Open Journal of Immunology
Vol.2 No.2(2012), Article ID:20128,6 pages DOI:10.4236/oji.2012.22009
α2-macroglobulin co-administered in vivo promotes antigen delivery and presentation
![]()
Department of Pathology, Duke University Medical Center, Durham, USA; *Corresponding Author: Pizzo001@mc.duke.edu
Received 10 March 2012; revised 1 April 2012; accepted 9 April 2012
Keywords: α2-Macroglobulin-Antigen Complexes; Antigen Presentation/Processing; Vaccination; Cytokines; Spleen
ABSTRACT
Administered in vivo, covalent receptor-recognized α2-macroglobulin (α2M*)-antigen complexes enhance humoral and cell-mediated immunity. We hypothesized that in vivo α2M*-encapsulation could be promoted in the setting of vaccines that co-deliver α2M* with unbound antigen, thereby eliminating the need to prepare complexes in vitro. Mice immunized intradermally with co-delivered α2M* and OVA demonstrated antigen-specific immune responses, including anti-tumor responses, similar to those elicited by conjugated α2M*-OVA complexes. Enhanced immunity appears to result from in vivo α2M*-encapsulation of antigen. This finding represents a significant advancement in the development of α2M* as an antigen delivery vehicle capable of enhancing the presentation of subunit vaccines.
1. INTRODUCTION
Previous studies have demonstrated that antigen encapsulation by α2-macroglobulin (α2M)1 enhances antigen-specific immune responses, both in vitro and in vivo [1-7 ]. For these studies, α2M-encapsulated antigen complexes were typically prepared by in vitro incubation of amine-activated α2M, designated α2M*, with an 8 to 100-fold-molar excess of antigen in the presence of heat [3,4,7 ]. These α2M*-antigen complexes were then purified by size-exclusion chromatography to remove unbound antigen. While producing α2M*-antigen complexes is not extremely difficult on a small scale, it would be more challenging to produce the complexes and perform the necessary quality control on an industrial scale.
While the maximal incorporation of antigen into α2M* occurs following 24 h of incubation at 37˚C, some incorporation of antigen occurs at much earlier time periods [8,9]. It is thought that α2M*-encapsulation occurs naturally in vivo as a mechanism of targeting antigen for uptake, processing, and presentation by professional antigen presenting cells [1]. It, however, is unknown whether in vivo α2M*-encapsulation could be promoted in the setting of a vaccine that co-delivers α2M* with unbound antigen. A high local concentration of antigen may be necessary to drive incorporation into α2M*. Because most routes of injection result in rapid dissipation of antigen, conditions that result in a depot effect, for example intradermal injection and alum absorption, should promote α2M*-encapsulation in vivo. In this study, we investigate the ability of α2M*, co-administered with unbound antigen, to enhance antigen-specific immune responses through in vivo encapsulation, resulting in enhanced antigen delivery.
2. MATERIALS AND METHODS
2.1. Purification and Activation of Murine and Human α2M
Purification and amine-activation of murine α2M was performed as previously described [7,8,10,11]. Human α2M was purified from fresh, frozen human plasma, obtained from the American Red Cross (Durham, NC, USA), according to a previously published protocol [12].
Native human α2M was converted to the “amine-activated” form (α2M*) by incubation in 200 mM ammonium bicarbonate at room temperature for 1 h. Buffer exchange into PBS was achieved using a 5 mL disposable de-salting column. Native human α2M was converted to the “trypsin-activated” form (α2M-T) by incubation with a 5-fold molar excess of trypsin (Worthington Biochemical, Lakewood, NH) for 1 h at room temperature. The Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL) was then added to inhibit proteolytic activity. Excess trypsin and protease inhibitors were removed by consecutive passes over a 100 kDa centrifugal concentrator (Pall Life Sciences, Ann Arbor, MI). Purified protein contained less than 10 pg of endotoxin per mg of protein, as determined by a commercial assay kit (Limulus Amebocyte Lysate Kinetic-QCL by Cambrex, Walkersville, MD).
2.2. Encapsulation of OVA into Murine α2M*
Complexes of amine-activated α2M* and Alexa FluorÒ 647-conjugated ovalbumin (OVA) (Molecular Probes, Eugene, OR) were prepared as previously described [7]. Molar ratio of incorporation was approximately 3:1 OVA: α2M*, as determined by fluorescence quantification.
