Open Journal of Marine Science
Vol. 2 No. 3 (2012) , Article ID: 21422 , 7 pages DOI:10.4236/ojms.2012.23013
Morphology of Gametes and Insemination in the Vestimentiferan Riftia pachyptila
1A.V. Zhirmunsky Institute of Marine Biology FEB RAS, ul. Palchevskogo, Vladivostok, Russia
2Far East Federal University, Vladivostok, Russia
3P.P. Shirshov Institute of Oceanology RAS, Nakhimovsky pr., Moscow, Russia
Email: *anatoliyld@mail.ru
Received February 7, 2012; revised March 7, 2012; accepted April 22, 2012
Keywords: Riftia; Sperm; Eggs; Insemination; Spermatozeugmata
ABSTRACT
Ultrastructure of gametes (sperm and eggs) of vestimentiferan tubeworms and external-internal insemination by means of spermatozeugmata in Riftia pachyptila were described. The spermatozoa of Riftia are threadlike, about 130 µm long, and have a diameter of about 0.7 µm, narrowing to 0.2 µm in the apical portion of the macrodome, and pointed at the end of the tail. Oocytes are produced by the ovaries at the first meiotic prophase stage. The early oocytes are small, hardly exceeding 10 µm in diameter, spherical cells with a poorly differentiated cytoplasm and large nuclei with a nucleolus. Completely formed oocytes reaching up to 130 µm in diameter leave the ovary, their germinal vesicle is unresorbed and has a nucleolus. They are coated by a yolk membrane of 1.2 µm. The eggs enter the oviduct, move along, and accumulate in its expanded anterior portion, the ovisack. The sperm is released in seawater as sperm packages, each having the shape of a torch. Then sperm moves to females and sperm packages at the posterior end of the oviduct surrounding of eggs. Inside the female tube, spermatozoa and, possibly, yet unsplit sperm packages, invade the oviducts through genital openings, where the unfertilized eggs are already present in the terminal portion of the egg sack.
1. Introduction
External-internal insemination by means of the spermatozeugmata occurs in nature enough often as in vertebrates [1,2] as in invertebrates [3-5]. There are many works about spermatozeugmata in annelids and related taxons [6-8].
Pogonophorans and vestimentiferans, or Siboglinids (Annelida) in modern taxonomy [9-11]—deep-sea worms that have chitinous tubes and inhabit reducing biotopes such as hydrocarbon seeps, hydrothermal vents, etc.— play an important role in many marine ecosystems. They have long attracted zoologists, with particular attention to their feeding and reproduction.
The reproductive organs of male and female Lamellibrachia barhami have been described at the histological level [12-14]. A light microscopic description of the gamete morphology of pogonophorans is given in a number of papers [12-15]. An electron microscopic study of sperm has been carried out on the pogonophoran Siboglinum ermani [16] and on the vestimentiferans Riftia pachyptila [17,18], Lamellibrachia luymesi, and Ridgeia piscesae [18,19].
Riftia pachyptila, the first vestimentiferan species described from deep-sea hydrothermal vents, has become a kind of model for the study of obturate pogonophoran biology [20]. In recent years, several papers have been published on the reproductive biology and development of this species [18,21,22]. Direct observations of Riftia ejecting both types of gametes into the seawater testify to external insermination in these animals [21]. At the same time, the sperm was found in the spermatheca of R. pachyptila and Lamellibrachia luymesi, suggesting internal fertilization in vestimentiferans [22].
Many questions remain open concerning the ultrastructure of mature spermatozoa in these species. The description of sperm of R. pachyptila was based on sections of the late spermatids [17]. The authors had at their disposal only cross-sections of mature spermatozoa. Marotta et al. [18] represented a different picture of the structure of the vestimentiferan sperm acrosome.
2. Materials and Methods
The vestimentiferans Riftia pachyptila (Polychaeta: Siboglinidae: Vestimentifera) were sampled using “MIR” manned submersibles during the 49th cruise of the RV “Akademik Mstislav Keldysh” in the rift zone of the East-Pacific Rise (9˚45’N; 103˚41’W) at a depth of about 2600 m and in the Guaymas Basin of the Gulf of California (27˚00’N; 111˚24’W) at a depth of about 2000 m. Live male and female gonads were analyzed under a light microscope. We picked up R. pachyptila specimens aboard and washed away mature eggs and sperm out of the tubes in the laboratory by an optical microscopy right away the worms were elevated to the deck from depth. We observed sperm motion and took its video record.
For electron microscopy, pieces of the gonads were prefixed in a 3% glutaraldehyde solution in cacodylate buffer with the addition of sodium chloride. Then they were postfixed in a 2% solution of osmium tetroxide in the same buffer, dehydrated in alcohols, and embedded in Epon-araldite resin. Sections were cut with a Reichert-Jung Ultracut E ultratome and analyzed with a JEOL 100 SX electron microscope.
