Advances in Biological Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17273 , 9 pages DOI:10.4236/abc.2012.21003
Growth inhibition of a human colon carcinoma cell, COLO 201, by a natural product, Vitex agnus-castus fruits extract, in vivo and in vitro
![]()
1Department of Clinical Molecular Genetics, School of Pharmacy, Tokyo University of Pharmacy & Life Sciences, Tokyo, Japan
2Laboratory of Physiological Chemistry, Institute of Medicinal Chemistry, Hoshi University, Tokyo, Japan
3Department of Cultural History, Seisen University, Tokyo, Japan
4Gastroenterological Center, Yokohama City University Medical Center, Yokohama, Japan
5Faculty of Pharmaceutical Sciences, Hoshi University, Tokyo, Japan
Email: *yuanbo@toyaku.ac.jp, *toyoda-h@toyaku.ac.jp
Received 8 November 2011; revised 21 December 2011; accepted 31 December 2011
Keywords: Colon Cancer; Apoptosis Induction; ER Stress; JNK; Flavonoids; Vitex agnus-castus
ABSTRACT
An extract from ripe fruit of Vitex agnus-castus (Vitex) has been used to treat patients with various obstetric and gynecological disorders in Europe. We have demonstrated that Vitex showed cytocidal effects on various types of cancer cell lines including a human colon carcinoma cell line, COLO 201. In this study, we extended our previous study to investigate the detailed mechanisms underlying cytocidal effects of Vitex on COLO 201. Furthermore, a possible clinical application of Vitex was also explored in vivo using nude mice xenografted with the cells. Treatment with Vitex induced apoptosis in COLO 201 in a time-dependent manner, accompanying with activation of caspase-9 and -3, but not caspase-8. An inhibitor for c-Jun NH2-terminal kinase (JNK), but not p38 mitogen-activated protein kinase (MAPK), significantly suppressed the apoptosis induction along with caspase-3 activation. Endoplasmic reticulum (ER) stressrelated genes were also upregulated by Vitex treatment. Most importantly, the in vivo efficacy of Vitex evaluated by assessing the tumor growth revealed that the administration of Vitex significantly suppressed tumor growth in COLO 201 xenografted mice. Collectively, current results suggest that apoptosis induction by Vitex in COLO 201 is mediated through the activation of JNK and caspase-9, -3 resulted from ER stress. Based on the current clinical application of Vitex, these results thus provide a new insight into the clinical use of Vitex and leave open a possibility of a new regimen as an alternative medicine approach for such devastating colon cancer treatment.
1. INTRODUCTION
Colon cancer is one of the most common cause of cancer death worldwide [1,2]. Treatment for the recurrent and metastatic disease remains a center of clinical attention. Combinational therapy, such as 5-fluorouracil (5-FU) and leucovorin with either irinotecan or oxaliplatin has been widely used for the treatment of patients with colorectal cancer [3,4]. Recently, various types molecular targetbased drugs, such as cetuximab and bevacizumab, are being used clinically. However, there is a growing concern about the side effects of these clinically used drugs. It is a noteworthy trend that botanical therapeutics has been receiving a great attention in order to reduce chemotherapy-associated side effects. In this regard, it is interesting to note that up to 60% of cancer patients use herbal supplements during or after chemotherapy in the USA [5]. Of those, flavonoids are known to display a wide variety of biological functions including antioxidative functions and anticancer activity [6].
