Open Journal of Ecology
Vol.06 No.06(2016), Article ID:66446,9 pages
10.4236/oje.2016.66030
A Theory of Ratio Selection―Lattice Model for Obligate Mutualism
Kei-Ichi Tainaka1, Tsuyoshi Hashimoto2
1Graduate School of Science and Technology, Shizuoka University, Hamamatsu, Japan
2Department of Information Engineering, National Institute of Technology, Matsue College, Matsue, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 February 2016; accepted 10 May 2016; published 13 May 2016
ABSTRACT
Mutualisms are cooperative interactions between members of different species. We focus on obligate mutualism, where each species cannot survive without the other. From a theoretical aspect, obligate mutualism is similar to the relationship between male and female. Empirical data indicate a sex-ratio selection: male and female have a specific ratio in their population sizes. In the present paper, we apply lattice model to obligate mutualism between two species, and present a theory of “ratio selection” which is a generalization of sex-ratio selection. Computer simulations are carried out by two methods: local and global interactions. In the former, interactions occur between neighbouring cells, while in the latter they occur between any pair of cells. Simulations in both interactions show the so-called Allee effect: both species can survive, when both densities are large in some extent. However, we find a large difference between local and global simulations. In the case of local interaction, restriction for survival is found to be extremely severe compared to global interaction. Both species require a proper ratio for their sustainability. This result leads to the theory of ratio selection: when interaction occurs locally, the ratio of both species is uniquely determined. We discuss that the ratio selection explains not only the evolution of endosymbionts from free-living ancestors but also the evolution from endosymbionts to organelles.
Keywords:
Obligate Mutualism, Population Dynamics, Ratio Selection, Allee Effect, Lattice Model, Sex-Ratio Selection, Endosymbiosis

