Open Journal of Obstetrics and Gynecology
Vol.07 No.03(2017), Article ID:74730,13 pages
10.4236/ojog.2017.73031
Evaluation of Steroid Receptors mRNA Fingerprints in Two Groups of Normozoospermic Patients: Men from Unexplained Infertility Couples vs. Men from Couples with Tubal Factor Infertility
Katarzyna Jarzabek1*, Agnieszka Mikucka-Niczyporuk2, Tomasz Bielawski1, Robert Milewski3, Jacek Z. Kubiak4,5,6, Slawomir Wolczynski2
1Department of Biology and Pathology of Human Reproduction in Bialystok, Institute of Animal Reproduction and Food Research in Olsztyn, Polish Academy of Sciences, Olsztyn, Poland
2Department of Reproduction and Gynecological Endocrinology, Medical University of Bialystok, Bialystok, Poland
3Department of Statistics and Medical Informatics, Medical University of Bialystok, Bialystok, Poland
4Institute of Genetics and Development of Rennes, Cell Cycle Group, CNRS, UMR 6290, Rennes, France
5Faculty of Medicine, University Rennes 1, UEB, IFR 140, Rennes, France
6Department of Regenerative Medicine and Cell Biology, Military Institute of Hygiene and Epidemiology, Warsaw, Poland

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 6, 2017; Accepted: March 12, 2017; Published: March 15, 2017
ABSTRACT
The study of sperm cellular components at molecular level is crucial for the diagnosis of male unexplained infertility. The aim of the study was to compare the molecular profile of steroid receptors and aromatase in spermatozoa obtained from two normozoospermic groups of patients issued from couples treated for infertility. We investigated 46 male patients from unexplained infertility couples and from men, 38 where female partners presented with tubal infertility. Sperm ERs (estrogen receptors: alpha and beta), GPER (G protein- coupled estrogen receptor), AR (androgen receptor) and aromatase mRNA expression levels by TaqMan qPCR were analyzed. AR transcript level was significantly lower in sperm of men from unexplained infertility couples vs. men from couples with tubal factor infertility (P = 0.04). Although the AR mRNA expression level did not had any effect on embryo development and its implantation, a significant correlation between AR mRNA levels and clinical pregnancy in unexplained infertility patients was observed. Taken together, AR transcript presence in ejaculated spermatozoa could be a potential marker for unexplained infertility.
Keywords:
Androgen Receptor, Unexplained Infertility, Spermatozoa mRNA

1. Introduction
The role of different RNA molecules in human mature ejaculated spermatozoa still remains unexplored. The previous dominant claim was that a population of residual mRNAs without any embryological and diagnostic values is presented in mature human sperm. However, current evidence strongly suggests that gene transcripts in ejaculated spermatozoa may help to determine the male fertility potential [1] . Routine diagnostics for infertility do not indicate any functional or organic pathology responsible for infertility in about 10% - 15% of couples in reproductive age. Most of these causes are defined as unexplained infertility. The unknown cause of pregnancy failure has recently been linked to molecular male factor but unrelated to sperm count, motility or morphology [2] .
Spermatozoa RNA may contain remnants of mRNAs reflecting only the proper course of spermatogenesis [3] [4] . On the other hand, it was shown that the spermatozoon can transmit to oocyte some of RNAs both in mice [5] [6] and human [7] and further contribute extragenomically to early embryonic development. Therefore it is highly likely that detailed sperm transcriptome analysis, could decode the role of RNAs population in mature spermatozoa, thus also in the diagnosis of male unexplained infertility. Spermatogenesis is a complex process requiring a precise and well-coordinated cascade of events leading to sperm differentiation and formation of mature spermatozoa capable of fertilization. Steroids (both estrogens and androgens) and their receptors play an important role in the male reproductive system, which may impact on controlling male fertility. A regulated balance between androgens and estrogens seems to be essential for normal testicular physiology and reproduction. Androgens are critical steroid hormones determining the expression of male phenotype as well as the initiation and maintenance of spermatogenesis [8] . Testicular estrogens are known to be important factors affecting the local testicular function and male reproductive tract [9] .
The effects of steroids on ejaculated spermatozoa have also been investigated in vitro. Efficacy of androgens in fertilization capacity maintenance is connected to their effects on mammalian sperm metabolism, which depends both on the maturity of spermatozoa and the nature of the androgen [10] . T (testosterone) and DHT (dihydrotestosterone) were both capable to inhibit the fertilizing capacity of human sperm inhibiting the penetration of zona-free hamster ova in vitro. This effect appeared not to be related to sperm motility [11] . Recent study showed that local excess of androgens inhibits phosphoinositide 3-kinase (PI3K) activity and affects negatively with human sperm survival [12] . The same research group hypothesized also a role for E2 in the sperm survival through the PI3K/AKT and ERK pathway activations [13] . There exist contradictory data on E2 effects on sperm. Pre-incubation treatment with E2 did not alter the ability of human spermatozoa to fuse with oocytes, or affect the enhancing effect of progesterone (P) action on this process [14] . On the contrary, studies on mouse sperm revealed a stimulatory effect of E2 on capacitation, acrosome reaction and fertilizing ability [15] . It is difficult to relate data obtained in vitro directly to the physiological conditions in the female genital tract. It is highly likely that several steroids act together with different other endocrine and paracrine factors in cervical mucus and oviduct fluid.