2.3. Time-Dependent Study of OVA Encapsulation into Human α2M
A 3-fold molar excess of OVA was incubated at 37˚C with either α2M* or α2M-T for 0.5 to 24 h. Samples were then immediately analyzed by native PAGE.
2.4. Cells and Cell Culture
The MO5 cell line, an OVA-transfected subclone of B16 melanoma, was a kind gift from Dr. Kenneth Rock (University of Massachusetts Medical School, Worcester, MA). MO5 cells were cultured in complete media supplemented with 2 mg/mL G418. Murine splenocytes were harvested and cultured as previously described [7].
2.5. Mice
Female C57/BL6 mice were obtained from Charles River Laboratories (Raleigh, NC). All mice were housed in the Duke University Animal Facility, an AAALAC approved facility. All experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol.
2.6. Intradermal Immunization and Tumor Challenge
Prior to tumor challenge, C57/BL6 mice were immunized by intradermal injection into the right ear pinna with 10 μL antigen or PBS, with or without the addition of α2M* or CpG 1826, 5’-TCCATGACGTTCCTGACGTT-3’ (Midland Certified Reagent Co., Midland, TX, USA). The treatment groups (n = 5; each receiving 1.35 μg OVA/ injection) included the following: OVA alone; OVA administered with 10 μg CpG 1826; α2M*-OVA; α2M*-OVA administered with 10 μg CpG 1826; α2M* co-administered with unbound OVA; and α2M* co-administered with unbound OVA with 10 μg CpG 1826. The quantity of α2M* administered with unbound OVA was equivalent to the amount of α2M* present in the α2M*-OVA preparations (6 μg).
Preparations of α2M* and OVA were kept in separate containers on ice until immediately prior to injection in order to minimize the possibility of in vitro α2M*-encapsulation. Mice were subsequently boosted at days 35 and 63, consistent with our previously published immunization protocol [7]. Control groups (n = 5) received intradermal injections of PBS, CpG 1826, or α2M* alone. Serum anti-OVA IgG was monitored every 2 weeks by ELISA, as previously described [7]. Mice were injected s.c. in the left flank with 104 MO5 tumor cells in Matrigel™ basement membrane matrix (BD Biosciences PharMingen) at week 14. Staining of mouse PBLs with iTAgÔ MHC Tetramer H2-Kb SIINFEKL-PE (Beckman Coulter, Fullerton, CA) and CaltagÔ FITC-conjugated Rat anti-Mouse CD8a antibody (Invitrogen Corp., Carlsbad, CA) was performed 2 weeks following tumor implantation, as previously described [7], in order to quantify the proportion of CD8+ T cells specific for the H2- Kb-restricted CTL epitope of OVA, the SIINFEKL peptide (OVA257-264). Tumor diameters were measured using digital calipers, and tumor volume was calculated using the equation V = 0.4 ab2, where a and b are the longest and shortest diameters, respectively. Mice were euthanized when tumor volume reached 2 cm3.
For the detection of fluorescently-labeled OVA encapsulated by α2M* in vivo, three mice were injected intradermally in the right ear pinnae with 3:1 OVA:α2M* (30 μg:10 μg) in PBS. For comparison, one mouse was injected with only OVA (30 μg) and another was injected with only α2M* (10 μg). After 1.5 h, mice were euthanized, and ear pinnae were flushed with 3 × 20 μL PBS. The collected fluid samples were analyzed by native PAGE, and incorporation of antigen was measured directly by fluorescence imaging using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
2.7. [3H]-Thymidine Proliferation Assay
Splenocytes harvested from mice that had been previously immunized with OVA and CpG 1826 (as above) were pulsed for 6 h with 2.5 μM OVA, either free or codelivered with amine-activated α2M* or trypsin-activated α2M-T (7.5 μM), in serum-free media. As controls, cells were also pulsed with Con A (5 μg/mL) and α2M* containing no antigen. Cells were then washed, resuspended in complete media, loaded at 2.5 × 105 cells per well onto a 96-well flat-bottom plate and cultured for 3 d at 37˚C in a humidified 5% CO2 incubator. Cultured cells were treated with 1 μCi/well [methyl-3H]thymidine (PerkinElmer, Waltham, MA) 18 h prior to harvesting. Incorporation of [3H]thymidine was measured using a Tri-Carb 2100 TR liquid scintillation counter (PerkinElmer, Boston, MA).
2.8. Statistical Analysis
For in vitro studies, the Student’s t-test was performed to determine P values and ascertain statistical significance between two treatments. For in vivo studies (antibody titers, tetramer staining, and tumor growth), ANOVA was performed, followed by multiple comparison procedures (Tukey) to determine differences between groups. Significance between Kaplan-Meier survival curves was determined by log-rank Mantel-Cox analysis. The level of significance used was 0.05.
