Open Journal of Genetics
Vol.05 No.02(2015), Article ID:57512,11 pages
10.4236/ojgen.2015.52006
Genetic Variation for Achene Traits in Cup Plant (Silphium perfoliatum L.)
Teshale Assefa1, Jixiang Wu2, Arvid Boe2*
1Iowa State University, Ames, USA
2South Dakota State University, Brookings, USA
Email: tmamo@iastate.edu, jixiang.wu@sdstate.edu, *arvid.boe@sdstate.edu
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 May 2015; accepted 26 June 2015; published 29 June 2015
ABSTRACT
Cup plant (Silphium perfoliatum L.) has demonstrated potential for biomass production in studies using transplants in poorly drained cropland not suitable for conventional crops, but little is known about its establishment from seeding. The success rate for stand establishment of perennial plants is usually positively correlated with seed weight. Therefore, objectives of this study were to determine contribution of genetic effects to variation in achene weight, seed weight, achene length, and achene width of cup plant in a population with high biomass potential. Seedlings of 33 half-sib (HS) families were transplanted at Brookings, SD in 1999 and 2010. Achene/seed traits were determined at seed maturity in 2000, 2011 and 2012. Narrow-sense heritability was higher for achene weight and seed weight than that for dimensional achene traits. Within-population genetic variation occurred for achene and seed weight, both of which varied in response to temporal variation in precipitation and temperature. Results of this study indicated the presence of sufficient additive genetic variation for progress from among-family selection for achene weight. Thus, since families with heavy achenes had higher percent seedling emergence and superior seedling vigor compared to families with light achenes, achene weight may be useful for indirect selection for enhanced seed quality in development of new cultivars of cup plant for biomass production on marginal crop land.
Keywords:
Cup Plant, Achene Traits, Heritability, Seedling Vigor, Seed Germination

1. Introduction
Cup plant (Silphium perfoliatum L.) is a perennial dicotyledonous plant that occurs in moist prairie areas from Ontario to South Dakota and south to Georgia, Mississippi, Missouri, and Oklahoma [1] [2] in North America (Figure 1). Cup plant is identified by its lance shaped opposite leaves, which are joined at the base and form a cup around the angular stem. Its disc flowers are sterile (staminate), whereas the yellow ray flowers produce achenes through cross pollination [1] (Figure 2). It has an indeterminate flowering habit, which makes harvesting a seed crop difficult since achenes shatter readily after ripening.
Cup plant is recognized as a bio-diversity enhancing and pollinator attracting plant but may also have medicinal and soil remediation uses [3] . Cup plant has been used as alternative forage and nectar crop in Europe and Asia [3] and recently in the United States for silage [4] [5] . Lehmkuhler et al. [6] showed that silage from cup plant had nutritional value lower than corn, but it could be incorporated as one component of feed to livestock with lower energy requirements. Similarly, Pichard [7] reported that cup plant could be incorporated as regular perennial summer feed crop to animals. Cup plant is also being used as a biogas crop in Germany [3] . Therefore, emphasis on the biomass and forage potential of cup plant has increased. However, development of new cultivars and the successful commercialization of those cultivars will require production of large amounts of high quality seed. Presently, there is scarcity of information on seed-related traits, such as achene and seed weights
Figure 1. Natural distribution of cup plant (Silphium perfoliatum L.) in North America. USDA-NRCS Plants Database (http://plants.usda.gov/core/profile?symbol=SIPE2). Cup plant occurs naturally in the colored US states and Canadian provinces. However, the colored are within each state is not indicative of the range.
Figure 2. Cup plant (left) growing with prairie cord- grass (Spartina pectinata Link) (back right) and switch- grass (Panicum vigatum L.) near Brookings, South Da- kota. Note the abundance of flower heads, indicative of the seed-production potential of the plant. The height of the cup plant > 2.5 m and biomass < 10 Mg∙ha−1.
and the relationship between achene weight and seedling vigor, an important trait for stand establishment of perennial grasses and forbs (Figure 3).