Our aim was to compare the molecular profile of selected transcripts of steroid receptors: classical ERs (estrogen receptors α, ESR1 and β, ESR2), membrane ER known as G protein-coupled estrogen receptor (GPER), AR (androgen receptor) and CYP19A1 coding aromatase in spermatozoa obtained from normozoospermic men suffering from unexplained infertility and from a group of men, where their female partners presented with tubal factor infertility.
2. Materials and Methods
2.1. Patients
The male patients of couple treated for infertility problems were recruited from IVF-ET program presented to the Department of Reproduction and Gynecological Endocrinology of the Medical University of Bialystok, Poland, between September 2010 and May 2012. All of patients were Caucasian from Polish origin. The study was approved by the ethical committee of the Medical University of Bialystok in Poland.
Two groups of male patients were compared: 1) male patients from unexplained infertility group (n = 46), and 2) male patients whose female partners presented infertility tubal factor (n = 38). In reality, pure unexplained male infertility group does not exist. However, term “unexplained infertility” refers to clearly male infertility remaining without explanation in contrast to the female one as based on research literature as well as on clinical practice. Therefore, the first group serves us as a group of patients with undefined infertility cause, while the second one serves us as a control group in which the female factor is unequivocally determined. Regarding the difficulties in obtaining the representative control group from healthy donors we decided to choose for our study these two groups highly contrasted by the origin of the fertility troubles. Differences found between these groups using statistical tests should be emphasized in comparison to a control obtained from healthy men. Diagnostic criteria were based on Recommendations for the diagnosis and treatment of infertility in Poland [16] . All participants characterized no features of hypogonadism and a normal karyotype. In the case of unexplained infertility, the routine diagnosis did not indicate any known infertility causes, all men presented with normozoospermia. Criteria for female partner were as follows for tubal factor infertility: Fallopian tubes obstruction, hydrosalpinx > 2.5 cm and scar tissue or adhesions around the tube; for female partner from couple with unexplained infertility: unblocked Fallopian tubes, proper tubal fimbrae, regular menstrual cycle, normal anatomy of the uterus, and lack of endometriosis foci. The ovarian reserve in all women patients was normal: FSH level in 3 day of cycles below 8 mU/ml; AFC (antral follicular count―the number of antral follicles in both ovaries counted the cycle preceding the ovarian stimulation) from 6 to 10. The FSH [mU/ml], LH [mU/ml] and T [ng/ml] serum levels in all males were normal. The hormones values were described in the following way: mean value ± SD (Standard Deviation), median value. For unexplained infertility group in males the hormone serum levels were as follows; FSH: 6.02 ± 2.05, 5.31; LH: 5.32 ± 1.88, 5.20; T 5.65 ± 1.62, 5.37. The hormone values for males from couples with tubal factor infertility were as follows; FSH: 5.34 ± 1.85, 4.88; LH: 5.74 ± 1.77, 5.20; T: 5.09 ± 1.39, 5.10. 80% of couples with unexplained infertility had undergone failed IUI (intrauterine insemination) before. Eligible men were between 25 and 50 years old, and their female partners age were between 24 and 36 years. The participants received a physical examination and completed a questionnaire with information about age, medical history, socioeconomic status, personal background, and lifestyle factors such as sexual, occupational, and environmental ones.
2.1.1. Semen Sample Collection, Preparation, and Analysis
Semen samples were obtained by masturbation after 3 days of sexual abstinence. After liquefaction at a room temperature for 30 - 60 min, semen parameters were studied according the WHO guidelines [17] . Semen samples were used for assisted reproduction treatment (ICSI―intracytoplasmic sperm injection). Aliquots of ejaculated samples were used for gene expression analysis. Samples with less than 15 × 106 cells∙ml−1 were excluded. Ejaculated liquefied sperms were fractionated on a SupraSperm gradient (MediCult, Jyllinge, Denmark) consisting of two layers with the densities of 95% and 47.5% to rule out the possibility of any contamination by somatic and residual cells, and spermatozoa without motion capability. After centrifugation (20 min at 300 g, 25˚C) motile spermatozoa represented at the top of the tube from 95% layer were transferred to sterile tube and a microscopic examination of sperm was performed to control the quality of the preparations. In order to eliminate remnants of SupraSperm, the pellet was washed 2 times with PBS through centrifugation (5 min, 10,000 rpm, 4˚C). The samples containing leucocytes and round cells, confirmed by PCR [18] , were excluded from the examination.