Vitex agnus-castus is a shrub of the Verbenaceae family and is found naturally in the Middle East and Southern Europe. Ripe fruit of V. agnus-castus has been used as a folk medicine for the treatment of various obstetric and gynecological disorders in Europe [7,8]. It has been reported that flavonoids are one of major components of an extract from dried ripe V. agnus-castus (Vitex) [9]. In fact, we have previously reported that the extract induced apoptosis in human gastric signet ring cell carcinoma (KATO-III) [10]. The facts that an increase in the level of intracellular reactive oxygen species (ROS) and the expression level of stress-related genes including hemoxygenase-1 (HO-1); an activation of caspase-9, -3 and caspase-8 as well as Bid; an abrogation of apoptosis induction by antioxidants such as N-acetyl-L-cysteine (NAC) and glutathione suggested that the apoptosis was induced by oxidative stress resulting in mitochondrial damage [10]. We also demonstrated that Vitex exhibited cytotoxic activities against other several types of human cancer cell lines including COLO 201, a colon carcinoma cell line [11,12]. Furthermore, we recently reported for the first time that 5-FU in combination with Vitex achieved an enhanced cytocidal effect on the cells, but lesser cytotoxic effect on human peripheral blood mononuclear cells, suggesting a new chemotherapeutic application of Vitex as a phytotherapeutics for the treatment of patients with colon cancer [13]. Our experimental data also demonstrated that apoptosis induction was involved in the mechanism underlying the cytocidal effect of Vitex in COLO 201 cells [11,12]. The lack of activity of NAC to abrogate apoptosis induction suggested that the mechanism was independent of oxidative stress, although a significant increase in HO-1 gene expression was observed in the cells treated with Vitex [12]. However, the detailed mechanisms underlying the cytocidal effect of Vitex on COLO 201 are not fully understood.
In the current study, we extended our previous study to investigate the detailed mechanisms underlying Vitexinduced apoptosis in COLO 201 cells. In order to predict a possible clinical application of Vitex, we further investtigated the effect of Vitex on the tumor growth of nude mice xenografted with COLO 201 cells.
2. MATERIALS AND METHODS
2.1. Reagents
Specific fluorogenic substrates for caspase-3 [Asp-GluVal-Asp-AFC (DEVD-AFC)], caspase-9 [Leu-Glu-HisAsp-AFC (LEHD-AFC)], caspase-8 [Ile-Glu-Thr-AspAFC (IETD-AFC)], and specific inhibitor for caspase-3, Z-DEVD-FMK, were purchased from BioVision Research Products (Mountain View, CA, USA). Boc-D-FMK, a pancaspase inhibitor, was purchased from Sigma (St. Louis, MO, USA). c-Jun NH2-terminal kinase (JNK) inhibitor, SP600125, and p38 mitogen-activated protein kinase (MAPK) inhibitor, SB203580, were purchased from Calbiochem (Darmstadt, Germany).
2.2. Cell Line and Culture Methods
COLO 201, a human colon carcinoma cell line [14], was purchased from the JCRB Cell Bank (JCRB 0226; Tokyo, Japan). Cells were cultured in RPMI-1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% heatinactivated fetal bovine serum (Bio-Whittaker, Walkersville, MD, USA) and antibiotics (100 U/ml of penicillin and 100 µg/ml of streptomycin (Gibco BRL, Gaithersburg, MD, USA)) at 37˚C in a humidified atmosphere (5% CO2 in air).
2.3. Vitex Treatment
Preparation of Vitex was carried out according to the methods described previously [10]. Vitex was dissolved in dimethyl sulfoxide (DMSO) at concentrations of 20, 100, and 200 mg/ml. COLO 201 cells (2 × 105 cells/ml) were precultured for 12 h, followed by the treatment with Vitex (final concentrations: 10, 50 and 100 µg/ml) at 37˚C for a designated time. Controls were prepared by treating cells with culture medium containing vehicle reagent, DMSO, only (final concentration: 0.05%).
2.4. DNA Preparation and DNA Fragmentation Analysis by Agarose Gel Electrophoresis
DNA preparation and agarose gel electrophoresis were carried out according to the methods previously reported [12]. Extracted DNA was dissolved in TE buffer (50 mM Tris-HCl, pH 7.8, 10 mM EDTA-2Na) in an approximate concentration of 1 μg DNA/μl. Twenty microliters of DNA solutions and Ready-LoadTM 100 bp DNA Ladder (Invitrogen, Carlsbad, CA, USA) were electrophoresed, respectively, on a 2% agarose gel (Agarose X, Nippon gene, Tokyo, Japan), and visualized by ethidium bromide (Sigma-Aldrich, Poole, UK) staining, followed by viewing under UV Light Printgraph (ATTO Corp, Tokyo, Japan).