1. Introduction
In recent years, the concern for mutualism is growing, since almost all species have mutualistic relationship with other species [1] - [4] . Microbial species influence on the abundances and ecological functions of related species [5] [6] . Many bacterial species coexist in a syntrophic association; that is, one species lives off the products of another species. Here, we pay attention to obligate mutualism between a pair of species; one species cannot survive without the other. The systems of obligate mutualism usually have some mechanism to avoid a sudden increase (or decrease) of one species [1] . We discuss the reason why such a mechanism is necessary.
The relationship of obligate mutualism has the similar behaviour as the male-female relationship [7] - [9] : female (or male) cannot get offspring without the partner. The sex ratio of many animals is nearly one-to-one. Fisher (1930) first explained the reason why the 1:1 sex ratio is optimal [10] . Later his argument was recognized as in the framework of evolutionarily stable strategy (ESS) [11] [12] . However, Fisher’s theory has some problems [13] [14] . For example, his theory may conflict with real data. In most animals, the sex ratio (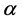 ) at birth is slightly biased (
) at birth is slightly biased (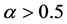 ) [15] . Consider a large population (resident) which is slightly biased (say
) [15] . Consider a large population (resident) which is slightly biased (say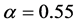 ). According to Fisher’s theory, the resident is easily beaten by all mutants which satisfy
). According to Fisher’s theory, the resident is easily beaten by all mutants which satisfy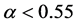 ; in particular, the mutant of asexual reproduction (
; in particular, the mutant of asexual reproduction (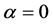 ) is optimal [16] .
) is optimal [16] .
It is known that ESS is the most powerful tool to obtain the optimal strategy which beats the other strategies [11] . To win such competitions may be one of most important driving forces in evolution, but it is not a necessary condition for evolution. For instance, the most sustainable strategy is advantageous for evolution [16] - [18] . Yoshimura et al. have explained the evolutionary origins of periodical cicadas in North America not by ESS but by sustainability [17] [18] . If the competition is dominant, real cicadas (13-and 17-year cycles) might be beaten by the other phenotypes with shorter cycles. The sustainability also explains the fact that the life span of species is not so long [19] . Similarly, Tainaka et al. (2006) have explained the evolution of 1:1 sex ratio on the basis of sustainability [8] . In the present paper, we extend this idea to obligate mutualism.
Lattice models have been applied to ecological problems, where simulations have been carried out by two methods: local and global interactions [20] [21] . In the former, interactions occur between neighboring sites, whereas in the latter they occur between any pair of lattice sites. The simulation of global interaction is often called “lattice gas model” [9] [22] [23] ; individuals contact like the collision of gas molecules. In most cases, the dynamics of global interaction can be represented by mean-field theory. However, for the local interaction, there are usually no theories; simulations are thus necessary to obtain various results.
The most famous model of population dynamics in ecology is Lotka-Volterra equations (LVEs) [24] [25] . However, they have a flaw, when we apply them to mutualism [4] [25] . For example, the population sizes of both species may increase infinitely (“divergence problem”) [25] . Moreover, LVEs never predict Allee effect for obligate mutualism [7] . Recently, Iwata et al. [9] introduced a simple population model for mutualism, applying the lattice gas model. They assume that all interactions occur between any pair of cells. In their model, the divergence has never occurred, and Allee effect has been derived in a simple form. The present work is the local-interaction version of Iwata’s model. Especially, we explore equilibrium densities to know the sustainability of populations.
In the next section, we explain our model and method of both local and global interactions. In Section 3, the prediction by mean-field theory is reported. We briefly describe the results obtained by Iwata et al. [9] , and we add new results, such as equilibrium densities. Section 4 is devoted to report simulation results. In the case of local interaction, the dynamics is similar to those for global interaction, such as Allee effect. However, there is a large difference between local and global interactions. In the former case, the sustainable range in parameter space is very narrow, compared to global interaction. Namely, the proportion (ratio) between both species is strongly restricted for their survival. In the final section, we present an idea of “ratio selection” which is an extension of the sex-ratio selection [12] - [15] .
2. Model and Methods
2.1. Model
Consider a system consisting of two mutualistic species X and Y (Figure 1). Each lattice site is labeled by X, Y or O, where O means the empty site. The empty cell is introduced to prohibit the divergence of population sizes [9] . The reactions are defined by
 (1a)
(1a)
Figure 1. Lattice model for obligate mutualism. The cell X (Y) indicates the occupation site (individual) of species X (Y), and O is empty. The empty cell is introduced to avoid the divergence of population sizes [13] .
 (1b)
(1b)
 (1c)
(1c)
 (1d)
(1d)
The reactions (1a) and (1c) respectively denote the birth and death processes of species X, and 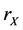 (
(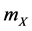 ) denotes the reproduction (mortality) rate of species X. Similarly, the reactions (1b) and (1d) mean the birth and death processes of species Y, respectively.
) denotes the reproduction (mortality) rate of species X. Similarly, the reactions (1b) and (1d) mean the birth and death processes of species Y, respectively.
2.2. Method
Simulations of lattice model are usually carried out by either local or global interaction [8] [20] . First, we explain the simulation procedure of local interaction. Reaction processes are performed in the following steps:
1) Initially, we distribute X, Y on a square lattice. Each cell is one of three sites: X, Y, and O (see Figure 1).
2) To update, we choose a target site randomly.
a) Reaction (1c) or (1d): If the target cell is X (or Y), then it becomes O by the rate 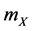 (or
(or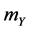 ).
).
b) Reaction (1a) or (1b): If the target site is O, we choose another cell from the neighboring 8 sites (Moore neighborhood) around the target cell. When the second site is X (or Y), then the target cell become X (or Y) by the rate 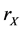 (or
(or
3) We repeat step 2) by 



4) We further continue the updates, until the system reaches stationary state.
The reproduction rates 



where we call 




Next, we explain the method of global interaction (lattice gas model). Almost all procedures are the same as local interaction. However, in the global simulations, the birth process [reaction (1a) or (1b)] occurs between any pair of lattice sites. In mean-field limit, the reproduction rates are replaced as follows:


where 

3. Prediction of Global Interaction
If the reaction (1a) occurs between any pair of lattice sites, and if the lattice size 


where the factor 


Note that this equation is almost the same as in the male-female system [7] [8] . Iwata et al. [9] proved that the Equation (5) led to one of two phases: extinction or Allee-effect phase (Figure 2). In the former case, both species always go extinct. The latter is called the survival/extinction (S/E) phase, because this phase has two stable equilibriums which means survival and extinction. The condition for the Allee-effect phase has been represented by