The relationship between achene size and germination rate has been documented for several members of the Asteracae [8] . For instance, large seeds may be favored because they produce vigorous seedlings with better chance of survival rate than small seeds [9] . Seed size is an important seed quality characteristic affected by variety, environment, and management practices [10] . In addition, seed size has been shown to affect many attri- butes of fitness including seedling competitiveness [9] . Meanwhile, dormancy also affects germination potential of the achene.
In general, expanding the agronomic and genetic information on cup plant is important as it has shown potential as an alternative forage crop and a dedicated bioenergy crop [3] [5] [7] [11] . Variation for size and weight in achenes for other members of the Asteracae, such as sunflower has been reported; however, variation for achene weight, length, and width has not been studied for cup plant. Therefore, since genetic variation for achene traits may be useful for establishment, propagation, production, and commercialization of cup plant, our objectives were to determine 1) whether genetic variation for achene weight, length, and width and seed weight occurred in a population of cup plant that had potential for bioenergy production in the northern Great Plains, and 2) if there were significant relationships between achene weight, seed weight, and seedling vigor in this population..
2. Materials and Methods
2.1. Plant Materials
The cup plant population used for this genetic study was composed of progenies of 33 parental plants derived from bulk seed produced by putative random mating among several plants from each of natural populations from Minnesota and Illinois [4] (seed was obtained from Dr. Walter Goldstein, Michael Fields Institute, East Troy, WI) in a spaced plant nursery at Brookings, South Dakota (SD). For convenience, these 33 progenies were named half-sib (HS) families and coded as F1-F33. Seeds of the 33 HS families were produced in 1998 from an isolated polycross nursery composed of the 33 parental spaced plants (0.9-m centers), established at Brookings in 1997. A ‘check’ population developed by K. Albrecht from open pollination of 26 natural populations from Wisconsin, Illinois, Iowa, and Minnesota and previously evaluated in forage production and dairy cattle (Bostaurus L.) feeding trials in Wisconsin was included for comparison to the HS families in the nursery that was established at Brookings, SD in 2010.
2.2. Methods
Experiment 1: Heritability using parent-offspring regression method
This first experiment was conducted during 1998 through 2000. Seedlings of the 33 HS families derived from seed produced in 1998 on the parental plants in the aforementioned polycross nursery were planted in replicated
Figure 3. Achenes (bottom left) and seeds (top right) of cup plant.
spaced-plant nurseries at Aurora (N 44.28˚, W 96.68˚) and Brookings (N 44.31˚, W 96.80˚), SD in May 1999. Soils were an Estelline silt loam (fine-silty over sandy or sandy-skeletal, mixed, superactive frigid calcic hapludolls) at Aurora and a Vienna (fine-loamy, mixed, superactive, frigid, calcic hapludolls)-Brookings (fine-silty, mixed, superactive, frigid pachichapludolls) complex. A randomized complete block design was used with three replicates of single-row plots composed of 5 plants with 0.8-m intra-plot spacing and 2.1-m inter-plot spacing (i.e., about 6300 plants ha−1) at both Aurora and Brookings. In 2000 bulk seed was collected at maturity from each family row at each of the two locations. The Wisconsin check population was not included in this first experiment. Seeds were removed from achenes by hand using forceps. Weights of 25 achenes and their associated 25 dehulled seeds were determined for each family row by an electronic balance with 0.001 g accuracy. Individual-achene length and width were measured using a Digimatic caliper with 0.1 mm accuracy. Family means, averaged across locations, for achenes/seeds produced in 2000 were regressed on their respective female parental means for achenes/seeds produced in 1998 to obtain estimates of narrow-sense heritability for the four achene/seed traits.
Experiment 2: Heritability using variance components method
The second experiment was conducted during 2011 and 2012 on the South Dakota State University Experimental Station Felt Farm near Brookings, SD (44˚19'N and 96˚42'W). The soil was a McInotsh (fine-silty, mixed, superactive, frigid aquiccaldiudolls)-Badger (fine, smectitic, frigid verticargiaquolls) silty clay loam. Precipitation data were obtained for each of the growing seasons for 2011, 2012, and 2013 at Brookings (Table 1).