2.1.2. Ovarian Stimulation
Controlled ovarian hyperstimulation (COH) was conducted according to the long GnRH agonist protocol. Pituitary desensibilisation was achieved by 100 µg daily dose of subcutaneous triptorelin (Gonapeptyl Daily, Ferring Pharmaceuticals) administered from day 21 of the previous cycle on. COH started on day 5 of the cycle. In all patients, the daily dose of 150 IU of recombinant FSH (Gonal F, Merck Serono) was kept throughout the whole stimulation, which continued until at least one of ovarian follicles reached 20 mm of size.
Transvaginal, ultrasound guided oocyte recovery was performed 36 hours after subcutaneous injection of 250 microgram of recombinant hCG (Ovitrelle, Merck Serono).
2.1.3. Oocyte Preparation for ICSI (Intracytoplasmic Sperm Injection)
The oocytes were denuded of their surrounding cumulus cells 3 - 4 hours after ovum pick-up using hyaluronidase (HYASE-10X, Vitrolife, Sweden) in G-MOPS buffered medium at the 1:10 dilution (Vitrolife, Sweden) for 10 to 15 seconds. After complete removal of the corona cells by repeated aspiration using denudation glass pipette, the oocytes were washed in clear G-MOPS buffered medium and transferred to ICSI dish. All dishes were prepared at least 4 hours before using and wormed in incubator without CO2 supplementation. Sperm injections were performed for oocytes at the metaphase II stage.
2.2. Gene Expression Analysis Using RT-qPCR (Quantitative Reverse Transcription PCR)
2.2.1. RNA Extraction and cDNA Synthesis
Total RNA was extracted from spermatozoa samples after preparation according to modified Chomczynski and Sacchi method [19] . RNA integrity was verified by amplification of housekeeping gene, β-actin. Equal volume of total RNA was used to prepare cDNA. cDNA synthesis was performed in 50 mM Tris-HCl (pH 8.5), 30 mM KCl, 8 mM MgCl2 (Roche, Basel, Switzerland), 1 mM dNTP mix, 1 µM oligo dT15, 20 U RNasin Ribonulease Inhibitor (Promega, Madison, WI, USA), 10 U Transcriptor Reverse Transcriptase (Roche, Basel, Switzerland) in a final volume of 20 µl using MJ Research Thermal Cycler (Model PTC-200, Watertown, Massachusetts, USA). For reverse transcription, the mixtures containing template RNA and oligo dT15 primers were first incubated at 65˚C for 10 min. After adding the remaining components, the mixtures were incubating at 55˚C for 30 min, followed by 5 min heating at 85˚C for 5 min and then finally cooled at 4˚C.
2.2.2. qPCR
In order to determine the amounts of studied transcripts, the standard curves were constructed with serially diluted PCR products. PCR products were obtained by amplification of cDNA from spermatozoa of a healthy donor using specific primers as follows: for ERα, 5’ TGC TTC AGG CTA CCA TTA TGG AGT CTG 3’ and 5’ GTC AGG GAC AAG GCC AGG CTG 3’; for ERβ, 5’ TTT AAA AGA AGC ATT CAA GGA CAT AAT G 3’ and 5’ CGG TGA AGG GCG CAC TG 5’ for GPER, 5’ CGA GAC TGT GAA ATC CGC AA 3’ and 5’ TCT GTT TTA AAT GGT GAA TCC ATC 3’; for aromatase, 5’ GCC ACT GAG TTG ATT TTA GC 3’ and 5’ CCA AAT GGC TGA AAG TAC C 3’; for AR, 5’ TTT GGA GAC TGC CAG GGA C 3’ and 5’ GCC TCT CCT TCC TCC TGT AGT T 3’; for β-actin, 5’ GGG ACG ACA TGG AGA AAA TCT G 3’ and 5’ TGC CAG TGG TAC GGC CA 3’.
In order to build the standards, PCR was carried out in a final volume of 50 μl using 25 pmol of each of the primers, 40 μM of each of dNTPs, 1 U HotStarTaq polymerase (Qiagen GmbH, Hilden, Germany) under the following conditions: 15 min at 95˚C, 1 min denaturation at 95˚C, 1 min annealing at 60˚C, 1 min extension at 72˚C for 40 cycles, with an additional 10 min extension for the last cycle. Amplified products were separated on a 2% (w/v) agarose gel, extracted and purified from agarose slices using a DNA Gel Extraction Kit (Millipore, Billerica, MA, USA), quantified by the use of One Dscan/Zero Dscan software (Scanalytics Inc., USA) and then serially diluted in sterile water.