2.5. Treatment of Cells with Inhibitors
Z-DEVD-FMK, a specific inhibitor for caspase-3, was dissolved in DMSO at a concentration of 10 mM. BocD-FMK, a pancaspase inhibitor; SP600125, a specific inhibitor for JNK; and SB203580, a specific inhibitor for p38 MAPK, were dissolved in DMSO at a concentration of 20 mM. COLO 201 cells (2 × 105 cells/ml) were cultured for 12 h prior to the addition of each of the following inhibitors, [Boc-D-FMK (final concentration: 50 μM), Z-DEVD-FMK (final concentration: 20 μM), and SP600125 or SB203580 (final concentration: 10 μM)], into culture medium, just before the addition of Vitex at a final concentration of 100 μg/ml, followed by the additional incubation for 48 h.
2.6. Measurement of Caspase-3, -9 and -8 Activities
Activity of caspase-3, -9, or -8 was measured using the caspase fluorometric assay kit (BioVision) according to the manufacturer’s instructions. Protein amount of 50 μg/50 μl was plated on a 96-well plate, followed by the addition of 50 μl of 2 × reaction buffer containing 10 mM DTT to each sample, and then 5 µl of 1 mM caspase substrate (final concentration of 50 µM). After incubation at 37˚C for 1-h, fluorescent intensity was measured with a 400 nm excitation filter and 505 nm emission filter using a microplate reader Safire (TECAN, Männedorf, Switzerland).
2.7. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA for RT-PCR analysis was extracted from Vitex-treated COLO 201 cells using an RNA extraction kit, ISOGEN (Wako Pure Chemical Industries, Osaka, Japan). Complementary DNA (cDNA) was synthesized from 1 µg of RNA using 100 pmol of random hexamers and 100 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a total volume of 20 µl according to the manufacture’s instruction manual. PCR was performed as described previously [12]. Sequences of sense and anti-sense primer pair for PCR were as follows: β-actin, 5’-CCT TCC TGG GCA TGG AGT CCT G-3’ and 5’-GGA GCA ATG ATC TTG ATC TTC-3’ [15]; HO-1, 5’-CCA GCA ACA AAG TGC AAG ATT C-3’ and 5’-TGC AGG AAC TGA GGA TGC TG-3’ [16]; GRP78, 5’-ATG AAG CCC GTC CAG AAA GT-3’ and 5’-GCT GTA TCC TCT TCA CCA GT-3’; CHOP, 5’-CAT ACA TCA CCA CAC CTG AAA G-3’ and 5’-CCG TTT CCT GGT TCT CCC TTG G-3’ [17]. PCR primer sequences for GRP78 gene were designed according to cDNA sequences published (GenBank accession No. BC020235). PCR products and Ready-LoadTM 100 bp DNA Ladder (Invitrogen) marker were electrophoresed, respectively, on a 2% UltraPureTM agarose gel (Invitrogen), and visualized by ethidium bromide staining, followed by viewing under UV Light Printgraph (ATTO Corp, Tokyo, Japan).
2.8. Treatment of COLO 201-Xenografted Mouse in Vivo
Specific pathogen-free (SPF) KSN mice (male, 5 weeks old) were purchased from Japan SLC Inc., (Shizuoka, Japan). Animal use and relevant experimental procedures were approved by the Tokyo University of Pharmacy and Life Sciences Committee on the Care and Use of Laboratory Animals. COLO 201 cells were cultured as described above, harvested, centrifuged at 1000 rpm for 3 min, washed and resuspended in sterile PBS. The total number of 5 × 106 cells in 0.2 ml was injected subcutaneously between the scapulae of each nude mouse housed under SPF condition. After transplantation, tumor size was measured using calipers and the tumor volume was estimated according to the formula: tumor volume (mm3) = L × W2/2, where L is the length and W is the width [18]. Once tumors reached a mean size of 200 mm3, animals received intraperitoneal injections of either 0.5 ml sterile PBS alone or 1 mg Vitex suspended in 0.5 ml sterile PBS/day for 4 weeks.
2.9. Statistical Analysis
Experiments were independently repeated three times, and the results were shown as mean ± standard deviation (S.D.) of three assays. Student t-test as well as Scheffe’s post-hoc test was applied, and p values less than 0.05 were considered as significant.
