Open Journal of Immunology
Vol.2 No.3(2012), Article ID:22543,5 pages DOI:10.4236/oji.2012.23013
Identification of a T-cell epitope in the Staphylococcus aureus Panton-Valentine LukS-PV component*
![]()
1Center for Infectious Disease, University of Texas School of Public Health, Houston, USA; #Corresponding Author: eric.l.brown@uth.tmc.edu
2Department of Pathology and Laboratory Medicine, University Texas-Houston Medical School, Houston, USA
3Institute of Biosciences and Technology, Texas A&M University Health Science Center, Houston, USA;
#Corresponding Author: bowdenm@uhd.edu
4Department of Natural Sciences, University of Houston-Downtown, Houston, USA
Received 30 April 2012; revised 25 May 2012; accepted 7 June 2012
Keywords: Staphylococcus; Cell Mediated Immunity; Panton-Valentine Leukocidin; T Cells
ABSTRACT
We previously observed the elicitation of a significant delayed-type hypersensitivity response to the Panton-Valentine leukocidin (PVL) LukSPV subunit following subcutaneous immunization (Brown et al., Clinical Microbiology and Infection, 2009, 156: 156-164). A LukS-PV-specific cell line (LST) was screened for proliferative responses against a panel of 25 amino acid-long peptides spanning the length of LukS-PV (amino acids 29 - 312). This analysis demonstrated that stimulation of LST with LukS-PV resulted in significant proliferative responses and adoptive transfer of LST into naïve mice conferred a LukSPV-specific DTH response following challenge. Challenge of mice adoptively transferred with LST with peptides 7 (149 - 173), 8 (169 - 193) and 14 (289 - 312) also elicited a measurable DTH response suggesting that these peptides contained T cell epitopes.
1. INTRODUCTION
Infections caused by Staphylococcus aureus have dramatically increased during the last decade, largely due to the emergence of strains that are resistant to numerous antibiotics and a significant change to the epidemiology of infections caused by this pathogen; namely the emergence of community-acquired (CA) infections [1]. Presentation of disease resulting from either CA or hospital acquired (HA) infections can range from minor skin infections to cellulitis, myositis, or deep bone and tissue infections [1,2].
2. RESULTS, MATERIALS AND DISCUSSION
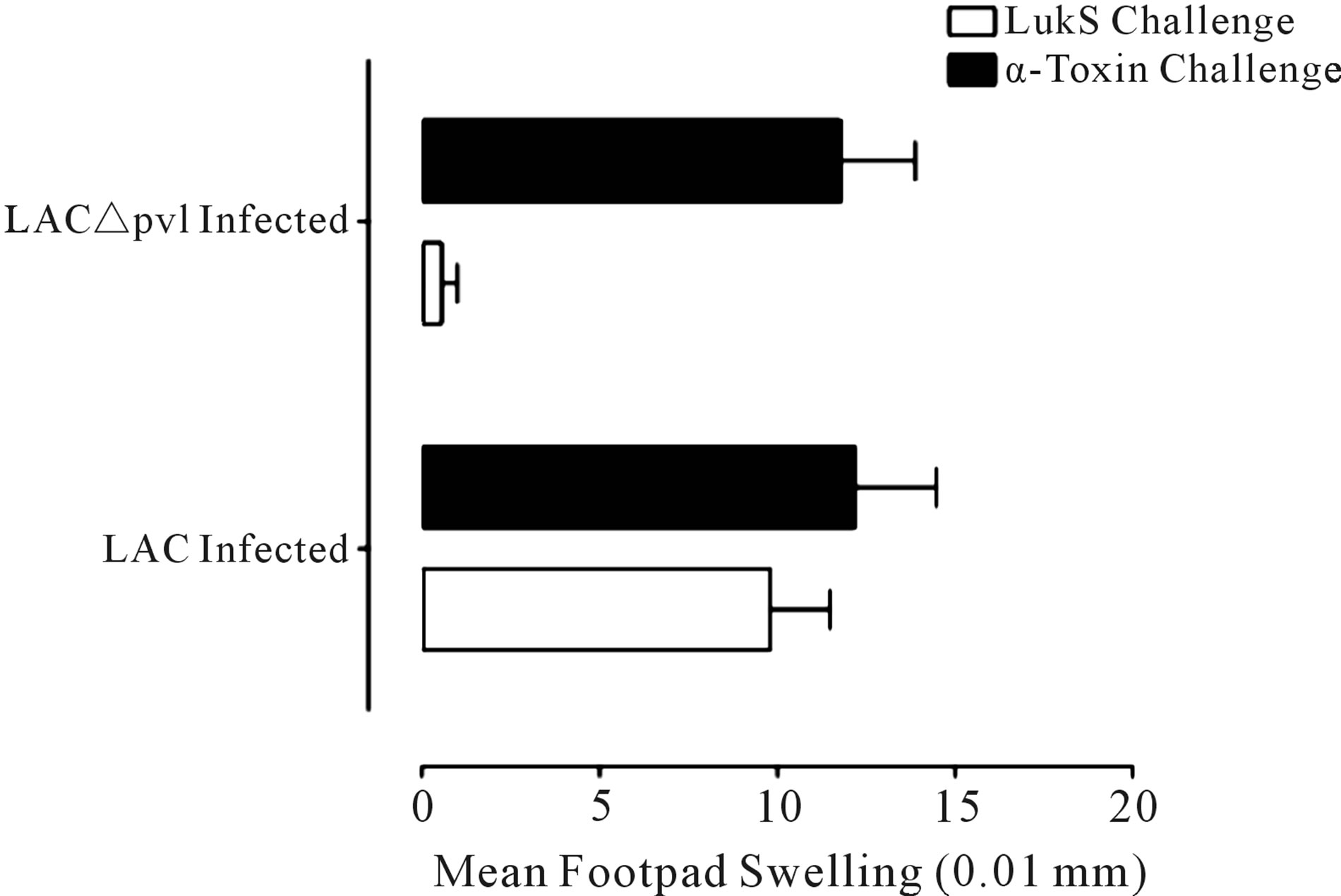