All qPCR reactions were performed using ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). For each qPCR run, a master mix was prepared with 10 μl 2x TaqMan Gene Expression PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 1 μl 20x Assays-on Demand Gene Expression Assay Mix (Applied Biosystems, Foster City, CA, USA), 5 μl cDNA or diluted standard and sterile water to final volume of 20 μl. To evaluate the levels of studied genes expression, the serially diluted curves ranged from fg to attg were used. All the results obtained (in fg or attg) were converted to fmol or attmol of transcripts calculated per number of spermatozoa, and then corrected by the level of β-actin mRNA.
2.3. Statistical Analysis
The mean values ± standard deviation (SD) and median with quartiles were calculated. The normal distribution was verified using the Kolmogorov-Smirnov test with the Lilliefors correction. To compare the differences between the two groups, the Mann-Whitney U test was used. The Spearman’s correlation coefficients were estimated. To check the associations between two categorical variables, Chi2 and the Fisher’s exact test was applied. The analysis was performed using the statistical software package Statistica 10.0 (StatSoft) with P < 0.05 indicating a statistically significant difference.
3. Results
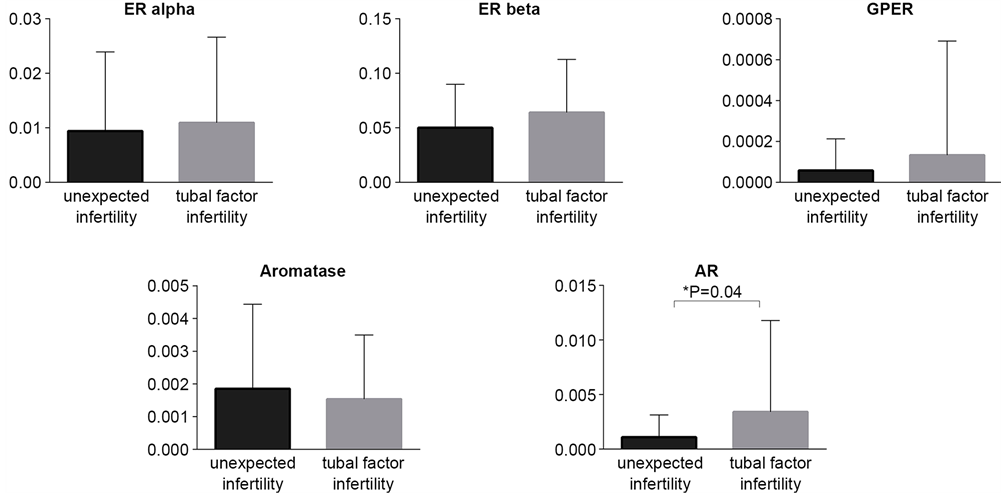
RT-qPCR analysis revealed the presence of the ERβ transcripts in the all studied sperm samples. ERα transcripts were presented in 99.9% of studied samples (only one sperm sample from unexplained fertility group was negative for ERα mRNA). The expression profile of studied genes between two groups revealed the comparable expression profiles of ERs, GPER and aromatase (Figure 1, Table 1). Only AR mRNA expression level showed significant differences between two groups. The semen samples from men of couples with unexplained infertility showed lower mRNA AR level comparing to semen samples from couples with tubal factor infertility (Table 1). In both studied groups the highest expression level was observed for ERβ mRNA, whereas the lowest was noticed for GPER (Table 1). However, only 34% and 26% of cases (unexplained infertility vs. tubal factor group, respectively) showed GPER mRNA expression. Among the sperm samples studied, 89.13% and 78.9% of cases showed aromatase mRNA expression; and 65.2% and 81.6% AR mRNA expression (unexplained infertility group and tubal factor group, respectively). Taking into consideration small amount of mRNAs in spermatozoa, it is difficult to conclude whether the absence of mRNA of studied genes resulted due to a disturbance in sperm maturation process or simply due to the method limitation.
In unexplained infertility group as well as in tubal infertility factor group we observed positive correlation between ERα and ERβ expression (P = 0.00005, r = 0.5; P = 0.00008, r = 0.65; respectively). Additionally, in the group with unexplained infertility we showed positive correlation between ERα and aromatase (P = 0.0044, r = 0.412) as well as ERβ and aromatase (P = 0.0002, r = 0.52).
We did not notice any difference between two studied groups in selected embryological data: in vitro fertilization rate (it was calculated as a ratio between obtained embryos and operated eggs), and good quality embryos. However, we observed a statistically important difference in pregnancy rate between two studied groups (Table 2).