3. RESULTS
3.1. Antigen Encapsulation by α2M* Occurs Rapidly and Can Be Detected Following as Little as 30 min of Incubation
Consistent with a previous report [8], maximal incorporation of antigen by α2M* occurs after 24 h of incubation at 37˚C (Figure 1). However, some association of antigen with α2M* can be observed at 30 min. This association likely represents covalent antigen incorporation by α2M* because it is not observed with proteolyticallyactivated α2M-T. Although proteolytic thiol ester cleavage in α2M is irreversible, thiol ester cleavage by primary amines can be reversed with heat, allowing the incorporation of new antigens by α2M [11]. A small association between OVA and α2M-T was observed following 24 h of incubation.
3.2. Co-Administration of α2M* with Unbound Antigen Enhances Humoral and Cell-Mediated Immunity to a Similar Degree as Conjugated α2M*-Antigen Complexes
To determine if co-delivery of antigen with unbound α2M* could enhance immune responses in vivo, groups of naïve C57/BL6 mice (n = 5) were immunized intradermally with OVA and unbound α2M*, with or without the addition of an immunostimulatory adjuvant, CpG 1826. This study was performed concurrently with a previously reported experiment investigating immune responses to prepared α2M*-OVA complexes [7]. Separate unbound α2M* and OVA preparations were kept on ice and combined immediately prior to injection in order to minimize the possibility of α2M*-encapsulation occurring in vitro. Following two booster injections (days 35 and 63), mice were challenged with a subcutaneously implanted OVAexpressing B16 melanoma flank tumor.
For both conjugated α2M*-OVA and unconjugated α2M* + OVA groups, the development of anti-OVA IgG antibody was first observed 8 weeks following initial injection (result not shown). End-point titers (antibody titers at the time of tumor implantation; week 14) are shown in Figure 2(a). OVA administered either with unbound α2M* or the well-characterized adjuvant CpG 1826 produced similar antibody titers in immunized mice.
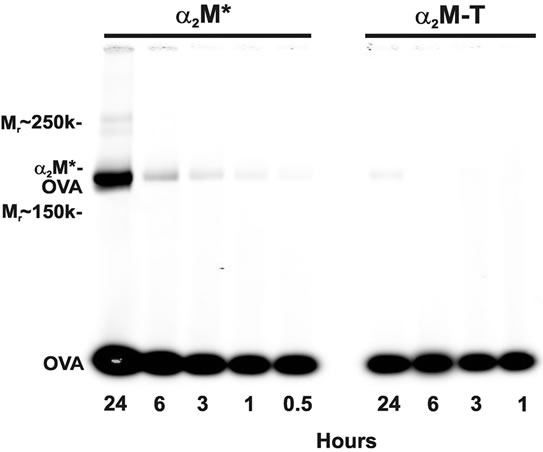
Figure 1. Time-dependence of α2M* incorporation of antigen. A 3-fold molar excess of OVA was incubated at 37˚C with either amine-activated α2M* or proteolytically-activated α2M-T for 0.5 to 24 h. Native PAGE demonstrates co-migration of fluorescently-labeled OVA (45 kDa) with α2M* dimers (360 kDa) after 30 min of incubation; maximal comigration can be observed after 24 h of incubation. For incubation periods less than 24 h, no association between OVA and α2M-T is observed. Previous studies have shown no incorporation of OVA into native α2M [7].
Although the mean antibody titers produced by conjugated α2M*-OVA were greater than those elicited with OVA and unbound α2M*, the difference between these groups was not found to be statistically significant.
Tetramer staining of PBLs was performed 2 weeks following tumor implantation in order to observe expansion of the antigen-specific CD8+ T cell population in these mice (Figure 2(b)). Mice treated with OVA and unbound α2M* with the addition of CpG, however, did elicit expansion of the OVA-specific CD8+ T cell population, which was significant compared to the control groups. Although the OVA with unbound α2M* without CpG group appeared to elicit some degree of expansion of the OVA-specific CD8+ T cell population, the tetramer staining population for this group was not significantly greater than that of the control groups (PBS, CpG, or α2M* alone). Conjugated α2M*-OVA treatment groups appeared to elicit greater expansion of antigen-specific CTLs than the OVA with unbound α2M* groups, the differences between these treatment groups were not found to be statistically significant. The greatest expansion of OVA-specific CD8+ T Cell population was observed in the α2M conjugated-OVA with the addition of CPG.