Previously, we demonstrated that intranasal or subcutaneous immunization of Balb/c mice with LukS-PV (the S component of the Panton Valentine leukocidin; PVL) conferred immunity against lung or skin infection, respectively, with S. aureus USA300 [3]. Immunization with LukF-PV did not confer protection [3]. Surprisingly, both immunization routes elicited cellular immune responses to LukS-PV measured by the significant delayed-type hypersensitivity (DTH) responses elicited in response to LukS-PV following challenge [3]. The antiLukS-PV DTH response was further examined here following infections with either S. aureus USA300 (LAC) or the PVL deletion mutant LAC∆pvl (strains generously provided by Dr. Frank DeLeo, National Institute of Allergy and Infectious Diseases) [4] that were maintained and cultured as described [3] (Figure 1(a)). Infections with either strain elicited a significant response to inactive α-toxin(H35L) which is expressed by both isolates, but elicitation of DTH was only observed in the mice infected with the LAC strain (Figure 1(a)).
To further define the role of cellular anti-LukS-PVspecific responses, a LukS-PV-specific T cell line (LST) was established and tested in vitro and in vivo for reactivity against a panel of peptides (Table 1) spanning the length of LukS-PV. Peptides (n = 14) 25 amino acids (aa) long with a 5 aa overlap spanning the entire length of the secreted protein were synthesized by Peptide 2.0 (Chantilly, VA) and ranged in purity from 90.31% - 98.1%. The signal peptide (SP) sequence was synthesized by Anaspec (San Jose, CA) (Table 1).
The LST line was derived from immunized Balb/c
 (a)
(a) (b)
(b)
Figure 1. Proliferative response to LukS-PV. (a) DTH response to LukS-PV and α-toxin following infection. Mice were infected with 5 × 107 S. aureus USA300 (LAC) or the isogenic LAC∆pvl. Two-weeks post infection mice were challenged in their right and left footpads with 2.5 µg LukS-PV and α-toxin(H35L), respectively. Footpads were measured at 0 and 24 h post challenge with a spring-loaded micrometer. The data are expressed as the mean ± SEM of 10 mice per group. *P < 0.005 vs. the LukS-PV response measured in LAC∆pvl mice using the unpaired t-test; (b) Characterization of the LST cell line. The LST cell line was obtained by purifying T cells from the popliteal lymph nodes of LukS-PV-immunized mice and maintained in culture in complete media containing IL-2. Proliferation of LST cells (1 × 104) in 96-well plates following stimulation with LukS-PV (1 µg) and APCs (1 × 105) for 48, 72 and 96. The data are expressed as the mean O.D. 590 nm ± SEM of triplicate wells for each treatment group. *P < 0.03 vs. all groups at the respective time points using the unpaired t test.