Figure 1. Comparative analysis of the transcript expression levels between two studied groups: unexpected infertility and tubal factor infertility. ER alpha, ER beta, GPER and aromatase mRNA levels showed similar profile in two studied groups. Lower AR mRNA level was shown in the group of patients with unexpected infertility factor.
Table 1. Comparative analysis of ERα, ERβ, GPER, aromatase, and AR mRNA expression levels in sperm from unexplained infertility couple (n = 46) and from men whose female partners presented tubal factor infertility (n = 38). Median, first quartile (Q1), third quartile (Q3) and mean ± standard deviation (SD) as well as P values were presented.
Table 2. Embryological data and results of the pregnancy outcome between two studied groups.
4. Discussion
Despite normal semen parameters in some male patients, sperm can show impaired fertilization ability related to hidden gametes defects. Although there is a significant advancement in genetics, transcriptomics, proteomics, and metabolomics research approaches in couples with unexplained fertility. Biomarkers of impaired fertilization capacity are still lacking. The comparison of spermatozoa transcriptomes between infertile and fertile men with similar sperm count revealed the existence of several molecular infertility factors involved in male gamete formation and maturation [20] .
Several transcripomic approaches confirmed the existence of mRNA molecules in spermatozoa. However, the biological role of the majority of identified transcripts in spermatozoa still remains to be established. It is still not clear which of the mRNAs function as a fingerprint of proper spermatogenesis process, which are transcribed into protein in mature spermatozoa or which are transferred to the egg during the fertilization process. Gur et al. proved that 55S mitochondrial ribosomes are actively involved in sperm protein translation [21] . One cannot exclude the possibility that some proteins stored in spermatozoa on ribosomes are not expelled with the cytoplasmic droplet, as mentioned by Anand-Ivell and Ivell [22] .
Taking into account the significance of the expression of classical ERs, GPER and aromatase in human mature sperm and its role in sperm maturation and proper function [23] [24] , we expected to see the differences in the expression levels of these genes between two studied groups. However, we do not observe any difference at the transcriptional levels of ERs, GPER and aromatase between the two studied groups.
It is well known that AR is expressed in Sertoli, Leydig, and peritubular myoid cells in human testis [8] . Unlike constant expression of AR in developing and adult Leydig and peritubular cell types, Sertoli cells display the differences in AR expression from AR absence in immature Sertoli cells in fetal-neonatal period through dynamic changes in AR expression during postnatal development and in the mature testis [25] . It was proposed that AR expressed in Sertoli cells plays a key role in meiotic-postmeiotic germ cell development [26] [27] [28] . The role of AR presence in male germ cells remains unresolved, although its expression has been documented in the midpiece and in mitochondria of human sperm [29] . It is possible that AR located in sperm mitochondria can be related rather to the metabolic activity of spermatozoa than with the sperm motility. AR was also described to play important role in the promotion of cell apoptosis [30] [31] [32] [33] , therefore, one cannot exclude the role of AR in sperm apoptosis. Interestingly, presence of AR transcript in mature human sperm and AR protein in both X- and Y-carrier haploid spermatozoa has been shown [34] . AR mRNA and protein coding by AR gene located on X chromosome in Y-carrier spermatozoa is probably the consequence of AR transit through the cytoplasmic bridges between elongated spermatids during cell division. Initially, AR had to be transcribed earlier either during the early steps of spermatogenesis by XY diploid spermatocytes or during spermiogenesis by X-carrier haploid spermatids [35] [36] .
Zalata et al. showed the significantly lower AR mRNA level in infertile men sperm compared with fertile men, especially in a group of infertile patients with varicocele [37] . In our study, we showed that infertile man classified to the unexplained factor infertility group with normal semen parameters express a lower amount of AR mRNA in mature sperm in comparison to men with comparable semen parameters from couples with tubal factor infertility. Whether AR mRNA could be a potential marker of fertilizing capacity of human spermatozoa or a biomarker of pregnancy rates remains still under discussion. Our results together with data from Zalata et al. [37] suggest that the assessment of transcriptional AR levels in human sperm might have the potential as a biomarker of fertilization.
ICSI can overcome the barrier of fertilization failure in the unexplained infertility group. In this group, we observed a proper embryo development and successful pregnancy rate vs. tubal infertility group. In the group of patients with tubal factor infertility we achieved similar rates of fertilization and a proper embryo development, but pregnancy success rates were statistically lower. It is possible that the tubal factor can impair embryo implantation process and as a consequence decrease pregnancy rate. Our results may suggest that AR transcript present in ejaculated spermatozoa could be associated with fecundity potential in the group of patients with unexplained infertility.
Advanced reproductive techniques like ICSI, but not IUI or classical in vitro fertilization could be treatment choice for men with low amount of AR transcript in spermatozoa, which may avoid unsuccessful infertility treatment and to shorten the treatment.
