figure 2(b)). Furthermore, the length distributions were not significantly different between the camera and laboratory derived krill length measurements (two-sample Kolmogorov-Smirnov test D = 0.18, p-value = 0.08).
2.2. Krill Behaviour
From three independent behavioural states we extracted movement metrics from ten randomly selected individuals with ten different individuals observed in each behavioural state. The three behaviour states were: 1) undisturbed, 2) feeding, and 3) escape response. Each behaviour state was measured after a period of 30 min had elapsed from deployment of the UVSC in the aquarium (settlement time). Behavioural states are as follows:
a) Krill undisturbed behaviour was recorded by observing behavioural metrics for two sec following the 30 min settlement time post UVSC deployment.
b) Feeding response was elicited through the introduction of a dense algal patch (a suspension of prawn larval feeds INVE Frippak Fresh CAR #1 and INVE Frippak Fresh CD #2) following the 30 min settlement time and then observed for 2 sec.
c) Escape behaviour was elicited by submerging and triggering a camera strobe in the krill tank following the 30 min settlement time. The escape response was deemed to have ended when krill returned to their undisturbed state (c. 1 sec post flash).
The behavioural metrics extracted from the USCS for individual krill were swimming speed, displacement length (Euclidian distance between the first and last path coordinates), tortuosity and vertical orientation (Figure 3). Tortuosity is the ratio between the length of an individual krill trajectory and the distance between start and end points. We found that swimming speed (ANOVA, F = 30.9, p-value = 1.8e−12) and displacement length (ANOVA, F = 43.76, p-value = 5.1e−08) were statistically sig-
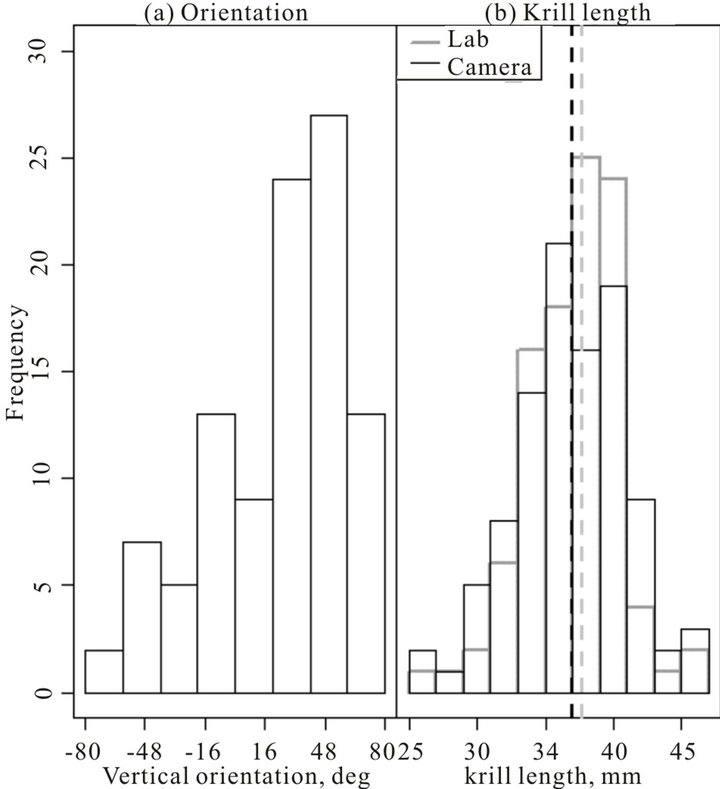
Figure 2. Krill vertical orientation and krill length measurements of 100 animals sampled at random. Panel (a): krill vertical orientation (measured from the horizontal plane). Panel (b): Histograms of krill length frequency distributions. The black histogram is the camera derived krill length frequency distribution (mean displayed as a black dashed vertical line). The grey histogram is the laboratory-based (labelled lab in the figure) krill measurements, with mean displayed as a grey dashed vertical line).
nificantly different for each behavioural state (Figures 3(a) and (b)). Tortuosity was statistically significantly different between the undisturbed state and the escape and feeding states (p-value = 2.5e−8) but not between the escape and feeding states (Figure 3(c)). Vertical orientation was not significantly different between behavioural states (ANOVA, F = 1.1, p-value = 0.33, Figure 3(d)).