This experiment employed narrower inter- and intra-plot spacing than did the first experiment. Individual family plots were single rows composed of six plants with 0.9 m between rows and 0.4 m between plants within rows (i.e., about 28,000 plants ha−1) [11] . Similar to the first experiment, seedlings from the 33 half-sib families with the addition of the ‘check’ population were transplanted to the field in June 2010 in a randomized complete block design with three replicates [11] .
The number of heads containing mature seeds collected from each family row varied from about 5 heads in 2012 to >10 heads in 2011. The growing season of 2011 was highly suitable for seed production and >100 heads were produced for each family row; whereas, in 2012 drought (Table 1) and feeding on the floral meristems by larvae of the giant eucosma moth (Eucosmagiganteana Riley) significantly reduced the number of heads per row. Achenes were hand-threshed from each head and bulked by family row. Twenty achenes from each family row were randomly selected to determine achene and seed weights, achene length, and achene width. Seed removal from the achenes by hand, and achene/seed weight and achene length and width data collection were as described for Experiment 1. The reason for reducing the number of achenes evaluated from each family plot from 25 sample−1 for the 2000 crop (i.e., Experiment 1) to 20 sample−1 for the 2011 and 2012 seed crops was simply to reduce the amount of time and hand labor required to excise seeds from achenes without breaking or chipping them in order to obtain accurate estimates of their weight.
Experiment 3: Germination/seedling emergence and vigor
In addition to the above mentioned genetic studies for estimating narrow-sense heritability for achene traits, an additional experiment to measure germination in the laboratory and seedling emergence and seeding vigor was conducted in the greenhouse.
The seed germination experiment was carried out at the South Dakota State University Seed Testing Lab. Three replicates of 25 achenes for each family produced from family rows in 2011 were planted on top of blot-
Table 1. Precipitation totals (mm) during the growing seasons of 2010, 2011, 2012, and 2013 at Brookings, South Dakota†.
†South dakota climate and weather (http://climate.sdstate.edu/climate_site/archive_data.html).
ters in covered plastic germination boxes in a germination chamber set at constant 20˚C in November 2013, using the Association of Official Seed Analysts [12] protocol for cultivated sunflower (Helianthus annuus L.). That protocol dictates the first reading for germination at four days after planting and the final reading at seven days after planting. Watering was as needed with deionized water.
Concurrent with the planting of the germination component, three replicates of single rows 30 cm long containing 20 equidistant seeds with 8 cm between rows were planted for each family in galvanized metal flats in the greenhouse at Brookings, SD. The planting medium was Miracle-Gro® Micromax potting mix. Achenes were planted at a depth of about 2 cm. Air temperature in the greenhouse fluctuated from about 10˚C during night to occasionally >30˚C during day in response to ambient atmospheric conditions, such as variation in cloudiness. Completely randomized designs were used both in the greenhouse and South Dakota State University Seed Testing Lab.
A seed was considered to be germinated when a radicle emerged. A seedling was considered emerged when the cotyledons appeared above the soil. Numbers of germinated seeds in the lab and emerged seedlings in the greenhouse were recorded daily from eight through 14 days after planting. Up to ten seedlings per family per replication were harvested on day 14 for the greenhouse experiment. Seedlings were dried at 60˚C for 72 hours to determine biomass production seedling−1. Seedling vigor was determined using a modified approach to the method described by Abdul-Baki and Anderson [13] . An index was calculated by multiplying percent emergence on day 14 by the mean seedling dry weight for each family and each replication.