3. Results
In hydrothermal sites of the East-Pacific Rise and in the Guaymas Basin, the giant (more than 1 m long) vestimentiferan Riftia pachyptila forms massive aggregations of dioecious individuals. Reproduction of R. pachyptila is asynchronous; however, ready-to-spawn animals with mature gametes are always present.
Ultrastructure of sperm. The spermatozoa of Riftia pachyptila are threadlike, about 130 µm long, and have a diameter of about 0.7 µm, narrowing to 0.2 µm in the apical portion of the acrosome, and pointed at the end of the tail. The sperm can be subdivided into three portions: an acrosome, a nucleus, and a tail, which includes the middle part of the sperm. The length of the acrosome is 6 µm, the length of nucleus is 26 µm, and that of the tail, including its middle part, is about 98 µm. The sperm mid portion is about 1 µm long (Figures 1(a)-(f)).
The sperm acrosome is formed by an elongated acrosomal vesicle, about 6 µm long, spirally twirled (4 gyres) around an electron-transparent subacrosomal material with sparse granules. The wall thickness of the acrosomal vesicle varies from 0.05 to 0.5 µm (Figures 1(a)-(c)). The acrosome main axis is located at an angle to the axis of the nucleus. A spiral thickening is present on the periphery of the acrosome. The basal part of the acrosome contains an electron-dense structure connecting it with the nucleus (Figures 1(b) and (c)). In one instance, the acrosome was seen to surround a hollow apical portion of the nucleus (Figure 1(a)).
The acrosome is followed by a region about 26 µm long that hosts the nucleus and mitochondria (Figures 1(a), (b) and (d)). The nucleus is made up of condensed chromatin and a short electron-transparent anterior portion of about 3 µm long. The apical portion of the nucleus is electron transparent and is merely enveloped by a nuclear membrane. The nuclear wall starts to thicken gradually, and the nucleus becomes filled with condensed chromatin and thus completely electron dense (Figures 1(a), (b) and (d)). There are two spiral parallel grooves in the nucleus (Figure 1(d)). One of the grooves hosts one or two mitochondria spirally twining round the nucleus, the other is filled with cytoplasm without any visible structures.
The sperm tail is threadlike, about 0.7 µm in diameter, and hosts an axoneme with a typical pattern, 9(2) + 2, of microtubules. It begins from a modified distal centriole (Figure 1(f)). The kinetosome is surrounded by a fibrillar root complex beginning from the middle portion of the kinetosome. Its electron dense fibrils surrounding the distal portion of the kinetosome and the apical portion of the axoneme separate and protrude out of the cytoplasm to form a special structure, the annulus (Figure 1(f)). The tail ends in a terminal portion, in which the doublets of microtubules are reduced gradually.
Ultrastructure of eggs. The ovaries of Riftia are very large and are located ventral to the trophosome in the paired gonocoel along the entire trunk and above the ventral blood vessel. Posteriorly the ovaries pass into the oviducts running forward and located ventrally to the ovaries.
In the middle part of the ovaries, three types of cells are observed: oogonia, oocytes at various stages of growth, and follicular cells (Figure 1(g)). The ovarian tissue is penetrated by anastomosing blood vessels surrounded by somatic cells. The oogonia are rather small, slightly more than 10 µm in diameter, and have a large nucleus with a transparent nucleoplasm.
The early oocytes are small, hardly exceeding 10 µm in diameter, spherical cells with a poorly differentiated cytoplasm and large nuclei having a nucleolus (Figures 1(g), (h) and (j)). Their cytoplasm contains elongate mitochondria, channels of endoplasmic reticulum, and cisterns of Golgi’s complex (Figure 1(k)). As they grow, the oocytes enlarge, cytoplasmic organelles increase in number, and, in addition to numerous mitochondria and dictyosomes, yolk granules of variable size of two types emerge in the cytoplasm: electron dense protein granules and electron transparent lipid ones (Figures 1(j) and (k)). At early stages of growth, oocytes are already surrounded by follicular cells with a large nucleus, about 10 µm in diameter, and a thin layer of cytoplasm (Figures 1(h), (j) and (k)). The plasma membrane of oocytes forms numerous microvilli: protrusions about 1.2 µm high and 0.01 µm thick, surrounded by an electron dense material, the glycocalix. The distal ends of microvilli closely contact follicular cells (Figures 1(h), (j) and (k)). In the cortical oocyte layer, besides numerous vesicles, no specialized cortical granules are observed that release their contents during the cortical reaction.
Completely formed oocytes reaching up to 130 µm in diameter leave the ovary; their germinal vesicle is unresorbed and has a nucleolus (Figures 1(i) and (k)). They are coated by a yolk membrane of 1.2 µm. The eggs enter the oviduct, move along, and accumulate in its expanded anterior portion, the ovisack (Figures 1(l) and (m)).
Insemination. When sperm released into seawater, the spermatozoa begin to move within the packages, so as the packages themselves start moving. Quickly moving sperm packages find their way into the chitinous tubes of the females.