3. RESULTS
3.1. Vitex-Induced Apoptosis Mediated through Caspase Activation in COLO 201 Cells
After treatment of the cells with 100 μg/ml Vitex for an indicated time period, a typical DNA fragmentation ladder representing apoptosis induction was observed after 24 h treatment (Figure 1(a)). The apoptosis induction was suppressed by the addition of 50 μM Boc-D-FMK, a pancaspase inhibitor, or 20 μM Z-DEVD-FMK, a specific inhibitor for caspase-3 (Figures 1(b) & 1(c)), indicating that caspase pathways play an important role in the Vitex-induced apoptosis. Furthermore, activation of caspase-9, and -3, but not caspase-8, was observed after 24 h treatment (Figure 1(d)).
3.2. Contribution of MAPK Pathways to Vitex-Induced Apoptosis in COLO 201 Cells
Addition of 10 µM SP600125, a specific inhibitor for JNK, clearly suppressed Vitex-induced apoptosis in COLO 201 cells, whereas no suppression was observed when 10 µM SB203580, a specific inhibitor for p38 MAPK, was added (Figures 2(a) & (b)). Furthermore, approximate 3-fold reduction of caspase-3 activity in Vitex-treated cells was observed in the presence of SP600125, indicating the contribution of JNK pathway to the caspase-3 activation (Figure 2(c)).
3.3. Vitex-Induced Upregulation of ER Stress-Related Gene Expression Levels in COLO 201 Cells
After treatment with 100 μg/ml Vitex for 12 and 48 h, the expression profiles of HO-1, CHOP and GRP78 were assessed by RT-PCR. Consistent with our previous report [12], the expression levels of HO-1 gene were strikingly upregulated in the Vitex-treated cells compared with those in controls at 12 h posttreatment, and the upregulation continued up to 48 h (Figures 3(a) & (b)). Compared with constitutive expression of CHOP gene in controls, Vitex also induced significant upregulation of the
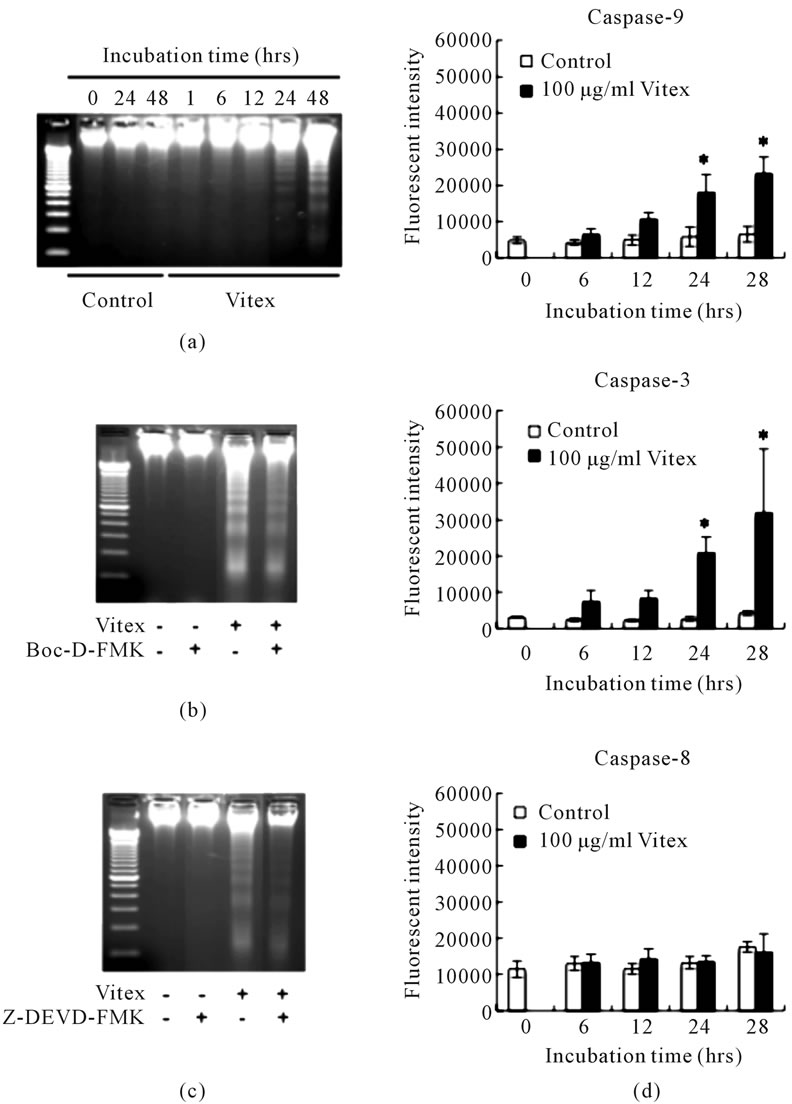
Figure 1. Vitex-induced apoptosis mediated through caspase activation in COLO 201 cells. Panels (a) to (c) show DNA electrophoresis patter of COLO 201 cells treated with 100 μg/ml of Vitex alone for an indicated time period (a); in the presence of 50 μM Boc-D-FMK, a pancaspase inhibitor (b); and in the presence of 20 μM Z-DEVD-FMK, a specific inhibitor for caspase-3 (c) for 48 h. A representative electrophoretic profile was shown from three independent experiments. Panel (d) shows the activity of caspase-9, -3 and -8 after treatment with 100 μg/ml of Vitex for 48 h, which was measured using a caspase fluorometric assay kit as described in Materials and Methods. Results are shown as mean ± S.D. (n = 3, *p < 0.05 compared to the control).