2.3. Data Analyses
Analyses of variance were performed to determine genetic and environmental effects for field experiments and for genetic effects for the greenhouse experiment using Statistix 9 [14] and SAS V9.3. All effects were considered to be random. F-tests were performed according to expected mean squares [15] . Differences among family means were tested by the protected least significant difference at the probability level of 0.05. Narrow sense heritability estimates were determined using variance components from analysis of variance for data from the second field experiment (i.e., 2011 and 2012 data) and by doubling the linear regression coefficient of HS progeny means on maternal parent means for the 1998 (i.e., parental polycross) and 2000 (i.e., HS family) data from the first field experiment [16] . Genetic correlation coefficients for the 2011 and 2012 data from the second field experiment were estimated utilizing the analysis of covariance procedure described by Hallauer and Miranda [17] , whereby the covariance components were calculated similar to the calculation of variance components from analysis of variance, which were used to calculate narrow-sense heritability estimates.
The formula for calculating narrow-sense heritability [16] was:

where
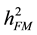 = narrow-sense heritability on a family mean basis with family the unit of selection;
= narrow-sense heritability on a family mean basis with family the unit of selection;
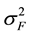 = among family variance component,
= among family variance component,
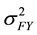 = family × year variance component,
= family × year variance component,
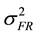 = family × replication variance component,
= family × replication variance component,
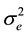 = error mean square, r = number of replications, and y = number of years.
= error mean square, r = number of replications, and y = number of years.
3. Results
3.1. Heritability Using Parent-Offspring Regression
Significant differences were found among the 33 parental plants in the polycross nursery and among the 33 families in the HS family nursery for all four of the achene/seed traits (ANOVA not shown). The range in parental means for 25-achene weight from achenes produced in 1998 in the polycross nursery was from 0.344 to 0.600 g. The range in family means from seed produced in 2000 was from 0.239 to 0.391 g.
3.2. Heritability Using Variance Components
The growing season of 2011 was relatively wet and cool compared to that of 2012. The average temperatures during June (18˚C) and July (24.3˚C) of 2011 were 3.3˚C and 1.0˚C lower than those in 2012. Total precipitation for June through August during flowering, pollination, and seed production was 225 mm in 2011 and 124 mm in 2012 (Table 1).
Significant differences were found between years for all four of the achene traits. All of the achene traits were substantially reduced in 2012 compared to 2011. Family differences were found for achene weight and seed weight. The family × year interaction was significant for all four traits (Table 2). Therefore, separate analyses of variance were also conducted for each year (Table 3). The across-year analysis of variance (Table 2) was used to generate variance components for estimating narrow-sense heritability as well.
Means for the 34 entries (33 HS families and a check population) for seed weight ranged from 0.186 to 0.323 g in 2011 and from 0.150 to 0.253 g in 2012; for achene length entry means ranged from 8.0 to 9.6 mm in 2011 and from 5.7 to 8.7 mm in 2012; and for achene width entry means ranged from 5.4 to 6.5 mm in 2011 and from 3.1 to 5.5 mm in 2012 (Table 3).
Coefficients of variation were higher for all traits in 2012 than those in 2011, except for seed weight (Table 3). Seven out of the top eight (i.e., top 25%) families for rank for achene weight and 6 out of the top 25% for rank for seed weight were common for both years. Twelve families had heavier achenes than the check in both years, averaging 26% and 33% heavier than the check in 2011 and 2012, respectively.
Significant genetic correlation coefficients were detected for all pairings of the four achene/seed traits (Table 4). Estimates of narrow-sense heritability were higher for the weights than for dimensional traits and were similar for variance component and parent-offspring regression methods (Table 5).
Highly significant year effects indicated phenotypic plasticity for achene/seed traits in response to temporal variation in environmental conditions (Table 2 and Table 6). However, the highly significant family × year interaction mean squares for all four achene/seed traits indicated ranks of families were not consistent between years (Table 2). For achene weight, >50% of the families exhibited differences between years; whereas, for seed weight, <50% exhibited differences between years. Only eight families had similar means between years for either trait (Table 3).