The packages in seawater do not retain their integrity, for more than 30 s, and then split into individual spermatozoa, each capable of independent wormlike motion. This is clearly seen with the light microscope during observations of live sperm packages and spermatozoa. Inside the female tube, spermatozoa and, possibly, yet unsplit sperm packages invade the oviducts through genital openings. Unfertilized eggs are already present in the terminal portion of the female egg sack (Figures 1(l) and (m)).
The sperm occur in seawater as sperm packages, each having the shape of a torch (Figure 2). Its cup is formed by acrosomes, a nuclear region of the sperm, and a middle region of the sperm. The long handle of the torch is formed by densely packed sperm tails. It has a pointed terminal region about 12.5 µm long formed by motile portions of the tails, which are only formed by axonemes not surrounded by a layer of cytoplasm. Initially, the packages are associated in groups, being connected by ends of the tails. The spermatozoa in packages are densely packed, and it is possible to distinguish three regions in the sperm package: an acrosomal region, a nuclear one, and a tail. There are about 340 - 350 spermatozoa in each package connected with each other by thin fibrils. The sperm packages are surrounded along the periphery by a thin layer of nanofibrils to ensure integrity.
Here, the sperm penetrates the eggs, and the maturation process is completed: the pronuclei merge to form the nucleus of a zygote.
4. Discussion
Various aspects of the reproduction and development of Riftia have earlier been studied on specimens sampled in vent sites of the Galapagos Rift [17] and the East Pacific Rise [18,21,22]. We also collected Riftia in the East-Pacific Rise rift zone, and, in addition, in the Guaymas Basin, the Gulf of California. Our observations on reproduction have been made on the East-Pacific material.
Our observations of the gamete morphology and insemination of Riftia mostly agree with the data of other authors who studied the gametes of Riftia [17-18,21-22]. There are discrepancies on the sperm acrosome structure in vestimentiferans described by different authors. Very unusual sperm with a unique structure of the acrosome have been described for Ridgeia piscesae [19]. These authors noted that the acrosomes of late spermatids sharply differ from those of mature sperm. The acrosome of late spermatids of Ridgeia is a spirally coiled ribbon-like cistern twining around the spindle-shaped process of spermatids that hangs down from its anterior end near the place of its connection with the cytoplasm of the cytophore. In the mature sperm of this species, the acrosome is surrounds the anterior region of the nucleus. Gardiner & Jones [17], who studied the ultrastructure of spermatogenesis in R. pachyptila, described only the formation of acrosomes in the spermatids as they had no mature spermatozoa at their disposal. Nevertheless, in a large review on vestimentiferans [23], these authors drew the conclusion that a similar displacement of the acrosome after completion of spermatogenesis might occur in R. pachyptila, too. The acrosome, which is lateral to the nucleus in late spermatids, moves onto the nucleus, surrounding its apical portion devoid of chromatin and the adjacent region of the nucleus containing chromatin. Such a unique structure of sperms in vestimentiferans greatly differs from that of perviate pogonophores, particularly Siboglinum [16], and this was considered as an argument in favor of the supposition that perviate pogonophores and obturate pogonophores or vestimentiferans may belong to absolutely different taxa [24]. It has been suggested to classify vestimentiferans as a phylum or as a subphylum Obturata with a single class— Vestimentifera [20]. But now zoologists consider all pogonophorans, among Polychaeta [9-11,25,26].
Our research shows that no change in the location of the sperm acrosome occurs at the end of spermiogenesis. In late spermatids, the acrosome is already anterior to the apical portion of the nucleus, being located at a slight angle to it. There is an electron dense structure between the acrosome and the nucleus that connects the acrosomal vesicle and the nucleus. Our observations confirm the results of electron microscopical and immunochemical studies by Marotta et al. [18] demonstrating that in mature sperm in sperm packages of Riftia pachyptila and Lamellibrachia luymesi the acrosome is located in front of the nucleus. In our material, the only instance where the acrosome is shifted onto the apical portion of the nucleus was probably an artifact of fixation.