3. Discussion
In this study, we demonstrated that a simple, economical underwater stereo camera system can successfully record krill morphometrics and behaviour in situ with repeatable and robust measurements. Kawaguchi et al. [4] demonstrated that captive krill could maintain their natural behaviour for long periods of time, thus we are confident that the aquarium-based observations presented here assessing USVS performance have external validity with regard to krill.
Some of the observations reported here have important implications. Whilst the 100 observations of krill in an undisturbed state showed large variation in vertical orientation (Figure 2(a)), there were no differences in mean vertical orientation between behavioural states. These results suggest that when calculating acoustically-derived
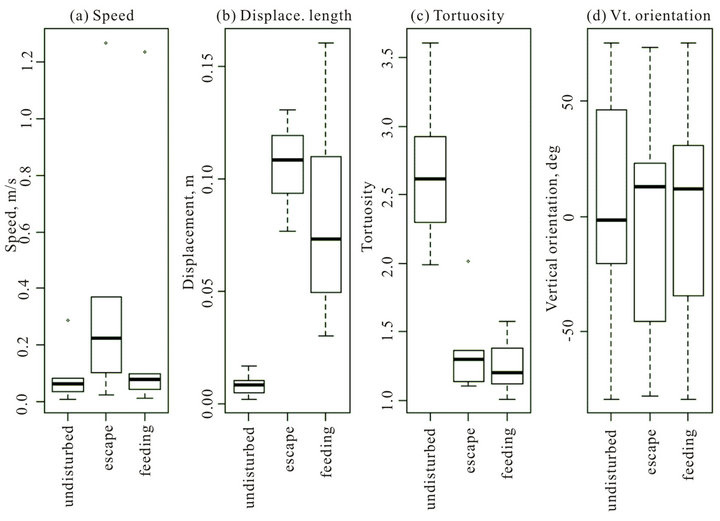
Figure 3. Krill behaviour metrics under three behavioural states: undisturbed, escape, and feeding. Behavioural metrics were: panel (a): swimming speed; panel (b): displacement length; panel (c): Tortuosity, and panel (d): vertical orientation.
krill biomass, it may be valid to apply a single distribution of vertical orientations throughout a survey region, noting however that caution should be used in generalising from this single experiment. Tank-based krill length and orientation observations cannot replace field-based observations for krill biomass estimation. However with field measurements made with the USVS, it should be possible to rapidly confirm or not the generalizability of this finding to wild krill.
We have demonstrated the efficacy of a low-cost underwater stereo video system to observe krill behaviour in an aquarium. This method clearly has applicability to other aquaria based studies; for instance, we envisage this method could readily be utilised in aquaria-based studies of krill for monitoring population growth, observing krill length and behaviour variation, e.g. escape response speed, under changing ocean conditions, such as acidification and warming. The USVS enables researchers to repeatedly observe krill behaviour and length frequency distributions so that a time-series can be established. As we have demonstrated in this study, measurement error can be quantified by observing underwater targets of known length and orientation, making it possible to correct for measurement error.
We have shown that a correction factor must be used in order to obtain sufficient precision to reliably measure krill. This correction factor is in part a function of the method employed by the stereo system [16] and in part a function of krill swimming behaviour. We suggest that if the USVS is used to monitor the growth rate of krill in an aquarium, a statistical distribution representing USVS observation error, such as the normal distribution, could be used to represent bias (e.g. the mean in a normal distribution) and random measurement error (e.g. the normal distribution variance parameter). Using the parameterised normal distribution in a simulation, the effect of USVS measurement error on krill growth rate estimates could be assessed to determine if the USVS measurements are fit for purpose.