Acknowledgements
This work was financially supported by the Polish Ministry of Higher Education (grant No. PBZ-MEiN-8/2/2006).
Conflict of Interest Statement
The authors declare no conflict of interest.
Cite this paper
Jarzabek, K., Mikucka-Niczyporuk, A., Bielawski, T., Milewski, R., Kubiak, J.Z. and Wolczynski, S. (2017) Evaluation of Steroid Receptors mRNA Fingerprints in Two Groups of Normozoospermic Patients: Men from Unexplained Infertility Couples vs. Men from Couples with Tubal Factor Infertility. Open Journal of Obstetrics and Gynecology, 7, 290-302. https://doi.org/10.4236/ojog.2017.73031
References
- 1. García-Herrero, S., Garrido, N., Martínez-Conejero, J.A., Remohí, J., Pellicer, A. and Meseguer, M. (2011) Differential Transcriptomic Profile in Spermatozoa Achieving Pregnancy or Not via ICSI. Reproductive BioMedicine Online, 22, 25-36.
https://doi.org/10.1016/j.rbmo.2010.09.013 - 2. Garrido, N., Zuzuarregui, J.L., Meseguer, M., Simón, C., Remohí, J. and Pellicer, A. (2002) Sperm and Oocyte Donor Selection and Management: Experience of a 10 Year Follow-Up of More Than 2100 Candidates. Human Reproduction, 17, 3142-3148.
https://doi.org/10.1093/humrep/17.12.3142 - 3. Miller, D., Briggs, D., Snowden, H., Hamlington, J., Rollinson, S., Lilford, R. and Krawetz, S.A. (1999) A Complex Population of RNAs Exists in Human Ejaculate Spermatozoa: Implications for Understanding Molecular Aspects of Spermiogenesis. Gene, 237, 385-392.
https://doi.org/10.1016/S0378-1119(99)00324-8 - 4. Ostermeier, G.C., Dix, D.J., Miller, D., Khatri, P. and Krawetz, S.A. (2002) Spermatozoal RNA Profiles of Normal Fertile Men. Lancet, 360, 772-777.
https://doi.org/10.1016/S0140-6736(02)09899-9 - 5. Hayashi, S., Yang, J., Christenson, L., Yanagimachi, R. and Hecht, N.B. (2003) Mouse Preimplantation Embryos Developed from Oocytes Injected with Round Spermatids or Spermatozoa Have Similar But Distinct Patterns of Early Messenger RNA Expression. Biology of Reproduction, 69, 1170-1176.
https://doi.org/10.1095/biolreprod.103.016832 - 6. Rassoulzadegan, M., Grandjean, V., Gounon, P., Vincent, S., Gillot, I. and Cuzin, F. (2006) RNA-Mediated Non-Mendelian Inheritance of an Epigenetic Change in the Mouse. Nature, 441, 469-474.
https://doi.org/10.1038/nature04674 - 7. Ostermeier, G.C., Miller, D., Huntriss, J.D., Diamond, M.P. and Krawetz, S.A. (2004) Reproductive Biology: Delivering Spermatozoan RNA to the Oocyte. Nature, 429, 154.
https://doi.org/10.1038/429154a - 8. Collins, L.L., Lee, H.J., Chen, Y.T., Chang, M., Hsu, H.Y., Yeh, S. and Chang, C. (2003) The Androgen Receptor in Spermatogenesis. Cytogenetic and Genome Research, 103, 299-301.
- 9. Carreau, S., Bouraima-Lelong, H. and Delalande, C. (2011) Estrogens in Male Germ Cells. Spermatogenesis, 1, 90-94.
https://doi.org/10.4161/spmg.1.2.16766 - 10. Voglmayr, J.K. and Amann, R.P. (1973) Glucose Metabolism and Lipid Synthesis of Cauda Epididymal and Ejaculated Bull Spermatozoa in the Presence of Selected Androgens. Acta Endocrinologica (Copenhagen), 73, 196-208.
- 11. Chan, S.Y., Tang, L.C., Tang, G.W. and Chan, P.H. (1983) Effects of Androgens on Fertilizing Capacity of Human Spermatozoa. Contraception, 8, 481-488.
https://doi.org/10.1016/0010-7824(83)90079-3 - 12. Aquila, S., Middea, E., Catalano, S., Marsico, S., Lanzino, M., Casaburi, I., Barone, I., Bruno, R., Zupo, S. and Andò, S. (2007) Human Sperm Express a Functional Androgen Receptor: Effects on PI3K/AKT Pathway. Human Reproduction, 22, 2594-2605.
