Open Journal of Obstetrics and Gynecology
Vol.4 No.13(2014), Article
ID:49533,6
pages
DOI:10.4236/ojog.2014.413104
Comparative Study of Sublingual and Vaginal Misoprostol in Second Trimester Induced Abortion
Rupali Modak1, Dilip Kumar Biswas2, Arindam Ghosh2, Amitava Pal2*, Tapan Kumar Mandal2
1Department of Obstetrics and Gynecology, R. G. Kar Medical College, Kolkata, India
2Department of Obstetrics and Gynecology, Burdwan Medical College, Burdwan, India
Email: *amitava.628@rediffmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 24 June 2014; revised 20 July 2014; accepted 17 August 2014
ABSTRACT
Background: To identify an effective misoprostol-only regime for the termination of second trimester pregnancy. Objectives: To compare the efficacy, safety and acceptability of sublingual and vaginal misoprostol for second trimester pregnancy termination. Methods: In a prospective randomized comparative study, over 138 pregnant women at 13 - 20 weeks (91 - 140 days) of gestation requiring medical abortion were randomly assigned to the sublingual or vaginal route for misoprostol administration with dose schedule of 400 mcg every 3 hours up to 5 doses within 24 hours. The course of misoprostol was repeated if the woman did not abort within 24 hours. Primary outcome was the efficacy of the treatment to terminate pregnancy completely at 24 and 48 hours. Secondary outcomes measured were induction-abortion interval, side effects, failure rate, and women’s perception to these treatments. Results: At 24 h, the complete abortion rate was 87.88% in the vaginal administration group and 79.41% in sublingual group (difference 8.5%, 95% CI: 3.8 to 13.2). No significant difference in the complete abortion rates was observed at 48 h (90.91% versus 88.24% difference: 2.7%, 95% CI: −0.04 to 5.4) when vaginal and sublingual groups were compared. Mean induction-abortion interval in sublingual and vaginal groups was 12.28 h (95% CI of mean 11.019 - 13.541 h) and 13.11 h (95% CI of mean 12.0301 - 14.1899 h) respectively; p = 0.485. The rates of side effects were similar in both groups except for fever, which was more common in vaginal group. Significantly more women in the sublingual group preferred the route as compared to vaginal administration (RR 1.618. 95% CI: 1.277 - 2.050; p < 0.0001). Conclusion: Both sublingual and vaginal routes of misoprostol are equally effective in medical termination of pregnancy in second trimester but sublingual route was preferred by the women.
Keywords
Misoprostol, Sublingual, Vaginal Route, Mid Trimester Abortion

1. Introduction
Termination of pregnancy is usually performed in first trimester, but second trimester termination contributes only 10% to 15% of all induced abortions cases and about two-thirds of all fatal complications are related to induced abortions [1] [2] . Second trimester abortion includes medical and surgical procedure. Surgical treatment is associated with quicker results but it can result in serious complications like uterine perforation, cervical incompetence, uterine synechiae and even maternal mortality [3] . Nowadays medical method is the treatment of choice for second trimester abortion. Misoprostol, a prostaglandin E1 analogue, is commonly used for termination of pregnancy in second trimester which is legalized in our country up to 20 weeks of gestation. It has been shown to be effective for this purpose with or without mifepristone—an antiprogesterone [4] -[6] . When mifepristone is not available; abortion can also be induced safely by misoprostol alone. Different doses and routes of administration of misoprostol alone for second trimester abortion have been investigated and the results are compared but optimal dose and route have not been identified [7] -[9] . It seems that doses more than 400 mcg did not significantly improve the efficacy but produced more side effects.
Vaginal administration of misoprostol for medical abortion has been shown to be more effective than oral route [10] . A pharmacokinetic study has revealed that sublingual route could achieve the peak concentration in the shortest time and had the highest bioavailability [8] .
The present study was undertaken to compare the efficacy, side effects, and acceptability of sublingual and vaginal misoprostol for second trimester induced abortion.