gene during the treatment period (Figures 3(a) & (c)). Similarly, the expression levels of GRP78 were upregulated by the Vitex treatment, although the degree of upregulation was slightly lower than those of the other two genes (Figures 3(a) & 3(d)).
3.4. Anti-Tumor Growth Effect of Vitex on COLO 201 Xenograft Mice
Human colon cancer xenograft was established by transplantation of COLO 201 cells into a 7-week old athymic nude mouse. Approximate required time for tumor growth to reach a mean size of 200 mm3 was two weeks. After reaching the mean size, animals received intraperitoneal injection of either 0.5 ml sterile PBS (control group) or 1 mg Vitex suspended in 0.5 ml sterile PBS to investigate anti-tumor effect of Vitex. A significant inhibition of tumor growth was observed from 26th day posttransplantation in Vitex-treated group compared to
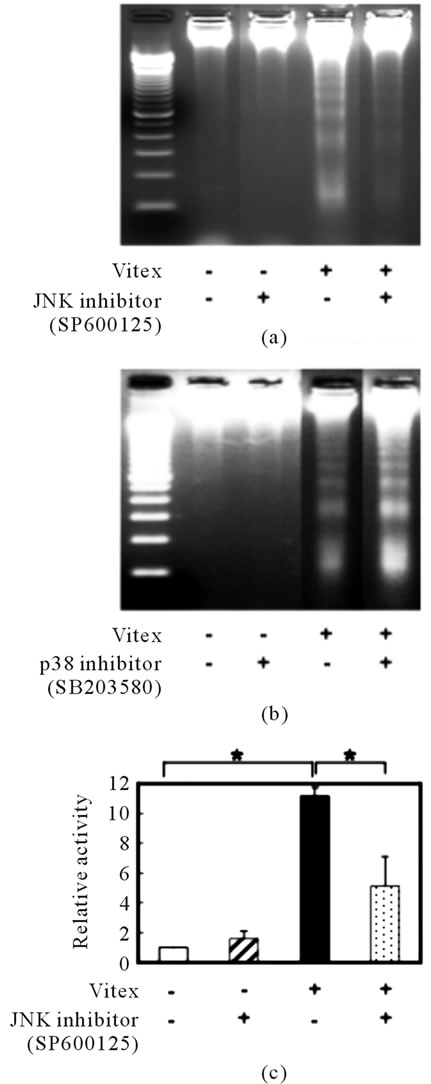
Figure 2. Contribution of MAPK pathways to Vitex-induced apoptosis in COLO 201 cells. After treatment with 100 μg/ml of Vitex in the presence or absence of 10 μM of SP600125 (an inhibitor for JNK) (a) or SB203580 (an inhibitor for p38 MAPK) (b), respectively, for 48 h, DNA fragmentation was analyzed by an agarose gel electrophoresis as described under Materials and Methods. A representative electrophoretic profile was shown from three independent experiments. After treatment with 100 μg/ml of Vitex in the presence or absence of 10 μM of SP600125 for 48 h, cell lysates were subjected to determination the activity of caspase-3 as described in the text (c). Relative activity of caspase-3 was calculated as the ratios against that control group. Experiments were carried out in triplicate, and results are shown as mean ± S.D. (*p < 0.05) using a caspase fluorometric assay kit as described in Materials and Methods. Results are shown as mean ± S.D. (n = 3, *p < 0.05 compared to the control).