3.3. Germination/Seedling Emergence and Vigor
Significant differences were found among families for percentage seedling emergence, dry seedling weight, and the seedling vigor index. Means among 34 families ranged from 70% to 98.3% for emergence and from 19.8 to 69.2 for the seedling vigor index, respectively. Emergence percentage increased as achene weight increased. Seven out of the top nine families for percent seedling emergence had greater than 90% emergence. In general, families with heavy achenes had higher percentage emergence and produced larger seedlings with greater seedling vigor than families with relatively light achenes (r = 0.76 and 0.81, respectively). Germination rate was low at constant 20˚C in a germination chamber, using the AOSA protocol for annual sunflower. After 14 days, 25% of the 34 entries had no germination, whereas 75 % had germination that ranged from 5% to 20%.
4. Discussion
The present research is the first agronomic study conducted on genetic variation for seed-related traits within the genus Silphium and, for that matter, as far as we know, for other perennial species within the family Asteraceae.
Table 2. Mean squares for random model analyses of variance for achene weight (AW), seed weight (SW), achene length (AL), and achene width (AWD) in 2011 and 2012 at Brookings, South Dakota.
*, **Significant at 0.05 or 0.01 probability level, respectively.
Table 3. Annual means for achene weight, seed weight, achene length and achene width at Brookings, South Dakota in 2011 and 2012.
LSD = Least significant different; CV = coefficient of variation.
Table 4. Genetic correlations among achene weight (AW), achene length (AL), achene width (AWD) and seed weight (SW) from seed produced at Brookings, South Dakota in 2011 and 2012.
*, **Significant at 0.01 level of probability.
Table 5. Estimates of narrow-sense heritability and standard errors (in parenthesis) from variance components and parent- offspring regression for achene and seed traits in cup plant from seeds produced at Brookings, South Dakota in 1998 and 2000 (parent-offspring) and 2011 and 2012 (variance components).
†Standard error of heritability estimate (Hallauer and Miranda, 1988).
Table 6. Phenotypic plasticity of 33 half-sib families of cup plant and a check population for achene weight (AW), seed weight (SW), achene length (AL) and achene width (AWD) in 2011 and 2012, at Brookings, South Dakota.
†F-test of difference between annual means significant at the 0.01 level of probability.
Therefore, the only published studies that are useful for drawing analogies between cup plant and related species are necessarily those conducted on cultivated annual sunflower, for which there are numerous genetic studies on seed size/weight and correlated traits. Several of those studies are useful for understanding the importance of genetic and environmental impacts on achene/seed weight in cup plant, relative to an important crop plant belonging to the Asteraceae family.
The impact of temporal variation in growing conditions was highly evident for achene/seed traits in cup plant. The environmental conditions during summer 2011 were much more favorable than in 2012 [11] and resulted in higher achene, seed weight, and achene dimensions. These values of the studied characters were similar in ranges for similar traits in sunflower [18] . Seed weight in sunflower responded to irrigation, with 27% increase in seed weight under irrigated compared to adjacent dryland conditions [19] .
Significant family × year interactions for achene weight and associated traits indicated that these 33 HS families did not rank the same across years. However, the relatively consistent performance of the top 25% of the families between years for achene and seed weights suggested that progress from selection for mean achene/seed weight could be expected in normal rainfall as well as drought stressed environments. Kwon and Torrie [20] found that estimates of genotype × year variance were larger than genotype × replication and genotype × location mean squares for most seed traits in soybean.
Moderate narrow-sense heritability estimates obtained for achene and seed weight from both variance components and parent-offspring regression methods indicated additive genetic variance was a significant fraction of the total phenotypic variance. Additive genetic variance also accounted for most of the genetic variance for seed weight in sunflower [19] [21] . Similarly, moderate to high narrow-sense heritability for 100-achene weight was reported in sunflower [22] [23] .
Significant associations between achene weight and germination percentage and seedling dry weight suggested that achene weight may be an important indirect selection criterion for improving stand establishment in cup plant. Numerous other studies of dicotyledonous plants have pointed out a positive relationship between seed size and germination success [24] . Vanisree et al. [25] found large variation among sunflower genotypes for achene weight. Habib et al. [26] reported sunflower genotypes with heavy achenes had progeny with better seedling emergence and growth than progeny that produced lighter achenes. Variation for dry seedling weight is thought to be related to variation in amount of food storage in cotyledons [27] .