In summary, two conditions are necessary for survival; one is the relation (6), and the other is that initial densities of two species are higher than the separatrix which is schematically shown in Figure 2(b).
We explicitly obtain equilibrium density in stable state, when


Figure 2. Schematic illustration of population dynamics. (a) extinction phase, and (b) Allee effect phase [13] .
From Equations (5) and (7), we get

where

From Equation (8), we can find the following relation: 


Inserting Equations (7) and (10) into (5), we have

where


Equation (11) denotes a typical Allee-effect equation [7] . According to the sign of D, the dynamics is classified into two phases: extinction phase for 




It is therefore found in the mean-field limit that 1) the maximum value of total density (



This inequality is the same as equation (6) under the condition of
4. Simulation Results
We report simulation results of individual-based (lattice) model. In the case of global interaction, the mean-field theory [Equation (5)] predicts that the dynamics has two phases. One is an extinction phase: both species always go extinct. The other is Allee-effect phase. A typical example of the latter phase is shown in Figure 3 (
In the case of local interaction, the dynamics are similar to the predictions of lattice gas theory. For instance, the Allee effect can be observed: unless both densities of species are considerably high, the population goes extinct. However, in the case of local interaction, simulation results exhibit a distinct difference from those of global interaction. In Figure 4, typical spatial distributions in stationary state are displayed (





In Figure 5, the steady-state densities are plotted against



Figure 3. Typical cases of population dynamics in Allee-effect phase (




Figure 4. Typical spatial patterns in stationary state (


Figure 5. Steady-state densities at stable equilibrium. (a) Global interaction, but (b) local interaction. The densities at stable equilibrium are plotted against





ratio of densities. However, it is necessary for the sustainability in local interaction that the value of 
When





5. Discussions
We have developed a lattice population of obligate mutualism. Local interaction strongly effects on the populations dynamics. The survival condition is extremely limited for local simulation. When both species have the same mortality rate (

The ratio selection is a generalization of sex-ratio selection. In fact, our model is also applicable to the male- female system. Equation (5) is almost the same as obtained in male-female system [7] [8] , where X (Y) corresponds to male (female) and 




Many empirical data suggest the ratio selection; two-species systems of obligate mutualism usually have some mechanism to avoid an abrupt increase of one species [1] . 1) Coral and algae: the ratio between cell number in stomach (“gastrodermis”) of coral and zooxanthellae number is just 1:1 [28] . When the density of algae is too high, the excess algae are excluded from coral. 2) Chlorella and Paramecium: the number of Chlorella (Chlorella) inside each body of Paramecium (Paramecium bursaria) is about 400 individuals [29] . 3) Yucca and