Table 1. LukS-PV peptidesa.
mice (Harlan, Indianapolis, IN) maintained in vitro as previously described [5]. All animal experiments were approved by the Institutional Animal Care Use Committee at the Texas A&M Health Science Center Institute of Biosciences and Technology. Briefly, LST were maintained in 24-well plates that contained 1 × 106 T cells and 5 × 106 mitomycin-treated APCs + 2 µg LukS-PV in a total volume of 1.5 ml complete medium [5]. T cells were cultured for 5 days in 7% CO2 - 93% air incubator at 37˚C. Cells were then pooled, washed, resuspended in complete medium containing 3 U/ml murine recombinant IL-2 (rIL-2 Boehringer-Mannheim) and distributed into 24-well plates at a concentration of 5 × 105 cells/ well in a total volume of 1.5 ml complete medium. The cultures were fed every 3 - 4 days with complete medium containing 5 U/ml rIL-2 and kept at a cell density of 5 × 105 - 1 × 106 cells/well. T cells were maintained in rest culture for 14 - 21 days, after which they were collected, washed and replated in 24-well cell culture plates with fresh mitomycin-treated APCs + 1 µg LukS-PV in a total volume of 1.5 ml complete medium/well. Three days after in vitro stimulation, 3 U of rIL-2 were added to each well. The cultures were then incubated for 3 more days, after which they were put into a rest cycle for another 14 - 21 days in medium containing 5 U/ml of rIL-2. At the end of each rest cycle, the T cells were tested for specificity by testing the proliferation response as follows:
 (a)
(a)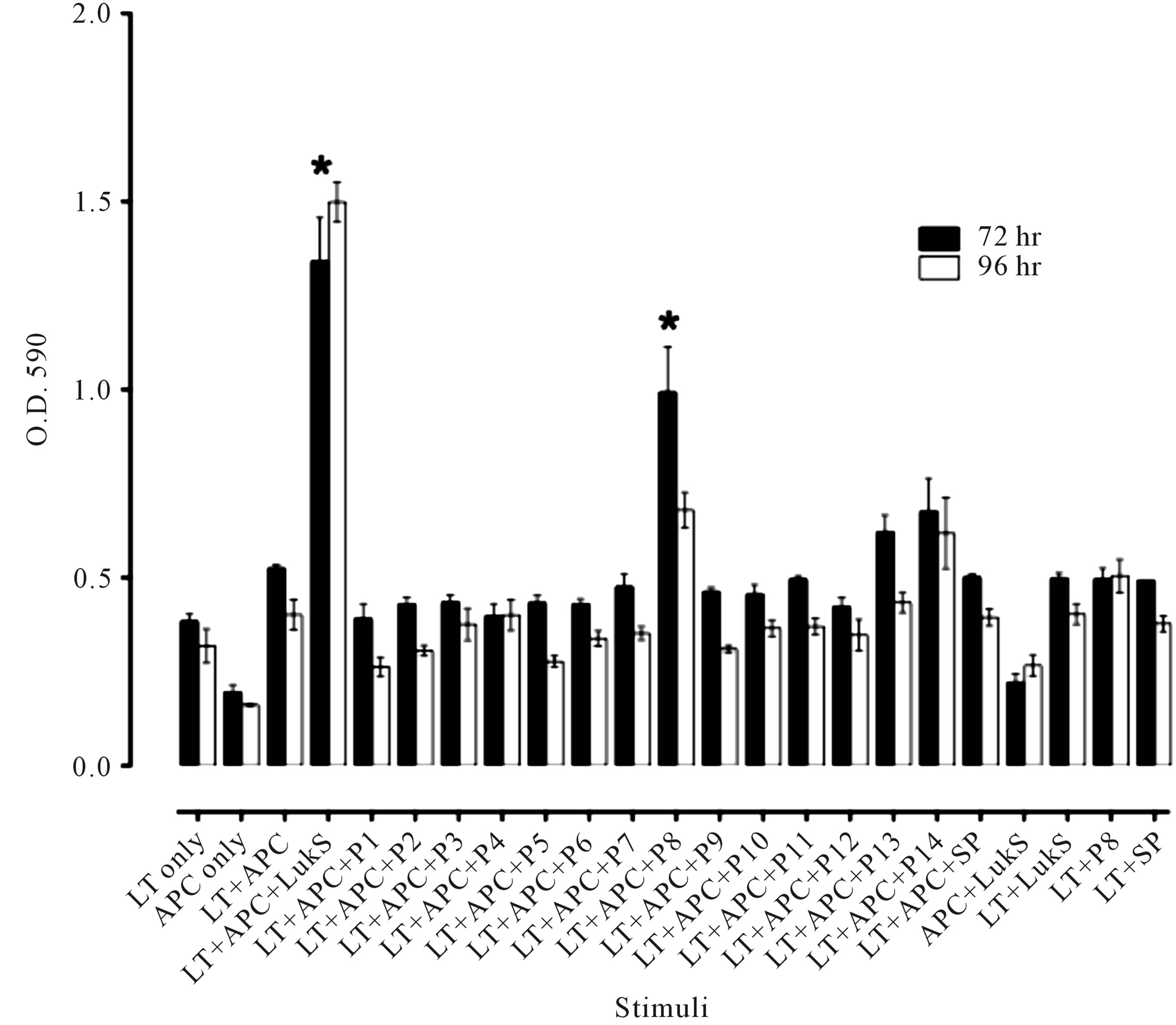 (b)
(b)
Figure 2. Reactivity of the LST cell line to LukS-PV and LukS-PV peptides. (a) Mice (n = 5 per group) adoptively transferred with LST cells (mice received 1 × 107 LST or a whole spleen equivalent [control]) 7 days post challenge with recombinant LukS-PV (0.5 µg) or peptides (2.5 µg each peptide in 50 µl PBS). Adoptively transferred LST mice and naïve mice challenged with LukS-PV served as positive and negative controls, respectively. The data are expressed as the mean ± SEM. *P < 0.05, **P < 0.0008 compared to the challenge only group. (b) Proliferation of LST cells in response to LukS-PV peptides. LST cells were stimulated with either LukS-PV (1 µg) or LukS-PV peptides (2 µg/well). Data are expressed as the mean O.D. 590 nm reading ± SEM of triplicate wells. *P < 0.007 vs. APC only, LST only, and LST + APC groups. This experiment was repeated twice with similar results.