3.3. Co-Administration of α2M* with Unbound Antigen Enhances Anti-Tumor Immune Responses
To investigate the anti-tumor response of co-administered α2M* vaccinated mice were challenged with OVA

Figure 2. Co-delivery of α2M* with antigen enhances tumor protection in vivo. End-point titers (a), tetramer staining of PBLs (b), and tumor growth (c) are shown for each mouse treatment group. For clarity, groups treated with α2M* co-administered with unbound OVA are shown in red. Values indicate mean ± SEM. P values (*P < 0.05; **P < 0.005) indicate differences between the respective treatment group and each of the three control groups (PBS, CpG, α2M*). The bracket in Panel A indicates a comparison between the indicated groups (*P < 0.05). (d) Kaplan-Meier plot depicts survival of treatment groups.
expressing BIG melanoma flank tumors. Anti-tumor responses elicited by the co-delivery of α2M* with unbound OVA were found to be similar to those observed with α2M*-OVA. Observable growth of OVA-expressing tumors over time is shown in Figure 2(c). Mice immunized with OVA and unbound α2M*, with or without CpG, demonstrated delayed tumor growth compared to each of the control groups, tumor growth for these groups was not significantly different from the α2M*-OVA treatment groups. Survival of mice treated with co-delivered α2M* and unbound OVA, either with or without CpG, was significantly prolonged (P < 0.005) compared to OVA treatment alone (Figure 2(d)). However, survival of these mice did not differ significantly from that of mice treated with conjugated α2M*-OVA.
3.4. Encapsulation of Antigen by α2M* Occurs in Vivo in the Setting of Intradermal Injection
We hypothesized that enhanced in vivo immune responses with α2M* and OVA co-delivery were the result of in vivo encapsulation of antigen. The conditions of antigen delivery, including the depot effect caused by intradermal injection and the 37˚C environment of the mouse, are similar to the conditions used to successfully incorporate antigen into α2M* in vitro [8]. To determine if such in vivo encapsulation of antigen could occur in this setting, we similarly intradermally injected the ear pinnae of mice with a 3:1 molar ratio of OVA:α2M*. For comparison, mice were injected with either OVA or α2M* alone. After 1.5 h, the mice were euthanized, and the ear pinnae were flushed with 3 × 20 μL PBS. The fluid that was recovered was analyzed by native PAGE (Figure 3). The detection of fluorescently labeled OVA co-migrating with α2M* dimers in these mice confirmed the occurrence of in vivo encapsulation of OVA into α2M*.
3.5. Enhanced Immune Responses with α2M* Co-Administration Result from Encapsulation of Antigen, Rather than Ligation of α2M* Receptors
The detection of in vivo α2M* encapsulation suggests a mechanism for the enhanced in vivo immune responses discussed above, it was also possible that ligation of the α2M* receptor, low-density lipoprotein receptor-related protein 1 (LRP-1)/CD91, in the absence of antigen encapsulation, may also contribute to this enhanced immunity. To investigate this possibility, splenocytes harvested from OVA-immunized mice were treated for 6 h with OVA and either unconjugated amine-activated α2M* or proteolytically-activated α2M-T. After 3 days, cell proliferation was measured by [3H]thymidine incorporation (Figure 4). Cell proliferation was increased approximately two-fold following co-delivery of α2M* with antigen. However, co-delivery of α2M-T, which is receptor-recognized but incapable of incorporating new antigen, did not enhance proliferation. Therefore, we concluded that this enhanced response is secondary to α2M*-encapsulation and not ligation of the α2M* receptor, (LRP-1)/CD91.
4. DISCUSSION
It has been suggested that new generation vaccines

Figure 3. Incorporation of antigen by α2M* occurs in vivo. Five mice received 10 mL intradermal injections in the left ear pinnae. One mouse (M1) was injected with only α2M*, three mice (M2, M3, M4) were injected with a 3:1 molar ratio of OVA:α2M*, and one mouse (M5) was injected with only OVA. After 1.5 h, mouse ears were flushed with 3 × 20 mL PBS, and collected fluid was analyzed by native PAGE (top: infrared fluorescence scan at l = 700 nm, bottom: Coomassie stain).