Figure 6. Same as Figure 5, but for




yucca moth: too many yucca moths become harmful for yucca plant [30] . 4) Chloroplast and mitochondria: the number of these organelles in a host cell is not so arbitrary [31] . The ratio selection comes from the requirement that all individuals of one species need a high local density of the other species. We consider such requirement evolutionarily leads to endosymbionts from free-living ancestors [32] . This is because endosymbiosis is more stable than free living to keep their proper ratio. Similarly, the ratio selection may be a driving force for the evolution from endosymbionts to organelles [33] [34] .
Acknowledgements
The authors sincerely thank to professors Jin Yoshimura for valuable comments. They also thank to Mr. Keiji Amemiya for the help of simulations.
Cite this paper
Kei-Ichi Tainaka,Tsuyoshi Hashimoto, (2016) A Theory of Ratio Selection—Lattice Model for Obligate Mutualism. Open Journal of Ecology,06,303-311. doi: 10.4236/oje.2016.66030
References
- 1. Begon, M., Townsend, C.R. and Harper, J.L. (2006) Ecology: From Individuals to Ecosystems. Wiley, New York.
- 2. Bashary, R. and Bronstein, J.L. (2004) Game Structures in Mutualisms: What Can the Evidence Tell us about the Kind of Models We Need? Advances in the Study of Behavior, 34, 59-101.
http://dx.doi.org/10.1016/S0065-3454(04)34002-7 - 3. Pellmyr, O. and Huth, C.J. (2002) Evolutionary Stability of Mutualism between Yuccas and Yucca Moths. Nature, 372, 257-260.
http://dx.doi.org/10.1038/372257a0 - 4. Yokoi, H., Uehara, T., Kawai, T., Tateoka, Y. and Tainaka, K. (2014) Lattice and Lattice Gas Models for Commensalism: Two Shellfishes in Intertidal Zone. Open Journal of Ecology, 4, 671-677.
http://dx.doi.org/10.4236/oje.2014.411057 - 5. Madigan, M.T., Martinko, J.M. and Parker, J. (2000) Biology of Microorganisms. Prentice Hall, Inc., New Jersey.
- 6. Goto, R., Okamoto, T., Kiers, E.T., Kawakita, A. and Kato, M. (2010) Selective Flower Abortion Maintains Moth Cooperation in a Newly Discovered Pollination Mutualism. Ecology Letters, 13, 321-329.
http://dx.doi.org/10.1111/j.1461-0248.2009.01425.x - 7. Berec, L. Boukal, D.S. and Berec, M. (2001) Linking the Allee Effect, Sexual Reproduction and Temperature-Dependent Sex Determination via Spatial Dynamics. American Naturalist, 157, 217-230.
http://dx.doi.org/10.1086/318626 - 8. Tainaka, K., Hayashi, T. and Yoshimura, J. (2006) Sustainable Sex Ratio in Lattice Populations. Europhysics Letters, 74, 554-559.
http://dx.doi.org/10.1209/epl/i2005-10558-3 - 9. Iwata, S., Kobayashi, K., Higa, S., Yoshimura, J. and Tainaka, K. (2011) A Simple Population Theory for Mutualism by the Use of Lattice Gas Model. Ecological Modelling, 222, 2042-2048.
http://dx.doi.org/10.1016/j.ecolmodel.2011.04.009 - 10. Fisher, R.A. (1930) The Genetical Theory of Natural Selection. Clarendon Press, Oxford.
http://dx.doi.org/10.5962/bhl.title.27468 - 11. Smith, J.M. (1968) Mathematical Ideas in Biology. Cambridge University Press, Cambridge.
http://dx.doi.org/10.1017/CBO9780511565144 - 12. Charnov, E.L. (1982) The Theory of Sex Allocation. Princeton University Press, Princeton.
- 13. Shaw, R.F. and Mohler, J.D. (1953) The Selective Advantage of the Sex Ratio. American Naturalist, 87, 337-342.
http://dx.doi.org/10.1086/281794 - 14. Karlin, S. and Lessard, S. (1986) Theoretical Studies on Sex Ratio Evolution. Princeton University Press, Princeton.
- 15. Cowgill, U.M. and Hutchinson, G.E. (1963) Differential Mortality among the Sexes in Childhood and Its Possible Significance in Human Evolution. Proceedings of the National Academy of Sciences of the United States of America, 49, 425-429.