2. Materials and Methods
Primarily 150 pregnant women were selected for this study attending OPD of Obstetrics & Gynaecology in Burdwan Medical College, Burdwan opting for medical termination of pregnancy between 13 to 20 weeks of gestation over a period of one year (March 2012 to February 2013). Sixteen mothers were excluded from the study due to refusal of enrolment (n = 9) and not meeting the inclusion criteria (n = 7). Finally a total of 134 pregnant women were randomly assigned into two groups [Group A (sublingual, n = 68 women) and Group B (vaginal, n = 66 women)]. The randomization was done by computer generated random numbers. All the pregnant women underwent detailed history-taking, clinical examination and routine investigations. Inclusion criteria included in the study were young healthy women with H/O regular menstrual cycle and knowledge of exact date of last normal menstrual period carrying 13 - 20 weeks of gestation as determined by first day of last normal menstrual period, clinical examination and confirmed by ultrasonography in case of doubt. Grand multipara, H/O allergy to prostaglandins, congenital uterine anomaly, women with medical disorders like severe anemia, cardio-vascular problem, bronchial asthma and disorders in pregnancy like previous uterine scar, hydatidiform mole, and period of gestation less than 13 weeks or more than 20 weeks were excluded from our study.
For the termination of pregnancy tablet misoprostol (400 mcg) was used at an interval of three hours, up to a maximum of five doses (5 × 400 mcg) within 24 hours in both the groups. Pregnant women in Group A were instructed to place misoprotol tablets under the tongue and to keep the drug in place until it dissolves and not to spit or swallow within an hour of administration. For Group B misoprostol tablets were placed in the posterior fornix of vagina after taking aseptic precautions. Before administration of subsequent doses of misoprostol vitals of mothers were checked. Vaginal bleeding appearance of side effects like nausea, vomiting, headache, diarrhea, shivering and fever were noted. An informed consent of all pregnant women was taken before enrollment and the study was approved by the ethical committee of the institution according to revised Helsinki 2000 protocol.
If a women failed to abort after 5 doses of misoprostol (400 mcg three hourly), the same protocol was repeated 24 hours later of first dose of misoprostol. After abortion, product of conception was checked for its completeness and induction-abortion interval was also noted. If the abortion was incomplete, uterine evacuation was performed promptly. Post abortal mothers were observed for 24 hours before discharge and failure of abortion was considered when a woman failed to abort within 48 hours of the start of treatment. Other methods of termination for second trimester of pregnancy were adopted in all such failure cases.
Primary outcome measure was abortion rate at 24 and 48 hour. The secondary outcome measures were induction-abortion interval (hours), number of failure cases, need for surgical evacuation in incomplete cases and side effects. The induction-abortion interval was defined as the interval between the time of administration of the first dose of misoprostol and the time when the fetus was aborted. Complete abortion rate was defined as the expulsion of both the fetus and placenta without operative intervention. Subjective assessment of patient’s satisfaction with sublingual and vaginal route was also compared.
Statistical analysis was performed by using software package of Stat Calc version 7.1.1. Student’s t-test and chi-square analysis were done to assess the significance of variables and p-value less than 0.05 was considered as statistically significant.
3. Result and Analysis
A total of 134 pregnant women were at 13 - 20 weeks of gestational age participated in this study. Sixty eight and sixty six women were included in sublingual (Gr A) and vaginal group (Gr B) respectively. Table 1 compares the demographic profile of both the groups. All the parameters in relation to age, body mass index, gravidity, base line hemoglobin level distribution were comparable. Mean gestational age in sublingual and vaginal groups were 15.5 weeks and 14.91weeks respectively; but this was not statistically significant.
At the end of 24 hours, success rate was higher in vaginal group (87.88%) as compared to sublingual group (79.41%) but the difference was not statistically significant (p = 0.186). Successful abortion rate at the end of 48 hours was almost similar in Group A and Group B (88.24% and 90.91% respectively) and was also not statistically significant (p = 0.613). Mean induction-abortion interval was similar in both the groups (12.28 v/s 13.11 hours). Sublingual group (Gr A) required more intervention for incomplete abortion in contrast to vaginal group (Gr B) (17.65% v/s 12.12%). Four women in each groups required blood transfusion for excessive bleeding. Eight women (11.76%) from sublingual group and six women (9.09%) from vaginal group failed to abort within 48 hours (Table 2). Side effects of misoprostol were more or less comparable in both the groups except fever, which was significantly higher (51.51%) in vaginal group, p = 0.035 (Table 3). Most of the pregnant women prefers sublingual route in comparison to vaginal administration, p < 0.0001 (Table 4).
Table 1. Demographic profile.
Results are expressed as mean ± standard deviation or number (%); BMI, body mass index.
Table 2. Comparison of treatment outcomes by misoprostol.
n (%), *p = 0.186, **p = 0.613, ***p = 0.613, *****p = 0.369.
Table 3. Incidence of side effects of misoprostol administration.
n (%), *p < 0.05, **watery stool more than 3 episodes.
Table 4. Subjective assessments of patients’ comfort to the route of administration.