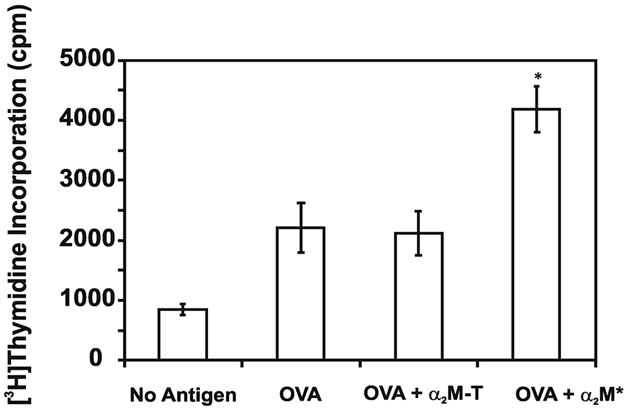
Figure 4. Co-delivery of α2M* with antigen enhances cell proliferation in vitro. Splenocytes harvested from OVAimmunized mice were treated for 6 h with OVA, either alone or in combination with α2M* or α2M-T. After 3 days of culture, cell proliferation was measured by [3H]-thymidine incorporation. As a control, incorporation by cells treated with no antigen is also shown. Each concentration was assayed in triplicate; values are mean ± SD. Results are representative of three experiments. *P < 0.05 compared to OVA or OVA + α2M-T.
will largely consist of purified recombinant proteins [13]. However, formulations of purified protein are frequently poor at eliciting humoral and cell-mediated immunity. Therefore, the development of adjuvants and antigen delivery vehicles that are efficacious, as well as costeffective and practical, is of extreme importance.
The highly conserved proteinase inhibitor α2M has received attention in recent years for its ability to entrap diverse macromolecules and target them for rapid receptor-mediated uptake by professional antigen presenting cells. Antigen delivery by α2M* elicits 100 to 1000-fold enhanced antibody titers against protein and peptide based vaccines and vaccine candidates, such as hepatitis B surface antigen [3] and the HIV envelope gp120 C4- V3 peptide [4]. Complexes of α2M* and trypanosomal proteinases have been shown to activate CD4+ T cells more efficiently than antigen alone [14] and to stimulate the production of antibodies that effectively inhibit activity of the enzyme [15]. Furthermore, our laboratory has recently demonstrated that α2M*-encapsulation enhances antigen-specific CTL responses and protection against antigen-presenting tumors [7]. Although these studies have established that α2M*-encapsulation can be achieved with relative ease on a small scale, assuming adequate resources and training in biochemical techniques, the large scale production of α2M*-antigen complexes may present new challenges. Therefore, achieving enhanced immunologic responses with co-administered α2M*, avoiding the steps of in vitro incorporation and isolation of complexes, represents a significant advance for this antigen delivery vehicle.
5. CONCLUSION
Our findings demonstrate that co-delivery of α2M* with unbound antigen can enhance humoral and cellmediated immunity, resulting in improved anti-tumor responses, to similar degree as α2M*-antigen complexes prepared in vitro. These enhanced immune responses with α2M* co-delivery appear to result from in vivo encapsulation of antigen, rather than a direct effect of ligating LRP by α2M* not carrying bound antigen α2M* receptors. The capacity of α2M* to promote antigen delivery in vivo results from the rapidity with which it encapsulates local macromolecules. Antigens encapsulated by α2M* are targeted for rapid receptor-mediated uptake by professional antigen presenting cells, resulting in efficient antigen processing and presentation. We conclude that administration of α2M* in the context of a high localized concentration of antigen, such as that which can be achieved with a depot, facilitates antigen delivery and presentation. These findings represent a significant advancement in the use of α2M* as an antigen delivery vehicle.
6. ACKNOWLEDGEMENTS
This work was supported by grant #HL-24066 from the National Heart, Lung, and Blood Institute. We thank Dr. K. Rock for his kind gift of the MO5 tumor cell line. Many thanks to Sturgis Payne, Yvonne Mowery, Steve Conlon, and Marie Thomas for their contributions to this work.
REFERENCES
- Chu, C.T., Oury, T.D., Enghild, J.J. and Pizzo, S.V. (1994) Adjuvant-free in vivo targeting. Antigen delivery by alpha 2-macroglobulin enhances antibody formation. Journal of Immunology, 152, 1538-1545.
- Chu, C.T. and Pizzo, S.V. (1993) Receptor-mediated antigen delivery into macrophages. Complexing antigen to alpha2-macroglobulin enhances presentation to T cells. Journal of Immunology, 150, 48-58.