control group (Figure 4(a)). Compared to the tumor size of 5356.3 ± 1330.6 mm3 in control group at 42nd day posttransplantation, the tumor size was 2430.0 ± 316.5 mm3 in Vitex-treated group, indicating more than 50% inhibition by the administration of Vitex (Figure 4(a)). Furthermore, the tumor weight of 5.2 ± 0.7 g in control group at the 42nd day posttransplantation was signifycantly reduced by approximate 50%, i.e., 2.6 ± 0.6 g, in Vitex-treated group (Figure 4(b)). In addition, body weight of mice was not changed during the period of administration of Vitex (data not shown).
4. DISCUSSION
We have been interesting in the effects of naturally derived flavonoids on cancer cell growth, particularly malignant cells in the gastrointestinal tract. In this regard, we have previously demonstrated that a crosstalk between intrinsic and extrinsic pathway via Bid activation as a result of oxidative stress plays a critical role in Vitexinduced apoptosis in KATO-III cell line, a human gastric signet ring cell carcinoma [10]. We also demonstrated that Vitex exhibited cytotoxic activities against several other types of human cancer cell lines including COLO 201, a colon carcinoma cell line [11,12]. Apoptosis induction was observed in Vitex-treated COLO 201, concomitantly with a significant increase in HO-1 gene expression as observed in KATO-III cells. On the other hand, unlike KATO-III, apoptosis induction was not abrogated in the presence of antioxidants, such as NAC, suggesting that apoptosis induction was independent of oxidative stress [12]. However, detailed mechanisms underlying the cytocidal effects of Vitex on COLO 201 are not fully understood.
In this study, apoptosis induction was reconfirmed in Vitex-treated COLO 201 cells, accompanying with activation of caspase-9 and -3, but not caspase-8 (Figures 1(a) & 1(d)). Furthermore, both a pancaspase inhibitor and a specific caspase-3 inhibitor clearly suppressed the apoptosis induction (Figures 1(b) & 1(c)), suggesting the involvement of caspase pathways in the apoptosis induction. We also demonstrated that the addition of a specific inhibitor for JNK not only clearly inhibited the activation of caspase-3 but also apoptosis induced by Vitex treatment, whereas it was not observed when a specific inhibitor for p38 MAPK was added (Figure 2). It is important to note that molecules of MAPK family such as JNK and p38 MAPK play a pivotal role in apoptosis induction [19-23]. Interestingly, genipin, one active compound found in gardenia fruit extract used in Traditional Chinese Medicine, has recently been reported to induce apoptosis in human leukemia K562 cells through the activation of JNK, but not p38 MAPK activation [23]. Furthermore, it has been demonstrated that luteolin, an
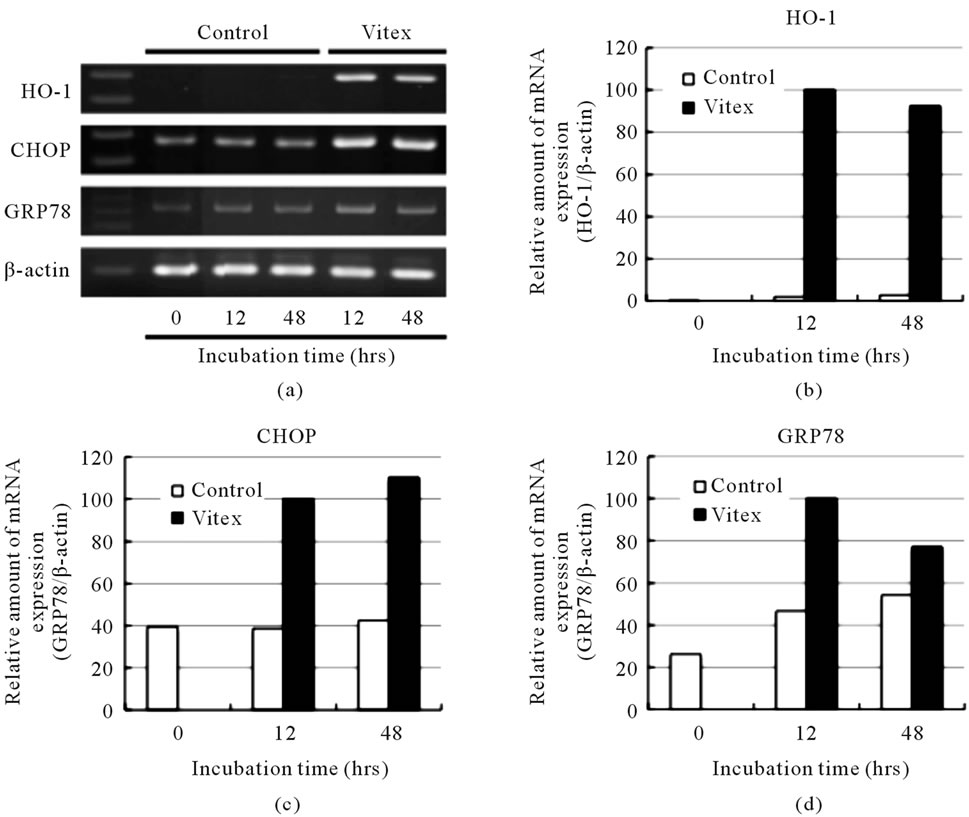