1 × 104 T cells were cultured in 96-well round-bottom plates along with 1 × 105 mitomycin-treated APCs in complete medium in a volume of 100 µl/well in the presence of 0.5 µg/well of LukS-PV. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide125 µg/well) was added to the wells and the plate incubated for another 2.5 h at 37˚C. Extraction buffer (50% dimethyl formamide, 20% sodium dodecyl sulfate in water) was added to dissolve the insoluble purple formazan product and the absorbance quantified at 590 nm in a spectrophotometer [5]. Using this procedure, the LST cell line was generated (Figure 1(b)). Significant proliferation was observed at 72 and 96 h (P < 0.003, Student’s t test) in response to LukS-PV compared to proliferation observed in the respective control groups (Figure 1(b)). Confirmation that the LST cell line was CD4+ was carried out by incubating LST cells with fluorescently-labeled antibodies specific to either CD3, CD4 or CD8. Twocolor flow cytometric analysis demonstrated that the LST cell line was >98% CD3+CD4+ (data not shown).
LukS-PV peptides were used to challenge mice adoptively transferred with LST cells (Figure 2(a)). Mice challenged with peptides 7 (149 - 173), 8 (169 - 193) or 14 (289 - 312) elicited significant responses compared to footpad swelling measurements observed following challenge with the remaining peptides, however, responses observed following challenge with either peptide 7 (149 - 173), 8 (169 - 193) or 14 (289 - 312) were significantly below the response elicited following challenge with LukS-PV (Figure 2(a)). Interestingly, in vitro stimulation of the LST cell line with the peptide panel did not reveal as well defined a proliferative response compared to the DTH data described above. Only peptide 8 (169 - 193) elicited significantly different proliferation of LST cells in vitro (Figure 2(b)). Peptides 7 (149 - 173), 10 (209 - 233) and 14 (289 - 312) elicited slightly elevated proliferative responses over background proliferation in vivo, but these peptides did not significantly stimulate LST proliferation in vitro (Figure 2(b)). These data suggest that an epitope contained in the peptide 8 sequence (N169YISEVERQNSKSVQWGIKANSFIT193) induced the highest levels of LST proliferation in vitro and in vivo (in mice adoptively transferred with LST cells). Further characterization of the epitope present within peptide 8 may further improve the anti-LukS-PV response if used as part of a vaccine.