https://doi.org/10.1093/humrep/dem243 - 13. Aquila, S., Sisci, D., Gentile, M., Middea, E., Catalano, S., Carpino, A., Rago, V. and Andò, S. (2004) Estrogen Receptor (ER) Alpha and ER Beta Are Both Expressed in Human Ejaculated Spermatozoa: Evidence of Their Direct Interaction with Phosphatidylinositol-3-OH Kinase/Akt Pathway. Journal of Clinical Endocrinology & Metabolism, 89, 1443-1451.
https://doi.org/10.1210/jc.2003-031681 - 14. Francavilla, F., Romano, R., Pandolfi, C., Macerola, B., Santucci, R., Necozione, S. and Francavilla, S. (2003) Evaluation of the Effect of 17alphaOH-Progesterone and 17beta-Oestradiol on Human Sperm Ability to Fuse with Oocytes: Comparison and Possible Interference with the Effect of Progesterone. International Journal of Andrology, 26, 342-347.
https://doi.org/10.1111/j.1365-2605.2003.00435.x - 15. Adeoya-Osiguwa, S.A., Markoulaki, S., Pocock, V., Milligan, S.R. and Fraser, L.R. (2003) 17beta-Estradiol and Environmental Estrogens Significantly Affect Mammalian Sperm Function. Human Reproduction, 18, 100-107.
https://doi.org/10.1093/humrep/deg037 - 16. Kuczyński, W., Kurzawa, R., Oszukowski, P., Pawelczyk, L., Poreba, R., Radowicki, S., Szamatowicz, M. and Wolczyński, S. (2012) Polish Gynecological Society and Polish Society for Reproductive Medicine Recommendations for the Diagnosis and Treatment of Infertility. Ginekologia Polska, 83, 149-154.
- 17. WHO (2010) Laboratory Manual for the Examination and Processing of Human Sperm. 5th Edition, World Health Organization, Geneva.
- 18. Lambard, S., Galeraud-Denis, I., Martin, G., Levy, R., Chocat, A. and Carreau, S. (2004) Analysis and Significance of mRNA in Human Ejaculated Sperm from Normozoospermic Donors: Relationship to Sperm Motility and Capacitation. Molecular Human Reproduction, 10, 535-541.
https://doi.org/10.1093/molehr/gah064 - 19. Chomczynski, P. and Sacchi, N. (1987) Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Analytical Biochemistry, 162, 156-159.
https://doi.org/10.1016/0003-2697(87)90021-2 - 20. Garrido, N., Martínez-Conejero, J.A., Jauregui, J., Horcajadas, J.A., Simón, C., Remohí, J. and Meseguer, M. (2009) Microarray Analysis in Sperm from Fertile and Infertile Men without Basic Sperm Analysis Abnormalities Reveals a Significantly Different Transcriptome. Fertility and Sterility, 91, 1307-1310.
https://doi.org/10.1016/j.fertnstert.2008.01.078 - 21. Gur, Y. and Breitbart, H. (2006) Mammalian Sperm Translate Nuclear-Encoded Proteins by Mitochondrial-Type Ribosomes. Genes, 20, 411-416.
https://doi.org/10.1101/gad.367606 - 22. Anand-Ivell, R. and Ivell, R. (2011) The Special Systems Biology of the Sperm. Biochemical Journal, 15, e3-e5.
https://doi.org/10.1042/bj20110766 - 23. Carreau, S., Wolczynski, S. and Galeraud-Denis, I. (2010) Aromatase, Oestrogens and Human Male Reproduction. Philosophical Transactions of the Royal Society B-Biological Sciences, 365, 1571-1579.
https://doi.org/10.1098/rstb.2009.0113 - 24. Prossnitz, E.R. and Barton, M. (2011) The G-Protein-Coupled Estrogen Receptor GPER in Health and Disease. Nature Reviews Endocrinology, 7, 715-726.
https://doi.org/10.1038/nrendo.2011.122 - 25. Hazra, R., Corcoran, L., Robson, M., McTavish, K.J., Upton, D., Handelsman, D.J. and Allan, C.M. (2013) Temporal Role of Sertoli Cell Androgen Receptor Expression in Spermatogenic Development. Molecular Endocrinology, 27, 12-24.
https://doi.org/10.1210/me.2012-1219 - 26. Chang, C., Chen, Y.T., Yeh, S.D., Xu, Q., Wang, R.S., Guillou, F., Lardy, H. and Yeh, S. (2004) Infertility with Defective Spermatogenesis and Hypotestosteronemia in Male Mice Lacking the Androgen Receptor in Sertoli Cells. Proceedings of the National Academy of Sciences of the United States of America, 101, 6876-6881.