High positive genetic correlations for achene/seed traits were similar to those for seed yield traits in sunflower reported by Habib et al. [26] . Positive correlations of achene and seed weight with achene length and width suggested achene/seed weight might be increased by selecting for dimensional traits, which can be easily measured.
As expected from the wide environmental variation between 2011 and 2012, most families showed highly plastic responses in achene/seed traits to temporal variation, with reduced expression in 2012, the drought year, compared to 2011. However, several families with similar means between years suggested genetic variation for type of response to large variations in environmental conditions for seed-related traits within this population of cup plant.
The large difference between Experiments 1 and 2 for plant population density was related to different objectives for each experiment reported here and to population densities in previous studies on cup plant successfully conducted in the Midwest. Experiment 1 was intended to evaluate the potential of individual plants within family rows to express their potential for biomass production, similar to previous studies in Wisconsin (K.A. Albrecht, personal communication). Whereas, Experiment 2 was intended to identify half-sib families that had the potential for biomass production in a planting density and arrangement similar to that used for commercial production of confectionary sunflowers in the northern Great Plains. In other words, for Experiment 1 the intended unit of selection was an individual plant, whereas for Experiment 2, the intended unit of selection was a half-sib family. More research is needed to determine the optimum plant population density for biomass and seed production in cup plant in North America. Preliminary data from on-going trials in South Dakota and Wisconsin have indicated that biomass response to variation in plant density is not independent of environment (K. Albrecht and A. Boe, unpublished data, 2014). Past studies with sunflower have shown increases in seed yield and decreases in seed weight in response to increases in plant population density [28] [29] .
Germination of cup plant seeds can be delayed due to dormancy, especially from seed planted within a year or two after harvest (Boe, unpublished data). However, in our study >70% of seeds of each family produced in the field in 2011 germinated under greenhouse conditions in 2013, suggesting that exposing cup plant seeds to wide ranges in diurnal temperatures and sunlight intensity, such as those that occurred in the greenhouse, might trigger germination. The positive linear relationships between seed weight and seedling emergence and seedling weight found in the present study have also been reported for sunflower [22] [30] . However, unlike the present study, those studies did not employ a comparison among families that differed for achene weight. Conversely, Kaya and Day [31] found that, although under large seeds of sunflower produced the most vigorous seedlings under non-saline conditions, small seeds produced faster germination rates and seedling growth under saline conditions. The laboratory germination percentage using the AOSA sunflower protocol at constant 20˚C produced very poor results and was not predictive of seedling emergence in the greenhouse. An alternative official protocol for sunflower germination dictates a 20˚C - 30˚C alternating temperature in a germination chamber [12] . More research is needed to determine if that protocol would be more effective for predicting seed viability and seedling emergence.
In summary, achene/seed traits in cup plant proved useful for quantifying among-family genetic diversity and phenotypic plasticity in fitness-related traits, as well as potential selection criteria to improve stand establishment. Of course, field studies are needed to determine if the genetic variation for seed related traits in this population is useful for developing new populations with improved agronomic performance related to seedling vigor and other agronomic traits. In addition, since annual variation in precipitation was shown to have a large impact on achene/seed weight (e.g., 2011 and 2012 seed crops), the potential influence of such environmentally-in- duced phenotypic variation on agronomic performance [32] needs investigation.
Acknowledgements
This research was supported by funding from the North Central Regional Sun Grant Center at South Dakota State University through a grant provided by the US Department of Energy Bioenergy Technologies Office under award number DE-FG36-08GO88073 and 08GO88073 and US Department of Agriculture award number 2010-38502-21861. We express appreciation to USDA-NIFA Hatch project 1005459 for providing funding for this research through the South Dakota Agricultural Experiment Station. Appreciation is also expressed to Dr. Brent Turnipseed and Mr. Dennis Ruhlman for providing facilities and expertise for the laboratory germination study.