3. CONCLUDING REMARKS
Due to the lack of success in human trials utilizing immunogens targeted for their potential to elicit humoral responses, future S. aureus vaccine candidate searches should consider targets with the potential of eliciting protective cellular and humoral responses; a two-pronged immune attack against this pathogen would most likely benefit containment and clearance of cutaneous disease [6]. To what end cellular immune responses may prevent infections or accelerate clearance of this pathogen following infections associated with blood or lung tissues remains to be examined, however, cellular responses, particularly those associated with elevated production of INFg are beneficial in protection against various types of S. aureus infections [7-9]. It is likely, that vaccinemediated induction of cellular immunity may bolster antibody responses generated following exposure (whether from infection, carriage, or contact) to S. aureus.
4. ACKNOWLEDGEMENTS
This work was supported in part by grant R21AI072439 and a gift from the Kleberg Foundation to ELB.
REFERENCES
- Klevens, R.M., Morrison, M.A., Nadle, J., Petit, S., Gershman, K., Ray, S., Harrison, L.H., Lynfield, R., Dumyati, G., Townes, J.M., Craig, A.S., Zell, E.R., Fosheim, G.E., McDougal, L.K., Carey, R.B. and Fridkin, S.K. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the united states. Journal of the American Medical Association, 298, 1763-1771. doi:10.1001/jama.298.15.1763
- Lowy F.D. (1998) Staphylococcus aureus infections. New England Journal of Medicine, 339, 520-532. doi:10.1056/NEJM199808203390806
- Brown, E.L., Dumitrescu, O., Thomas, D., Badiou, C., Koers, E.M., Choudhury, P., Vazquez, V., Etienne, J., Lina, G., Vandenesch, F. and Bowden, M.G. (2009) The panton-valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clinical Microbiology and Infection, 156, 156- 164. doi:10.1111/j.1469-0691.2008.02648.x
- Voyich, J.M., Otto, M., Mathema, B., Braughton, K.R., Whitney, A.R., Welty, D., Long, R.D., Dorward, D.W., Gardner, D.J., Lina, G., Kreiswirth, B.N., DeLeo, F.R. (2006) Is panton-valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? Journal of Infectioius Diseases, 194, 1761-1770. doi:10.1086/509506
- Pride, M.W., Brown, E.L., Stephens, L.C., Killion, J.J., Norris, S.J. and Kripke, M.L. (1998) Specific Th1 cell lines that confer protective immunity against experimental Borrelia burgdorferi infection in mice. Journal of Leukocyte Biology, 63, 542-549.
- Proctor, R.A. (2012) Challenges for a universal Staphylococcus aureus vaccine. Clinical Infectious Diseases, 54, 1179-1186. doi:10.1093/cid/cis033
- Gomez, M.I., Sordelli, D.O., Buzzola, F.R. and Garcia, V.E. (2002) Induction of cell-mediated immunity to Staphylococcus aureus in the mouse mammary gland by local immunization with a live attenuated mutant. Infection and Immunity, 70, 4254-4260. doi:10.1128/IAI.70.8.4254-4260.2002
- Guillen, C., McInnes, I.B., Vaughan, D.M., Kommajosyula, S., Van Berkel, P.H., Leung, B.P., Aguila, A. and Brock, J.H. (2002) Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. Journal of Immunology, 168, 3950-3957.
- Lin, L., Ibrahim, A.S., Xu, X., Farber, J.M., Avanesian, V., Baquir, B., Fu, Y., French, S.W., Edwards, J.E. and Spellberg, B. (2009) Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathogens, 5, e1000703. doi:10.1371/journal.ppat.1000703
NOTES
*Authorship: ELB and MGB generated the LST cell lines and carried out the cell culture and animal infection and DTH experiments. KCS carried out the flow cytometric analysis. All authors participated in manuscript preparation.
Disclosures: The authors report no conflict of interest.