https://doi.org/10.1073/pnas.0307306101 - 27. De Gendt, K., Swinnen, J.V., Saunders, P.T., Schoonjans, L., Dewerchin, M., Devos, A., Tan, K., Atanassova, N., Claessens, F., Lécureuil, C., Heyns, W., Carmeliet, P., Guillou, F., Sharpe, R.M. and Verhoeven, G. (2004) A Sertoli Cell-Selective Knockout of the Androgen Receptor Causes Spermatogenic Arrest in Meiosis. Proceedings of the National Academy of Sciences of the United States of America, 101, 1327-1332.
https://doi.org/10.1073/pnas.0308114100 - 28. Holdcraft, R.W. and Braun, R.E. (2004) Androgen Receptor Function Is Required in Sertoli Cells for the Terminal Differentiation of Haploid Spermatids. Development, 131, 459-467.
https://doi.org/10.1242/dev.00957 - 29. Solakidi, S., Psarra, A.M., Nikolaropoulos, S. and Sekeris, C.E. (2005) Estrogen Receptors Alpha and Beta (Eralpha and Erbeta) and Androgen Receptor (Ar) in Human Sperm: Localization of Erbeta and AR in Mitochondria of the Midpiece. Human Reproduction, 20, 3481-3487.
https://doi.org/10.1093/humrep/dei267 - 30. Heisler, L.E., Evangelou, A., Lew, A.M., Trachtenberg, J., Elsholtz, H.P. and Brown, T.J. (1997) Androgen-Dependent Cell Cycle Arrest and Apoptotic Death in Pc-3 Prostatic Cell Cultures Expressing a Full-Length Human Androgen Receptor. Molecular and Cellular Endocrinology, 126, 59-73.
https://doi.org/10.1016/S0303-7207(96)03970-6 - 31. King, K.J., Nicholson, H.D. and Assinder, S.J. (2006) Effect of Increasing Ratio of Estrogen: Androgen on Proliferation of Normal Human Prostate Stromal and Epithelial Cells, and the Malignant Cell Line LNCaP. Prostate, 66, 105-114.
https://doi.org/10.1002/pros.20327 - 32. Olsen, N.J., Viselli, S.M., Fan, J. and Kovacs, W.J. (1998) Androgens Accelerate Thymocyte Apoptosis. Endocrinology, 139, 748-752.
https://doi.org/10.1210/en.139.2.748 - 33. Shetty, G., Wilson, G., Hardy, M.P., Niu, E., Huhtaniemi, I. and Meistrich, M.L. (2002) Inhibition of Recovery of Spermatogenesis in Irradiated Rats by Different Androgens. Endocrinology, 143, 3385-3396.
https://doi.org/10.1210/en.2002-220206 - 34. Zuccarello, D., Garolla, A., Ferlin, A., Menegazzo, M., De Toni, L., Carraro, M., Veronese, C. and Foresta, C. (2009) Androgen Receptor Is Expressed in Both X- and Y-Carrier Human Spermatozoa. Fertility and Sterility, 91, 193-200.
https://doi.org/10.1016/j.fertnstert.2007.11.040 - 35. Braun, R.E., Behringer, R.R., Peschon, J.J., Brinster, R.L. and Palmiter, R.D. (1989) Genetically Haploid Spermatids Are Phenotypically Diploid. Nature, 337, 373-376.
https://doi.org/10.1038/337373a0 - 36. Dym, M. and Fawcett, D.W. (1971) Further Observations of Spermatogonia, Spermatocytes, and Spermatids Connected by Intercellular Bridges in the Mammalian Testis. Biology of Reproduction, 4, 195-215.
https://doi.org/10.1093/biolreprod/4.2.195 - 37. Zalata, A.A., Mokhtar, N., Badawy, Ael.-N., Othman, G., Alghobary, M. and Mostafa, T. (2013) Androgen Receptor Expression Relationship with Semen Variables in Infertile Men with Varicocele. The Journal of Urology, 189, 2243-2247.
https://doi.org/10.1016/j.juro.2012.11.112
List of Abbreviations
AFC (antral follicular count), AKT (serine/threonine-specific protein kinase, also known as protein kinase B (PKB)), AR (androgen receptor), COH (controlled ovarian hyperstimulation), DHT (dihydrotestosterone), E2 (estradiol), ER (estrogen receptor), ERK (extracellular signal-regulated kinase), FSH (follicle- stimulating hormone), GPER (G protein-coupled estrogen receptor), hCG (human chorionic gonadotropin), ICSI (intracytoplasmic sperm injection), IUI (intrauterine insemination), IVF-ET (in vitro fertilization-embryo transfer), LH (luteinizing hormone), P (progesterone), PBS (Phosphate Buffered Saline), PI3K (phosphoinositide 3-kinase), qPCR (quantitative polymerase chain reaction), RT (reverse transcription), SD (standard deviation), T (testosterone), WHO (World Health Organization).

Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact ojog@scirp.org