References
- McGregor, R.L., Barkley, T.M., Brooks, R.E. and Schofied, E.K. (1986) Flora of the Great Plains. University Press of Kansas, Lawrence.
- Geoffrey, S. (1990) Silphium perfoliatum (Cup-Plant) as a New Forage. In: Smith, D.D. and Jacobs, C.A., Eds., 12th North American Prairie Conference, University of Northern Iowa, Cedar Falls, 33-37.
- Gansberger, M., Montgomery, L.F.R. and Liebhard, P. (2015) Botanical Characteristics, Crop Management and Potential of Silphium perfoliatum L. as a Renewable Resource for Biogas Production: A Review. Industrial Crops and Products, 63, 362-372. http://dx.doi.org/10.1016/j.indcrop.2014.09.047
- Albrecht, K.A. and Goldstein, W. (1997) Silphium perfoliatum: A North American Prairie Plant with Potential as a Forage Crop. The XVIII International Grassland Congress, Winnipeg, 8-19 June 1997, 167-168.
- Han, K.J., Albrecht, K.A., Muck, R.E. and Kim, D.A. (2000) Moisture Effect on Fermentation Characteristics of Cup-Plant Silage. Asian-Australasian Journal of Animal Sciences, 13, 636-640. http://dx.doi.org/10.5713/ajas.2000.636
- Lehmkuhler, J.W., Ramos, M.H. and Albrecht, K.A. (2007) Cup-Plant Silage as a Replacement for Corn Silage in Growing Beef Cattle Diets. Forage & Grazinglands, 5.
- Pichard, G. (2012) Management, Production and Nutritional Characteristics of Cup-Plant (Silphium perfoliatum) in Temperate Climates of Southern Chile. Ciencia e Investigación Agraria, 39, 61-77.
- van Mölken, T., Jorritsma-Wienk, L.D., van Hoek, P.H. and de Kroon, H. (2005) Only Seed Size Matters for Germination in Different Populations of the Dimorphic Tragopogon pratensis Subsp. pratensis (Asteraceae). American Journal of Botany, 92, 432-437. http://dx.doi.org/10.3732/ajb.92.3.432
- Howe, H.F. and Richter, W.M. (1982) Effects of Seed Size on Seedling Size in Virola surinamensis; a within and between Tree Analysis. Oecologia, 53, 347-351. http://dx.doi.org/10.1007/BF00389011
- Robinson, R.G. (1978) Production and Culture. In: Carter, J.F., Ed., Sunflower Science and Technology, American Society of Agronomy, Madison, 89-143.
- Assefa, T., Wu, J., Albrecht, K.A., Johnson, P.J. and Boe, A. (2015) Genetic Variation for Biomass and Related Morphological Traits in Cup Plant (Silphium perfoliatum L.). American Journal of Plant Sciences, 6, 1098-1108. http://dx.doi.org/10.4236/ajps.2015.68114
- Association of Official Seed Analysts (2014) AOSA Rules for Testing Seeds. Vol. 1. Principles and Procedures. Association of Official Seed Analysts, Inc., Washington DC.
- Abdul-Baki, A.A. and Anderson, J.D. (1973) Vigor Determination in Soybean Seed by Multiple Criteria. Crop Science, 13, 630-633. http://dx.doi.org/10.2135/cropsci1973.0011183X001300060013x
- Statistix (2009) Statistix 9: Analytical Software. Tallahassee.
- Satterthwaite, F.E. (1946) An Approximate Distribution of Estimates of Variance Components. Biometrics Bulletin, 2, 110-114. http://dx.doi.org/10.2307/3002019
- Nguyen, H.T. and Sleper, D.A. (1983) Theory and Application of Half-Sib Mating in Forage Grass Breeding. Theoretical and Applied Genetics, 64, 187-196. http://dx.doi.org/10.1007/BF00303763
- Hallauer, A.R. and Miranda, J.B. (1988) Quantitative Genetics in Maize Breeding. Iowa State University Press, Ames.