- Cianciolo, G.J., Enghild, J.J. and Pizzo, S.V. (2001) Covalent complexes of antigen and alpha2-macroglobulin: Evidence for dramatically-increased immunogenicity. Vaccine, 20, 554-562. doi:10.1016/S0264-410X(01)00361-9
- Liao, H.X., Cianciolo, G.J., Staats, H.F., Scearce, R.M., Lapple, D.M., Stauffer, S.H., Thomasch, J.R., Pizzo, S.V., Montefiori, D.C., Hagen, M., Eldridge, J. and Haynes, B.F. (2002) Increased immunogenicity of HIV envelope subunit complexed with alpha2-macroglobulin when combined with monophosphoryl lipid A and GM-CSF. Vaccine, 20, 2396-2403. doi:10.1016/S0264-410X(02)00090-7
- Binder, R.J., Karimeddini, D. and Srivastava, P.K. (2001) Adjuvanticity of alpha2-macroglobulin, an independent ligand for the heat shock protein receptor CD91. Journal of Immunology, 166, 4968-4972.
- Binder, R.J., Kumar, S.K. and Srivastava, P.K. (2002) Naturally formed or artificially reconstituted non-covalent alpha2-macroglobulin-peptide complexes elicit CD91-dependent cellular immunity. Cancer Immunity, 2, 16.
- Bowers, E.V., Horvath, J.J., Bond, J.E., Cianciolo, G.J. and Pizzo, S.V. (2009) Antigen delivery by alpha(2)- macroglobulin enhances the cytotoxic T lymphocyte response. Journal of Leukocyte Biology, 86, 1259-1268. doi:10.1189/jlb.1008653
- Gron, H. and Pizzo, S.V. (1998) Nonproteolytic incorporation of protein ligands into human alpha2-macroglobulin: Implications for the binding mechanism of alpha 2-macroglobulin. Biochemistry, 37, 6009-6014.
- Adlakha, C.L., Hart, J.P. and Pizzo, S.V. (2001) Kinetics of nonproteolytic incorporation of a protein ligand into thermally activated alpha2-macroglobulin: Evidence for a novel nascent state. The Journal of Biological Chemistry, 276, 41547-41552. doi:10.1074/jbc.M106357200
- Bhattacharjee, G., Gron, H. and Pizzo, S.V. (1999) Incorporation of non-proteolytic proteins by murine alpha2- macroglobulin. Biochimica et Biophysica Acta (BBA) — Protein Structure and Molecular Enzymology, 1432, 49- 56. doi:10.1016/S0167-4838(99)00072-2
- Gron, H., Thogersen, I.B., Enghild, J.J. and Pizzo, S.V. (1996) Structural and functional analysis of the spontaneous re-formation of the thiol ester bond in human alpha 2-macroglobulin, rat alpha1-inhibitor-3 and chemically modified derivatives. Biochemical Journal, 318, 539-545.
- Chu, C.T. and Pizzo, S.V. (1994) alpha2-Macroglobulin, complement, and biologic defense: Antigens, growth factors, microbial proteases, and receptor ligation. Laboratory Investigation, 71, 792-812.
- O’Hagan, D.T. and De Gregorio, E. (2009) The path to a successful vaccine adjuvant—“The long and winding road”. Drug Discovery Today, 14, 541-551.
- Morrot, A., Strickland, D.K., Higuichi Mde, L., Reis, M., Pedrosa, R. and Scharfstein, J. (1997) Human T cell responses against the major cysteine proteinase (cruzipain) of Trypanosoma cruzi: Role of the multifunctional alpha 2-macroglobulin receptor in antigen presentation by monocytes. International Immunology, 9, 825-834. doi:10.1093/intimm/9.6.825
- Huson, L.E., Authie, E., Boulange, A.F., Goldring, J.P. and Coetzer, T.H. (2009) Modulation of the immunogenicity of the Trypanosoma congolense cysteine protease, congopain, through complexation with alpha(2)-macroglobulin. Veterinary Research, 40, 52. doi:10.1051/vetres/2009036
ABBREVIATIONS
α2M: α2-macroglobulin;
α2M*: amine-activated α2M;
α2M-T: trypsin-activated α2M;
α2M*-OVA: α2M*-encapsulated ovalbumin;
CpG 182: 5’-TCCATGACGTTCCTGACG-TT-3’;
LRP-1: low-density lipoprotein receptor-related protein 1;
OVA: ovalbumin;
OVA257-264: H2-Kb-restricted CTL epitope of OVA (SIINFEKL peptide).