Figure 3. Vitex-induced upregulation of ER stress-related gene expression levels in COLO 201 cells. Cells were treated with or without 100 µg/ml of Vitex for an indicated time. The expression levels of ER stress-related mRNAs were analyzed by RT-PCR, followed by an agarose gel electrophoresis as described in Materials and Methods (a). The relative amount of mRNA was determined based on the results in (a): expression levels of ER stress-related gene/β-actin at each time point was divided by the relative ratio at 12-h time point using ImageJ 1.42q. A representative result of three separate experiments is shown in panels (b) for HO-1, (c) for CHOP and (d) for GRP78.
important component of Vitex [9], induces apoptosis in human hepatoma HepG2 cells mediated through mitochondria damage and activation of JNK, rather than p38 MAPK [21]. Based on these studies and our present results, it is suggested that JNK predominantly contributed to Vitex-induced apoptosis, resulting in the activation of caspase family molecules.
It is well-known that JNK pathway is involved in the regulation of caspase-3 via activation of caspase-8 [24, 25]. However, the activation of caspase-8 was not observed in the Vitex-treated COLO 201, while the activetion was observed in Vitex-treated KATO-III [10]. These experimental results thus suggest that different mechanisms are involved in the apoptosis induction in different types of cancer cells. Moreover, it is significant to note that the activation of JNK leads to a caspase-8-independent activation of Bid and the subsequent release of the pro-apoptotic protein Smac (second mitochondria-derived activator of caspase)/DIABLO (direct inhibitor of apoptosis proteins (IAP) binding protein with low pI) from mitochondria in TNF-α-induced apoptosis in HeLa cells [26]. Moreover, it is interesting to note that a similar experimental results have been reported in a human breast cancer cells, MDA-MB-231, stably transfected with human insulin-like growth factor-binding protein-5 (IGFBP- 5), which is a member of a family of high-affinity binding proteins that modulate the mitogenic and antiapoptotic effects of IGFs [27]. In the IGFBP-5-overexpressing cells treated with TNF-α, an enhanced phosphorylation of JNK along with Bid activation was observed. However, the Bid activation was blocked by a JNK-specific inhibitor, SP600125, but not by a caspase-8-specific inhibitor, z-ITED-fmk, suggesting that the Bid activation is mediated via a caspase-8-independent and JNK-dependent pathways. Taking these previous findings and our observations into account, we suggest that the mitochondrial pathway of apoptosis, namely, the activation of caspase-9 probably attributed to the activation of Bid via JNK pathway, most likely to contribute to the activation of caspase-3, although the analysis of the activation of Bid is obviously needed.