Oogenesis of R. pachyptila is similar to oogenesis of annelids, which has been adequately studied in various groups: polychaetes [27,28], oligochaetes [29], and leaches [30]. Our observations on oogenesis in R. pachyptila are consistent with the earlier description [23]. Oogonia and oocytes at different stages of growth surrounded by flattened follicular cells are observed in the ovaries of R. pachyptila. Blood vessels are also found, but trophocytes
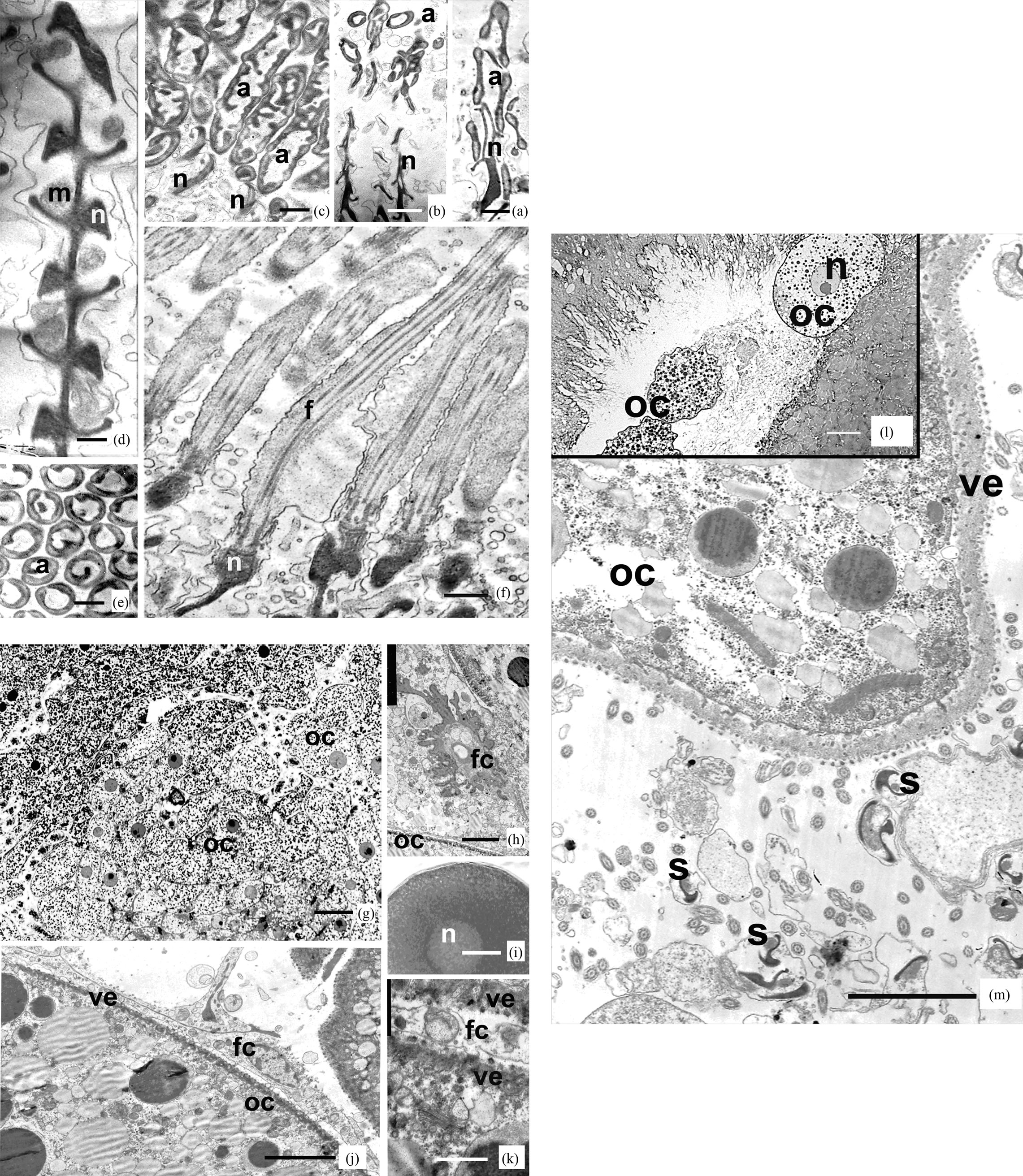
Figure 1. Longitudinal ((a)-(d), (f)) and cross-cut (e) sections of spermatozoa of vestimentiferan Riftia pachiptila. (a) Sperm acrosomes and apical parts of nucleus. Scale: 0.25 μm; (b) Acrosomes and nucleus of sperm of Riftia. Scale: 0.5 μm; (c) Lon-gitudinal sections of sperm acrosomes. Scale: 0.25 μm; (d) Middle part of nucleus and mitochondria. Scale: 0.25 μm; (e) Cross sections of acrosomes. Scale: 0.25 μm; (f) Tail parts of sperm of Riftia. Scale: 0.5 μm; Oocytes ((g)-(j)) and mature egg (i) of vestimentiferan Riftia pachiptila; (g) Oocytes of Riftia. Scale: 20 μm; (h) Oocyte cytoplasm at a stage of growth. Scale: 10 μm; (i) Mature egg of Riftia, washed up from female oviduct. Scale: 10 μm; (j) The follicular cells, surrounding oocytes. Scale: 10 μm; (k) Vitelline envelope of oocytes. Scale: 1 μm; (l) and (m) Mature eggs of Riftia pachyptila in female oviduct, sur-rounded with sperm. (l) Scale: 40 μm; (m) Scale: 5 μm.
Abbreviations for figgures. (a)-(m): a: acrosome, f: flagellum, fc: folliculare cell, m: mitochondria, n: nucleus, oc: oocyte, s: sperm, ve: vitelline envelope.