Results are expressed in number and (%), relative risk (RR), 1.618, 95% CI: 1.277 - 2.050; odds ratio (OR) 6.250, 95% CI: 2.586 - 15.107; p < 0.0001.
At 24 hour the vaginal group had a higher success rate than sublingual group (87.88% versus 79.41%, difference 8.5%, 95% CI: 3.8 to 13.2). Evidence of the two arms cannot be concluded since the confidence interval crosses the 10% pre-established margin. Both treatments can be claimed to be equivalent at 48 h within the 10% margin established [90.91% (vaginal) versus 88.24% (sublingual), difference 2.7%, 95% CI: −0.04 to 5.4)] (Figure 1).
4. Discussion
Abortions during second trimester represent only 10% - 15% of all induced abortions but it provoke two-third of all serious complications, half of the deaths related to the practice [1] [2] [11] . Development of safe, effective, widely available and acceptable method for second trimester abortions has become a major clinical challenge. Misoprostol, with or without mifepristone has been investigated for second trimester medical abortion. Pre treatment with mifepristone resulted in a shorter induction-abortion interval compared with regimens without mifepristone [4] -[6] . However, use of mifepristone would increase the cost. Therefore, there was a need to develop an effective regimen without mifepristone. Abortifacient properties of misoprostol for termination in the second trimester have been reported in the medical literature since 1993 [12] . Several numbers of studies have demonstrated that it is safe and efficient drug for achieving fetal expulsion. Moreover it is inexpensive and can be stored in room temperature [6] . Due to lack of evidences from well controlled randomized studies, consensus is yet to be reached regarding optimal dose, route and time interval of misoprostol administration for induction of medical abortion. Oral route is easy to administer, but there is increased incidence of gastrointestinal side effects along with increased induction-abortion interval. Vaginal route had been found to be more promising than oral route probably due to an accumulating plasma level with fewer gastrointestinal side effects [7] [10] . On the other hand, vaginal absorption was inconsistent with large individual variations. In contrast, misoprostol is rapidly absorbed when administered sublingually and Tang et al. had compared the pharmacokinetics of misoprostol by sublingual, oral and vaginal routes and found that sublingual route achieved significantly higher serum peak concentration [8] .