The USCS approach allows a large proportion of an aquarium population to be repeatedly sampled whilst minimising stress to the animals. Krill are notoriously difficult to rear in captivity and any method that can reduce stress levels yet facilitate monitoring and experimentation is of high importance [23]. The USCS not only provides a robust non-invasive method for monitoring important physical parameters such as size, but can simultaneously provide effective behavioural metrics such as orientation, which clearly cannot be observed by removing individuals from aquaria.
3.1. Applicability to Wild Krill
We envisage the low-cost of the Gopro cameras will make it possible to simultaneously deploy multiple camera systems within field survey sites to approximately 60 m. Such deployments could be particularly useful for observing krill lengths and orientation during acoustic surveys of krill biomass [11]. The UCVS can also observe krill presence/absence in shallow-water (less than 15 m deep), a region that is not typically sampled during conventional acoustic surveys yet likely important to biomass estimates due to shoaling nature and vertical distribution of krill [12].
Word count = 2435.
4. Acknowledgements
We are grateful to Lloyd Groves for assistance using the EventMeasure software (SeaGIS, Pty Ltd.), and preparation of the movie clips. Engineering support was provided by Steven Whiteside from the Australian Antarctic division instrument workshop. David Borchers provided the R code to create the histograms in Figure 3. Work was funded by Australian Antarctic Division science programme Project 4037 (Experimental Krill Biology: Response of krill to environmental change), and Project 4050 (Assessing change in krill distribution and abundance in Eastern Antarctica. MJC was supported by ARC grant FS11020 0057. TBL was supported by the Marine Biodiversity Hub through the Australian Government’s National Environmental Research Program (NERP).
REFERENCES
- D. M. Bailey, N. J. King and I. G. Priede, “Cameras and Carcasses: Historical and Current Methods for Using Artificial Food Falls to Study Deep-Water Animals,” Marine Ecology Progress Series, Vol. 350, 2007, pp. 179- 191. doi:10.3354/meps07187
- T. B. Letessier, J. J. Meeuwig, K. Kemp, L. Groves, P. Bouchet, L. Chapuis, G. M. S. Vianna, M. Gollock and H. Koldewey, “Assessing Pelagic Fish Populations: The Application of Demersal Video Techniques to the Mid-Water Environment,” Methods in Oceanography, in Press.
- D. L. Watson, M. J. Anderson, G. A. Kendrick, K. Nardi and E. S. Harvey, “Effects of Protection from Fishing on the Lengths of Targeted and Non-Targeted Fish Species at the Houtman Abrolhos Islands, Western Australia,” Marine Ecology Progress Series, Vol. 384, 2009, pp. 241-249. doi:10.3354/meps08009
- S. Kawaguchi, R. King, R. Meijers, J. E. Osborn, K. M. Swadling, D. A. Ritz and S. Nicol, “An Experimental Aquarium for Observing the Schooling Behaviour of Antarctic Krill (Euphausia superba),” Deep Sea Research Part II: Topical Studies in Oceanography, Vol. 57, No. 7-8, 2010, pp. 683-692. doi:10.1016/j.dsr2.2009.10.017
- W. F. Dolphin, “Prey Densities and Foraging of Humpback Whales, Megaptera novaeangliae,” Experientia, Vol. 43, No. 4, 1987, pp. 1-4. doi:10.1007/BF01940459
- W. M. Hamner and P. P. Hamner, “Behavior of Antarctic Krill (Euphausia superba): Schooling, Foraging, and Antipredatory Behavior,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 57, No. 3, 2000, pp. 192-202. doi:10.1139/f00-195
- M. Cox, J. Waaren, D. Demer, G. Gutter and A. S. Brierley, “Three-Dimensional Observations of Swarms of Antarctic Krill (Euphausia superba) Made Using a MultiBeam Echosounder,” Deep Sea Research Part II: Topical Studies in Oceanography, Vol. 57, No. 7, 2010, pp. 508- 518. doi:10.1016/j.dsr2.2009.10.003
- S. Kawaguchi, R. Kilpatrick, L. Roberts, R. A. King and S. Nicol, “Ocean-Bottom Krill Sex,” Journal of Plankton Research, Vol. 33, No. 7, 2011, pp. 1134-1138. doi:10.1093/plankt/fbr006
- K. Schmidt, A. Atkinson, S. Steigenberger, S. Fielding, M. C. M. Lindsay, D. W. Pond, G. A. Tarling, T. A. Klevjer, C. S. Allen, S. Nicol and E. P. Achterberg, “Seabed Foraging by Antarctic Krill: Implications for Stock Assessment, Bentho-Pelagic Coupling, and the Vertical Transfer of Iron,” Limnology and Oceanography, Vol. 56, No. 4, 2011, pp. 1411-1428. doi:10.4319/lo.2011.56.4.1411
- R. P. Hewitt and D. Demer, “Krill Abundance,” Nature, Vol. 353, 1991, p. 310. doi:10.1038/353310b0
- L. Calise and G. Skaret, “Sensitivity Investigation of the SDWBA Antarctic Krill Target Strength Model to Fatness, Material Contrasts and Orientation,” CCAMLR Science, Vol. 18, 2011, pp. 97-122.