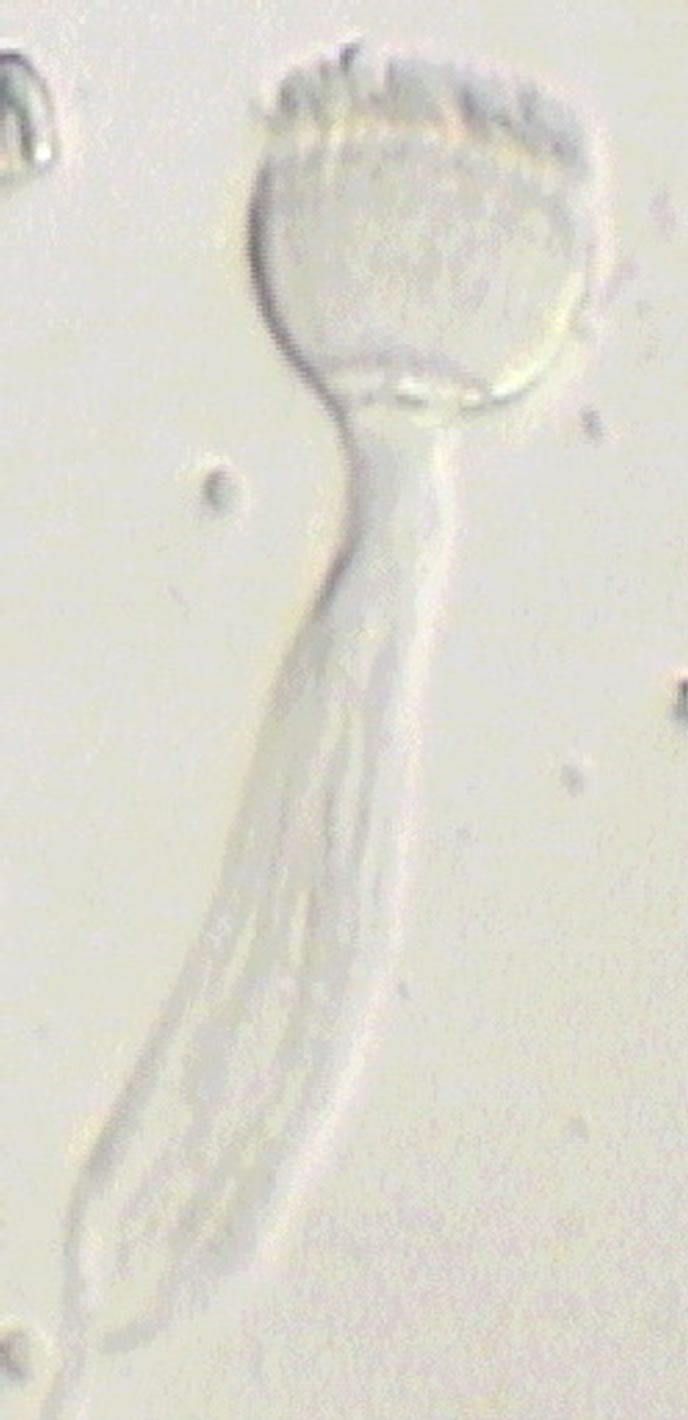
Figure 2. The sperm package of R. pachyptila in sea water has the shape of a torch. Scale: 10 µm.
are absent. Follicular cells that perform the protein-synthesizing and nutritive functions have been described for many polychaetes [27,28]. They can also participate in oocyte resorption. Similarly to previous observations, the blood vessels are usually surrounded by various somatic cells, which, in addition to the feeding function, have the structuring function and support the ovarian tissue. It is possible to summarize that Riftia exhibits the follicular type of oogenesis which occurs in many polychaetes.
It is generally believed that insemination is internal in R. pachyptila, a view first put forward by Gardiner and Jones [17] and now shared by many authors [19,21,22, 31-33]. As R. pachyptila typically form dense populations of hundreds of dioecious individuals, Malakhov and Galkin [20] assumed that the males could transfer sperm masses to the females by extending the tentacular region with adhering sperm packages out of the tube and bending it toward the females.
In our opinion, insemination in R. pachyptila should be classified as the external-internal type, and it is accomplished by means of spermatozeugmata. During spermiogenesis, R. pachyptila forms packages consisting of 340 - 350 spermatozoa. The anterior part of sperm packages comprised of acrosomes, nuclei, middle parts of spermatozoa, and short basal portions of tails are stuck together by means of an extracellular nanofibrillar matrix and form a uniform structure. The remaining greater part of sperm tails are stick together more losely.
As the sperm packages consist of homogeneous cells, they cannot be classified as spermatophores, and such formations are referred to as the spermatozeugmata [19]. To date, insemination by means of the spermatozeugmata has been described for a large variety of animals: fishes [1,2,34-35], annelids [6,7,36], gastropods [37,38]. The spermatozeugmata is also found in animals with external insemination, namely bivalves [39,40], bryozoans [4,5], and sipunculans [8].
The spermatozeugmata is thought to provide a more effective transport of sperm. For example, in oyster, the eggs usually remain close to motionless females at a significant distance from males [40]. Males of freshwater bivalve unionids release the spermatozeugmata into the water as spherical aggregations of 8000 - 9000 spermatozoa with their heads turned to the center of the sphere [41]. In the bryozoan Membranipora membranacea, the colonies eject into seawater individual eggs and spermatozeugmata containing 32 - 64 spermatozoa [4].