In the present study, we have compared the clinical outcomes of sublingual and vaginal misoprostol for termination of pregnancy between 13 and 20 weeks of gestation. Success rate of sublingual and vaginal misoprostol at 24 hours (79.41% versus 87.88%) is similar to the study by Tang et al. (72% versus 86%) [13] . At 48 hours, success rate of both the routes are similar (88% versus 91%) in our study which also corresponds with the study by Tang et al. (91% versus 95%) [13] and Bhattacharjee N. et al. (79.14% versus 82.01%) [14] . Mean
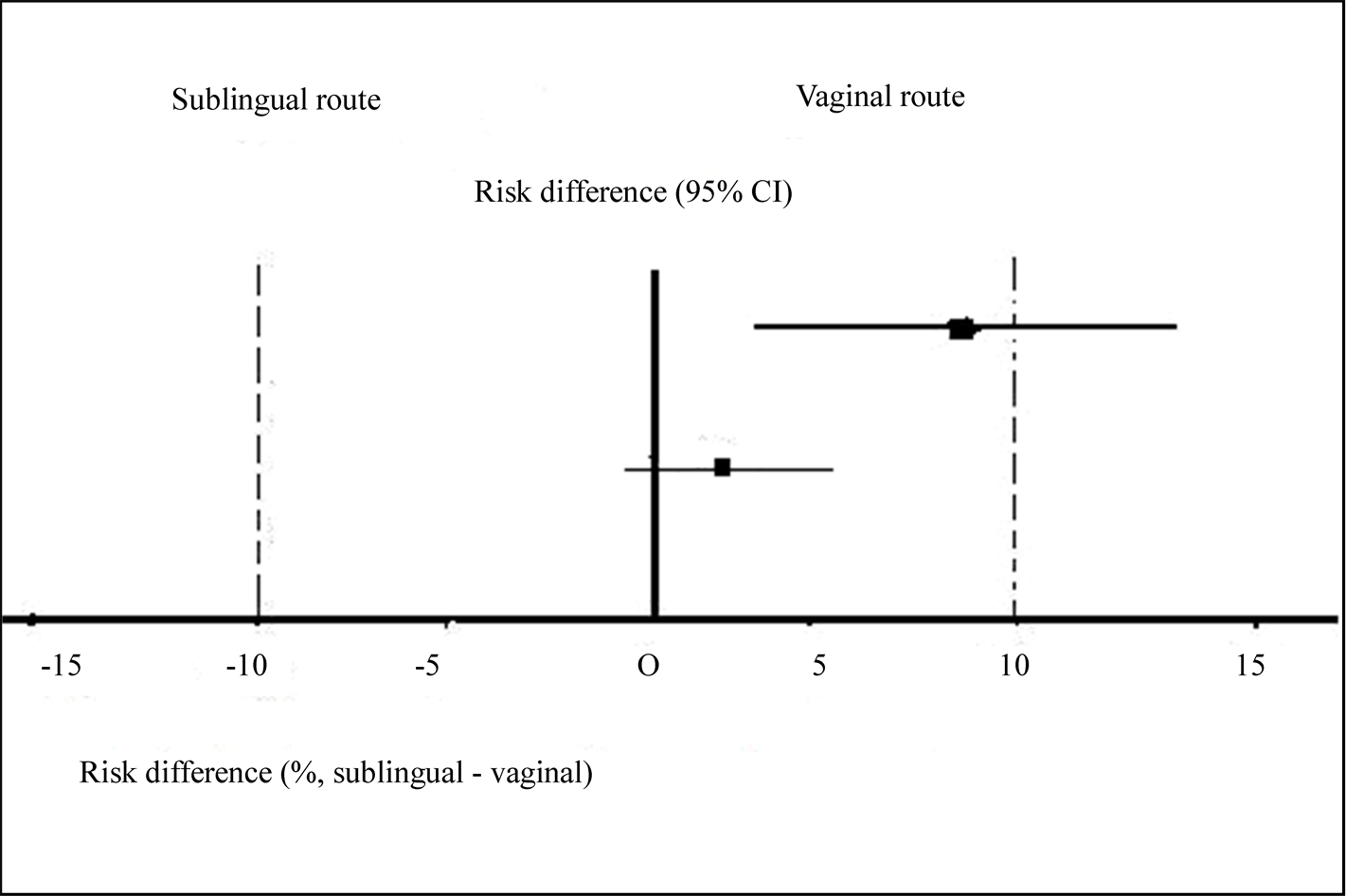
Figure 1. Risk difference to achieve abortion at 24 hour (above) and 48 hour (below). The Y-axis corresponds to the treatment effect (where risk difference = 0 represents neutral effect). The horizontal lines ( ) are the 95% confidence interval of risk difference.
) are the 95% confidence interval of risk difference.
induction-abortion interval was 12.2 hours and 13.1 hours in sublingual and vaginal groups in our study corresponds with the studies by Bhattacherjee N. et al. (14.1 hours and 14.5 hours) [14] where as Hertzen H.V. et al. and Tang et al. showed that median induction-abortion-interval were 12.0 hours vs 12.3 hours and 12.2 hours vs 10.5 hours in sublingual and vaginal groups respectively [13] [15] . Failure rates in sub lingual and vaginal groups in our study (11.76% and 9.09% respectively) were also similar to the study by Bhattacherjee N. et al. (12.23% and 9.42%) [14] , but higher when compared to the result of Ganguly et al. (sublingual versus vaginal, 3.6% vs 8.3%) [16] . High failure rate in the present study in sublingual group might be due to swallowing of the dissolved misoprostol tablet by some patient prior to proper absorption by sublingual route despite proper instruction.
Side effects were not uncommon to misoprostol irrespective of route of administration. Incidence of fever was significantly higher in vaginal (51.51%) than sublingual group (26.42%). This is due to the higher bio availability associated with repeated administration of vaginal misoprostol. In our study satisfaction rate in sublingual group was significantly higher than vaginal group (88.24% versus 54.55%, RR 1.618; p < 0.0001) because of pain and discomfort related to vaginal examination could be avoided in former group. The observation in respect to advantage of sublingual administration of misoprostol has some similarity with those of some other studies [13] [14] [16] .
5. Conclusion
Both sublingual and vaginal administration of misoprostol are almost equally effective for terminating early second trimester pregnancies; mean induction-abortion interval, need for surgical evacuation, failure rates are also similar but sublingual route gives better patient acceptance.
References
- World Health Organization (1997) Medical Methods for Termination of Pregnancy: Report of a WHO Scientific Group. Who Technical Report Series No. 871, World Health Organization, Geneva.