- Dua, R. and Yadava, T. (1985) Genetics of Seed Yield and Its Components in Sunflower. Proceedings of 11th International Sunflower Conference, Mar del Plata, 10-13 March 1985, 627-632.
- Alza, J.O. and Fernandez-Martinez, J.M. (1997) Genetic Analysis of Yield and Related Traits in Sunflower (Helianthus annuus L.) in Dryland and Irrigated Environments. Euphytica, 95, 243-251. http://dx.doi.org/10.1023/A:1003056500991
- Kwon, S. and Torrie, J. (1964) Heritability of and Interrelationship among Traits of Two Soybean Populations. Crop Science, 4, 196-198. http://dx.doi.org/10.2135/cropsci1964.0011183X000400020023x
- Miller, J.F., Hammond, J.J. and Roath, W.W. (1980) Comparison of Inbred vs. Single-Cross Testers and Estimation of Genetic Effects in Sunflower. Crop Science, 20, 703-706. http://dx.doi.org/10.2135/cropsci1980.0011183X002000060007x
- Khan, A., Malik, N.J., Khan, M.I. and Riaz, S. (2001) Growth Performance, Heritability and Inter Relationship in Some Quantitative Traits in Sunflower. Journal of Biological Science, 1, 895-897. http://dx.doi.org/10.3923/jbs.2001.895.897
- Khair, I., Hussain, M. and Mehdi, S. (1992) Heterosis, Heritability and Genetic Advance in Sunflower. Pakistan Journal of Agricultural Research, 13, 232-238.
- Stanton, M.L. (1984) Seed Variation in Wild Radish: Effect of Seed Size on Components of Seedling and Adult Fitness. Ecology, 65, 1105-1112. http://dx.doi.org/10.2307/1938318
- Vanisree, G., Ananthasayana, K., Nagabhushanam, G. and Jagadish, C. (1988) Correlation and Path Coefficient Analysis in Sunflower (Helianthus annuus L.). Journal of Oilseeds Research, 5, 46-51.
- Habib, H., Mehdi, S.S., Anjum, M.A., Mohyuddin, E. and Zafar, M. (2007) Correlation and Path Analysis for Seed Yield in Sunflower (Helianthus annuus L.) under Charcoal Rot (Macrophomina phaseolina) Stress Conditions. International Journal of Agricultural Biology, 9, 362-264.
- Zareian, A., Hamidi, A., H., S. and Jazaeri, M. (2013) Effect of Seed Size on Some Germination Characteristics, Seedling Emergence Percentage and Yield of Three Wheat (Tritucum aestivum L.) Cultivars in Laboratory and Field. Middle East Journal of Scientific Research, 13, 1126-1131.
- Massey, J.H. (1971) Effects of Nitrogen Rates and Plant Spacing on Sunflower Seed Yields and Other Characteristics. Agronomy Journal, 63, 137-138. http://dx.doi.org/10.2134/agronj1971.00021962006300010043x
- Zubriski, J.C. and Zimmerman, D.C. (1974) Effects of Nitrogen, Phosphorus, and Plant Density on Sunflower. Agro- nomy Journal, 66, 798-801. http://dx.doi.org/10.2134/agronj1974.00021962006600060024x
- Robinson, R.G. (1974) Sunflower Performance Relative to Size and Weight of Achenes Planted. Crop Science, 14, 616-618. http://dx.doi.org/10.2135/cropsci1974.0011183X001400050003x
- Kaya, M.D. and Day, S. (2008) Relationship between Seed Size and NaCl on Germination, Seed Vigor and Early Seedling Growth of Sunflower (Helianthus annuus L.). African Journal of Agricultural Research, 3, 787-791.
- Copeland, L.O. (1976) Principles of Seed Science and Technology. Burgress Publishing Company, Minneapolis.
NOTES
*Corresponding author.