During spawning, sperm packages or spermatozeugmata are released from the males into the water where they actively move to the tubes of females. In the laboratory, the spermatozeugmata split within 15 - 30 seconds into individual spermatozoa. However, it is quite possible that in specific conditions of hydrothermal vents the sperm packages keep their integrity for a longer period. When inside the tube of female, the spermatozoa penetrate through the genital opening (gonopore) into the eggsack, where they can be stored and fuse with the eggs. Sperm packages cannot move within the limited space of the oviducts, but spermatozoa are capable of vermicular movements in the oviducts. For adaptation to this type of motion, R. pachyptila sperm contain a plenty of cytoplasm in the nuclear and tail regions. Only the acrosome is surrounded by the plasma membrane and it has no layer of cytoplasm.
Such a type of insemination should be considered external-internal because during spawning the sperm is released into the seawater, swim to the females, and penetrate into the female tubes to carry out fertilization by merging with the eggs in the oviducts. R. pachyptila eggs, being of a medium size, about 130 µm, have merely a thin yolk envelope and are adapted for internal insemination and development up the larval stage in the ovisacks or in the tube cavity. The lack of well-developed cortical granules suggests that at fertilization no apparent cortical reaction occurs in the eggs of R. pachyptila and no fertilization membrane is formed, i.e., the embryos have no other protective coating than a thin yolk envelope.
It is safe to assume that the spermatozeugmata are capable of chemotaxis—directional movement inside the female tube. The middle region of sperm contains numerous actin microfilaments [18]. This suggests that the tail is motile relative to the nucleus and that the spermatozoa, as well as the sperm package can change the direction of movement.
In summary, it can be concluded that the gametes of R. pachyptila are adapted for external-internal insemination and embryonic development inside the females. By their development and morphology, gametes of R. pachyptila are similar to those of many annelids that exhibit insemination by means of the spermatozeugmata. This undoubtedly points to the affinity of the vestimentiferans with the polychaetes. This viewpoint has long been advanced by mophologists [11,43-45] and has repeatedly been confirmed by molecular biologists [9,10,25,26].
4. Acknowledgements
This work was supported by a grant of Cariplo Foundation & Landau Network-Centro Volta (Como, Italy). We thank Prof. Marco Ferraguti and Roberto Marotta (University of Milan) for help in studying and Tat’yana Koznova for help in translating from Russian into English.
REFERENCES
- A. L. Downing, J. R. Burns, “Testis morphology and spermatozeugma formation in Three Genera of Viviparous Halfbeaks: Nomorhamphus, Dermogenys, and Hemirhamphodon (Teleostei:Hemiramphi-dae),” Journal of Morphology, vol. 225, No. 3, 1999, pp. 329-343. doi:10.1002/jmor.1052250305
- A. Pecio, J. R. Burns and S. H. Weitzman, “Sperm and Spermatozeugmata Ultrastructure in the Inseminating Species Tyttocharax cochui, T. tambopatensis, and Scopaeocharax rhinodus (Pisces: Teleostei: Characidae: Glandulocaudinae: Xenurobrynconini),” Journal of Morphology, vol. 263, No. 2, 2005, pp. 216-226. doi:10.1002/jmor.10299
- J. M. Healy and B. G. M. Jamieson, “Euspermatozoa, paraspermatozoa and spermatozeugmata of Littoraria (Palustorina) articulata (Prosobranchia: Caenogastropoda) with Special Reference to the pseudotrich,” Acta Zoologica, vol. 74, No. 4, 1993, pp. 321-330. doi:10.1111/j.1463-6395.1993.tb01246.x
- M. H. Temkin and S. Bortolami, “The Waveform Dynamics of spermatozeugmata during the transfer from paternal to Maternal Individuals of Membranipora membranacea,” Biological Bulletin, vol. 206, No. 1, 2004, pp. 35-45. doi:10.2307/1543196
- Å. Franzén, “Spermiogenesis, Sperm Structure and spermatozeugmata in the Gymnolaematous Bryozoan Electra pilosa (Bryozoa, Gymnolaemata),” Invertebrate Reproduction and Development, vol. 34, No. 1, 1998, pp. 55- 63. doi:10.1080/07924259.1998.9652353
- M. Ferraguti, G. Bernadini, G. Melone and R. Dallai. “Structure and function of the metachronal wave in Tubifex tubifex spermatozeugmata (Annelida, Oligochaeta),” Journal of Ultrastructure and Molecular Structure Research, vol. 99, No. 1, 1988, pp. 79-95. doi:10.1016/0889-1605(88)90035-3
- M. Ferraguti, G. Grassi and C. Erséus, “Different models of Tubificid Spermatozeugmata,” Hydrobiologia, vol. 180, No. 1, 1989, pp. 73-82. doi:10.1007/BF00027539
- A. S. Maiorova and A. V. Adrianov, “The first finding of spermatozeugmata in Sipunculids (Sipuncula), animals with External Fertilization,” Doklady Biological Sciences, vol. 402, No. 1-6, 2005, pp. 214-216. doi:10.1007/s10630-005-0092-z
- K. Fauchald and G. W. Rouse, “Polychaete systematics: past and present,” Zoologica Scripta, vol. 26, No. 2, 1997, pp. 71-138. doi:10.1111/j.1463-6409.1997.tb00411.x
- G. Rouse, “A cladistic analysis of Siboglinidae Caullery, 1914 (Polychaeta, Annelida): formerly the phyla Pogonophora and Vestimentifera,” Zoological journal of the Linnean Society, vol. 132, No. 1, 2001, pp. 55-80. doi:10.1111/j.1096-3642.2001.tb02271.x
- A. Schulze, “Phylogeny of Vestimentifera (Siboglinidae, Annelida) inferred from morphology,” Zoologica scripta, vol. 32, No. 4, 2002, pp. 321-342. doi:10.1046/j.1463-6409.2003.00119.x
- A. V. Ivanov, “Pogonophore, Fauna SSS (Fauna of the USSR), vol. 75,” Akademiya Nauk SSSR, Moscow, 1960 (in Russian).