http://dx.doi.org/10.1073/pnas.49.4.425 - 16. Ito, H., Uehara, T., Morita, S., Tainaka, K. and Yoshimura, J. (2011) Slightly Male-Biased Sex Ratios for the Avoidance of Extinction. Evolutionary Ecology Research, 13, 759-764.
- 17. Yoshimura, J. (1997) The Evolutionary Origins of Periodical Cicadas during Ice Ages. The American Naturalist, 149, 112-124.
http://dx.doi.org/10.1086/285981 - 18. Tanaka, Y., Yoshimura, J., Simon, C., Cooley, J.R. and Tainaka, K. (2009) The Allee Effect in the Selection for Prime-Numbered Cycles in Periodical Cicadas. Proceedings of the National Academy of Sciences of the United States of America, 106, 8975-8979.
http://dx.doi.org/10.1073/pnas.0900215106 - 19. Tainaka, K. (2003) Perturbation Expansion and Optimized Death Rate in a Lattice Ecosystem. Ecological Modelling, 163, 73-85.
http://dx.doi.org/10.1016/S0304-3800(02)00414-3 - 20. Tainaka, K. (1988) Lattice Model for the Lotka-Volterra System. Journal of the Physical Society of Japan, 57, 2588-2590.
http://dx.doi.org/10.1143/JPSJ.57.2588 - 21. Tainaka, K. and Araki, N. (1999) Press Perturbation in Lattice Ecosystems: Parity Law and Optimum Strategy. Journal of Theoretical Biology, 197, 1-13.
http://dx.doi.org/10.1006/jtbi.1998.0829 - 22. Frisch, U., Hasslacher, B. and Pomeau. Y. (1986) Lattice-Gas Automata for the Navier-Stokes Equation. Physical Review Letters, 56, 1505-1508.
http://dx.doi.org/10.1103/PhysRevLett.56.1505 - 23. Hagiwara, T., Ushimaru, T., Tainaka, K., Kurachi, H. and Yoshimura, J. (2011) Apoptosis at Inflection Point in Liquid Culture of Budding Yeasts. PLoS ONE, 6, e19224.
http://dx.doi.org/10.1371/journal.pone.0019224 - 24. Hofbauer, J. and Sigmund, K. (1998) Evolutionary Games and Population Dynamics. Cambridge University Press, Cambridge.
http://dx.doi.org/10.1017/CBO9781139173179 - 25. Takeuchi, Y. (1996) Global Dynamical Properties of Lotka-Volterra Systems. World Scientific, Singapore.
- 26. Hardy, I.C.W. (2002) Sex Ratios: Concepts and Research Methods. Cambridge University Press, Cambridge.
http://dx.doi.org/10.1017/CBO9780511542053 - 27. Hamilton, W.D. (1967) Extraordinary Sex Ratios. Science, 156, 477-488.
http://dx.doi.org/10.1126/science.156.3774.477 - 28. Rosenberg, E., Koren, O., Reshef, L., Efrony, R. and Zilber-Rosenberg, I. (2007) The Role of Microorganisms in Coral Health, Disease and Evolution. Nature Reviews Microbiology, 5, 355-362.
http://dx.doi.org/10.1038/nrmicro1635 - 29. Miwa, I. (2009) Regulation of Circadian Rhythms of Paramecium Bursaria by Symbiotic Chlorella Species. In: Fujishima, M., Ed., Endosymbionts in Paramecium, Springer-Verlag, Berlin, 83-110.
http://dx.doi.org/10.1007/978-3-540-92677-1_4 - 30. Fiegna, F., Yu, Y.-T.N., Kadam, S.V. and Velicer, G.J. (2006). Evolution of an Obligate Social Cheater to a Superior Cooperator. Nature, 441, 310-314.
http://dx.doi.org/10.1038/nature04677 - 31. Milo, R. and Philips, R. (2014) Cell Biology by the Number. http://book.bionumbers.org/
- 32. Hosokawa, T., Ishii, Y., Nikoh, N., Fujie, M., Satoh, N. and Fukatsu, T. (2016) Obligate Bacterial Mutualists Evolving from Environmental Bacteria in Natural Insect Populations. Nature Microbiology, 1, Article Number: 15011.
http://dx.doi.org/10.1038/nmicrobiol.2015.11 - 33. Sagan, L. (1967) On the Origin of Mitosing Cells. Journal of Theoretical Biology, 14, 255-274.
http://dx.doi.org/10.1016/0022-5193(67)90079-3 - 34. Margulis, L. and Sagan, D. (2001) Marvellous Microbes. Resurgence, 206, 10-12.