- T. Jarvis, N. Kelly, S. Kawaguchi, E. van Wijk and S. Nicol, “Acoustic Characterisation of the Broad-Scale Distribution and Abundance of Antarctic Krill (Euphausia superba) off East Antarctica (30-80 degrees E) in January-March 2006,” Deep Sea Research Part II: Topical Studies in Oceanography, Vol. 57, No. 9, 2010, pp. 916- 933. doi:10.1016/j.dsr2.2008.06.013
- D. A. Demer and S. G. Conti, “New Target-Strength Model Indicates more Krill in the Southern Ocean,” ICES Journal of Marine Science, Vol. 62, No. 1, 2005, pp. 25- 32. doi:10.1016/j.icesjms.2004.07.027
- ASAM, “CCAMLR SG-ASAM-10: Fifth Meeting of the Subgroup on Acoustic Survey and Analysis Methods, Cambridge, UK,” ASAM, Cambridge, 2010.
- G. L. Lawson, P. Wiebe, C. J. Ashjian, D. Z. Chu and T. K. Stanton, “Improved Parameterization of Antarctic Krill Target Strength Models,” Journal of the Acoustical Society of America, Vol. 119, No. 1, 2006, pp. 232-242. doi:10.1121/1.2141229
- E. S. Harvey and M. Shortis, “A System for Stereo-Video Measurement of Sub-Tidal Organisms,” Marine Technology Society Journal, Vol. 29, 1996, pp. 10-22.
- E. S. Harvey and M. Shortis, “Calibration Stability of an Underwater Stereo-Video System: Implications for Measurement Accuracy and Precision,” Marine Technology Society Journal, Vol. 329, No. 329, 1998, pp. 3-17.
- SeaGIS, “PhotoMeasure. SeaGIS Pty, Bacchus Marsh,” 2008. www.seagis.com.au
- S. Rao Jammalamadaka and A. Sengupta, “Topics in Circular Statistics, Section 2.2.7,” World Scientific Press, Singapore, 2001
- C. Agostinelli and U. Lund, “R Package ‘Circular’: Circular Statistic (Version 0.4-3),” 2011. http://r-forge r-project org/projects/circular
- R. C. Team, “R: A Language and Environment for Statistical Computing,” R Foundation for Statistical Computing, Vienna. http://www.R-project.org
- D. J. Morris, J. L. Watkins, C. Ricketts, F. Buchholz and J. Priddle, “An Assessment of the Merits of Length and Weight Measurements of Antarctic Krill Euphausia superba,” British Antarctic Survey Bulletin, No. 79, 1988, pp. 27-50.
- S. Nicol, J. Kitchener, R. King, G. Hosie and W. Delamare, “Population Structure and Condition of Antarctic Krill (Euphausia superba) off East Antarctica (80 - 150˚E) during the Austral Summer of 1995/1996,” Deep Sea Research Part II: Topical Studies in Oceanography, Vol. 47, No. 12, 2000, pp. 2489-2517. doi:10.1016/S0967-0645(00)00033-3
NOTES
*Corresponding author.