- A. V. Ivanov, “Pogonophora,” Academic Press, London, 1963.
- M. Webb, “Studies on Lamellibrachia barhami (Pogonophora). II—The Reproductive Organs,” Zoologische Jahrbuecher Abteilung fuer Anatomie und Ontogenie der Tier, vol. 97, 1977, pp. 455-481.
- J. Van der Land and A. Nørrevang, “Structure and relationships of Lamellibrachia (Annelida, Vestimentifera),” Kongelige Danske Videnskabernes Selskab, Biologist Skrifter, vol. 21, 1977, pp. 1-102.
- Å. Franzén, “The spermatozoon of Siboglinum (Pogonophora),” Acta Zoologica, vol. 54, No. 3, 1973, pp. 179- 192. doi:10.1111/j.1463-6395.1973.tb00453.x
- S. L. Gardiner and M. L. Jones, “Ultrastructure of spermiogenesis in the vestimentiferan tube worm Riftia pachyptila (Pogonophora: Obturata),” Transactions of the American Microscopical Society, vol. 104, No. 1, 1985, pp. 19-44. doi:10.2307/3226354
- R. Marotta, G. Melone, M. Bright and M. Ferraguti, “Spermatozoa and Sperm Aggregates in the Vestimentiferan Lamellibrachia luymesi. Compared with those of Riftia pachyptila (Polychaeta: Siboglinidae: Vestimentifera),” Biological Bulletin, vol. 209, No. 3, 2005, pp. 215-226. doi:10.2307/3593111
- E. C. Southward, K. A. Coates, “Sperm masses and spermtransfer in a vestimentiferan, Ridgeia piscesae Jones, 1985 (Pogonophora: Obturata),” Canadian Journal of Zoology, vol. 67, No. 11, 1989, pp. 2776-2781. doi:10.1139/z89-393
- V. V. Malakhov and S. V. Galkin, “Vestimentiferans— Gutless Invertebrates of Sea Depths,” KMK, Moscow, 1998 (in Russian).
- C. L. Van Dover, “In Situ Spawning of Hydrothermal Vent Tubeworms (Riftia pachyptila),” Biological Bulletin, vol. 186, No. 1, 1994, pp. 134-135. doi:10.2307/1542043
- A. Hilario, C. M. Young and P. A. Tyler, “Sperm Storage, Internal Fertilization, and Embryonic Dispersal in Vent and Seep Tubeworms (Polychaeta: Siboglinidae: Vestimentifera),” Biological Bulletin, vol. 208, No. 1, 2005, pp. 20-28. doi:10.2307/3593097
- S. L. Gardiner, M. L. Jones, “Vestimentifera,” In: F. W. Harrison and M. E. Rice, eds., Microscopic Anatomy of Invertebrates. 12. Onycophora, Chilopoda, and Lesser Protostomata, Wiley-Liss, New York, 1993, pp. 371-460.
- A. L. Drozdov and V. N. Ivankov, “Gamete morphology of animals. Meaning for systematics and phylogenetics,” Round years, Moscow, 2000 (in Russian).
- D. McHugh, “Molecular Evidence That Echiurans and Pogonophorans Are Derived Annelids,” Proceedings of the National Academy of Sciences of the United States of America, vol. 94, No. 15, 1997, pp. 8006-8009. doi:10.1073/pnas.94.15.8006
- K. J. Peterson and P. J. Eernisse, “Animal Phylogeny and the Ancestry of Bilaterians: Inferences from Morphology and 18S rDNA Gene Sequences,” Evolution & Development, vol. 3, No. 3, 2001, pp. 170-205. doi:10.1046/j.1525-142x.2001.003003170.x
- K. J. Eckelbarger, “Oogenesis and Female Gametes,” In: W. Westheide and C. O. Hermans, eds., The ultrastructure of Polychaeta. 4. Microfauna Marina, Fischer Verlag, Stuttgart, 1988, pp. 291-307.