- Tietza, C. (1981) Second-Trimester Abortion: A Global View. In: Berger, G.S., Berger, W.E., Keith, L.G., Eds., Second-Trimester Abortion, John Wright, Boston, 1-11. http://dx.doi.org/10.1007/978-94-009-8293-2_1
- Gomez Ponce de Leon, R. and Wing, D.A. (2009) Misoprostol for Termination of Pregnancy with Intrauterine Fetal Demise in the Second and Third Trimester of Pregnancy—A Systematic Review. Contraception, 79, 259-271. http://dx.doi.org/10.1016/j.contraception.2008.10.009
- El-Rafaey, H. and Templeton, A. (1995) Induction of Abortion in the Second Trimester by a Combination of Misoprostol and Mifepristone: A Randomized Comparison between Two Misoprostol Regimens. Human Reproduction, 10, 475-478.
- Ho, P.C., Chan, Y.E. and Lau, W. (1996) Misoprostol Is as Effective as Gemeprost in Termination of Second Trimester Pregnancy When Combined with Mifepristone: A Randomized Comparative Trail. Contraception, 53, 281-283. http://dx.doi.org/10.1016/S0010-7824(96)00061-3
- Wong, K.S., Ngai, C.S.W., Yeo, E.L.K. and Tang, L.C.H. (2000) A Comparison of Two Regimens of Intravaginal Misoprostol for Termination of Second Trimester Pregnancy: A Randomized Comparative Trail. Human Reproduction, 15, 709-712. http://dx.doi.org/10.1093/humrep/15.3.709
- Zieman, M., Fong, S.K., Benowitz, N.L., Banskter, D. and Darney, P.D. (1997) Absorption Kinetics of Misoprostol with Oral or Vaginal Administration. Obstetrics & Gynecology, 90, 88-92. http://dx.doi.org/10.1016/S0029-7844(97)00111-7
- Tang, O.S., Schweer, H., Seyberth, H.W., Lee, S.W.H. and Ho, P.C. (2002) Pharmacokinetics of Different Routes of Administration of Misoprostol. Human Reproduction, 17, 332-336. http://dx.doi.org/10.1093/humrep/17.2.332
- Meckstroth, K.R., Whitaker, A.K., Bertisch, S., et al. (2006) Misoprostol Administered by Epithelial Routes: Drug Absorption and Uterine Response. Obstetrics & Gynecology, 108, 582-590. http://dx.doi.org/10.1097/01.AOG.0000230398.32794.9d
- Ho, P.C., Ngai, S.W., Liu, K.L., Wong, G.C. and Lee, S.W. (1997) Vaginal Misoprostol Compared with Oral Misoprostol in Termination of Second Trimester Pregnancy. Obstetrics & Gynecology, 90, 735-738. http://dx.doi.org/10.1016/S0029-7844(97)00419-5
- Ngai, S.W., Tang, O.S. and Ho, P.C. (2003) Prostaglandins for Induction of Second Trimester Termination and Intrauterine Death. Best Practice Research Clinical Obstetrics Gynaecology, 17, 765-775. http://dx.doi.org/10.1016/S1521-6934(03)00068-3
- El-Refeay, H., Hinshaw, K. and Templeton, A. (1993) The Abortifacient Effect of Misoprostol in Second Trimester. A Randomized Comparison with Gemeprost in Patients Pretreated with Mifepristone (RU486). Human Reproduction, 8, 1744-1746.
- Tang, O.S., Lau, W.N.T., Chan, C.C.W. and Ho, P.C. (2004) A Prospective Randomized Comparison of Sublingual and Vaginal Misoprostol in Second Trimester of Pregnancy. BJOG, 111, 1001-1005. http://dx.doi.org/10.1111/j.1471-0528.2004.00222.x
- Bhattacharjee, N., Saha, S.P., Ghoshroy, S.C., Bhowmik, S. and Barui, G. (2008) A Randomised Comparative Study on Sublingual versus Vaginal Administration of Misoprostol for Termination of Pregnancy between 13 to 20 Weeks. Australian and New Zealand Journal of Obstetrics and Gynaecology, 48, 165-171. http://dx.doi.org/10.1111/j.1479-828X.2008.00831.x
- Hertzen, H.V., Piaggio, G., Wojdyla, D., Huong, N.T.M., Marions, L. and Okeov, G. (2009) Comparison of Vaginal And Sublingual Misoprostol for Second Trimester Abortion: Randomized Controlled Equivalence Trial. Human Reproduction, 24, 106-112. http://dx.doi.org/10.1093/humrep/den328
- Ganguly, R.P., Saha, S.P., Mukhopadhyay, S., Bhattacharjee, N., Bhattacharyya, S.K. and Patra, K.K. (2010) A Comparative Study on Sublingual Versus Oral and Vaginal Administration of Misoprostol for Late First and Early Second Trimester Abortion. Journal of the Indian Medical Association, 108, 283-286.
NOTES

*Corresponding author.