- K. J. Eckelbarger, “Polychaeta: Oogenesis,” In: F. W. Harrison and S. L. Gardiner, eds., Microscopic Anatomy of Invertebrates. 7. Annelida, Wiley-Liss, New York, 1992, pp. 109-127.
- E. Siekierska, “The structure of the ovary and oogenesis in the earthworm, Dendrobaena veneta (Annelida, Clitellata),” Tissue & Cell, vol. 35, No. 4, 2003, pp. 252-259. doi:10.1016/S0040-8166(03)00038-7
- J. Fernández, V. Téllez and N. Olea, “Hirudinea,” In: F. W. Harrison and S. L. Gardiner, Eds., Microscopic anatomy of Invertebrates. 7. Annelida, Wiley-Liss, New-York, 1992, pp. 323-394.
- S. C. Cary, H. Felbeck and N. D. Holland, “Observations on the Reproductive Biology of the Hydrothermal Vent Tube Worm Riftia pachyptila,” Marine Ecology Progress Series, vol. 52, 1989, pp. 89-94. doi:10.3354/meps052089
- C. L. Van Dover, “The Ecology of Deep-Sea Hydrothermal Vents,” Princeton University Press, Princeton, 2000.
- I. R. MacDonald, V. Tunnicliffe and E. C. Southward, “Detection of Sperm Transfer and Synchronous Fertilization in Ridgeia piscesae at Endeavour segment,” Juan da Fuca Ridge, Les Cahiers de Biologie Marine, vol. 43, 2002, pp. 395-398.
- H. L. Pratt Jr. and S. Tanaka, “Sperm storage in male elasmobranchs: A Description and survey,” Journal of Morphology, vol. 219, No. 3, 1994, pp. 297-304. doi:10.1002/jmor.1052190309
- M. Girard, P. Rivalan and G. Sinqlin, “Testis and Sperm Morphology in Two Deep-Water Squaloid Sharks, Centroscymnus coelolepis and Centrophorus squamosus,” Journal of Fish Biology, vol. 57, No. 6, 2000, pp. 1575- 1589. doi:10.1111/j.1095-8649.2000.tb02233.x
- B. D. Vanpraagh, “Reproductive-Biology of Megascolides australis Mccoy (Oligochaeta, Megascolecidae),” Australian Journal of Zoology, vol. 43, No. 5, 1995, pp. 489- 507. doi:10.1071/ZO9950489
- J. A. Buckland-Nicks and F.-S. Chia, “On the nurse cell and spermatozeugmata in Littorina sitkana,” Cell Tissue Research, vol. 179, No. 3, 1977, pp. 347-356. doi:10.1007/BF00221105
- J. M. Healy and B. G. M. Jamieson, “Euspermatozoa, paraspermatozoa and spermatozeugmata of Littoraria (Palustorina) articulata (Prosobranchia: Caenogastropoda) with Special Reference to the pseudotrich,” Acta Zoologica, vol. 74, No. 4, 1993, pp. 321-330. doi:10.1111/j.1463-6395.1993.tb01246.x
- D. Ó Foighil, “Sperm transfer and storage in the brooding bivalve Mysella tumida,” Biological Bulletin, vol. 169, No. 3, 1985, pp. 602-614. doi:10.2307/1541302
- D. Ó Foighil, “Role of spermatozeugmata in the spawning ecology of the Brood Oyster Ostrea edulis,” Gamete Research, vol. 24, No. 2, 1989, pp. 219-228. doi:10.1002/mrd.1120240209
- D. L. Waller and B. A. Lasee, “External Morphology of Spermatozoa and Spermatozeugmata of the Freshwater Mussel Truncilla truncata (Mollusca: Bivalvia: Unionidae),” American Midland Naturalist, vol. 138, No. 1, 1997, pp. 220-223. doi:10.2307/2426669
- M. H. Temkin and S. Bortolami, “The Waveform Dynamics of spermatozeugmata during the transfer from paternal to Maternal Individuals of Membranipora membranacea,” Biological Bulletin, vol. 206, No. 1, 2004, pp. 35-45. doi:10.2307/1543196
- N. A. Liwanov and N. A. Porfirjeva, “About annelid hypothesis of Pogonophorans Origin,” Zoologicheskiy zhurnal, vol. 44, 1965, pp. 161-175 (in Russian).
- N. A. Liwanov and N. A. Porfirjeva, “Die Organization der Pogonophoren und deren Beziehungen zu den Polychäten,“ Biologisches Zentralblatt, vol. 86, 1967, pp. 177-204.
- E. C. Southword, A. Schulze and S. Gardiner, “Pogonophora (Annelida): form and Function,” In: Developments in Hydrobiology. Morphology, Molecules, Evolution and Phylogeny in Polychaeta and Related Taxa, vol. 179, 2005, pp. 227-251.
NOTES
*Corresponding author.