We further demonstrated that the expression of HO-1
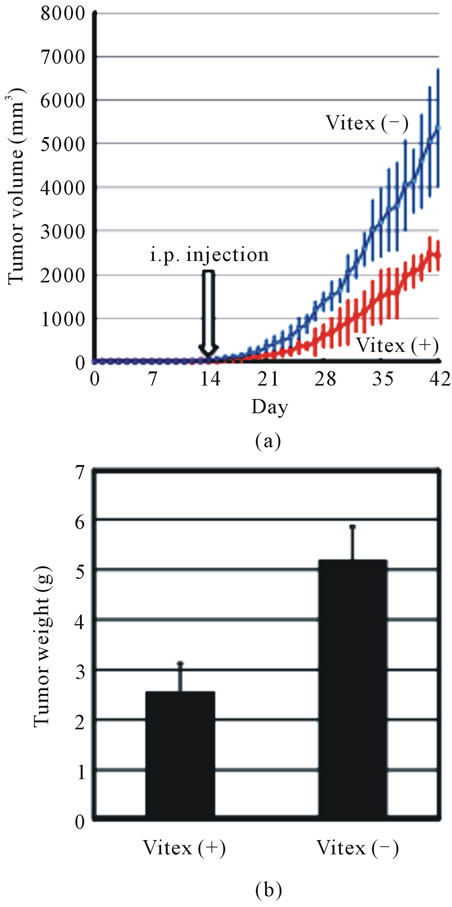
Figure 4. Anti-tumor growth effect of Vitex on COLO 201 xenograft mice. The total number of 5 × 106 COLO 201 cells in 0.2 ml of sterile PBS was injected subcutaneously once between the scapulae of each 7-week old nude mouse under SPF conditions. After transplantation, tumor size was measured using calipers and the tumor volume was estimated as described under Materials and Methods. It took about two weeks to reach tumor volume with a mean size of 200 mm3. Then, animals received intraperitoneal injections of either 0.5 ml sterile PBS or 1 mg Vitex suspended in 0.5 ml sterile PBS per day for additional 4 weeks. At the 42nd day after the experiment start, animals were sacrificed, and tumor volume (a) and weight (b) were assessed. Results are shown as mean ± S.D. (n = 4, *p < 0.05 Vitex-treated group (Vitex (+) vs control group without Vitex treatment (Vitex (–)).
gene was upregulated substantially by Vitex treatment (Figures 3(a) & 3(b)), similar to our previous report [12]. It should be noted that the expression of HO-1 has been observed in the endoplasmic reticulum (ER) [28]. HO-1 upregulation in various cell types has been demonstrated when ER stress was induced by a variety of experimental agents [28]. Indeed, Liu et al. recently reported that ER stress induced apoptosis in vascular smooth muscle, accompanying with an increase in HO-1 gene expression [29]. Taking both these reports and our previous observations into account, we hypothesized the involvement of ER stress in the Vitex-induced apoptosis in COLO 201 cells. In a logical extension of our hypothesis, the expression of GRP78 and CHOP genes, which are known to be associated with ER stress pathways [28-30], were also upregulated during the treatment period (Figures 3(a), (c) & (d)). In fact, it has been reported that JNK pathways are activated by the ER stress [31,32]. Collectively, our results suggested that Vitex-induced apoptosis in COLO 201 was mediated through the activation of JNK, and caspase-9 and -3 as a result of ER stress. In support of this hypothesis, our microarray analysis data also demonstrated that besides CHOP and GRP78 genes, the expression of another representative ER-stress related gene, DnaJ (Hsp40) homolog subfamily B member 9 gene [33] was also upregulated by Vitex treatment (unpublished observation).
Most importantly, our in vivo experimental data revealed that the administration of Vitex significantly suppressed tumor growth in COLO 201 xenograft mice (Figure 4), although more studies must be conducted to understand detailed in vivo pharmacological characterization of Vitex treatment. Of note, Vitex has been used to treat patients with various obstetric and gynecological disorders in Europe [7,8]. Moreover, it is interesting to note that Vitexins, which is isolated from the seed of Chinese herb Vitex Negundo and bears a basic flavonoid structure, shows cytotoxic and antitumor effects against breast, prostate and ovarian cancer cells through apoptosis induction via a intrinsic pathway based on in vitro and in vivo xenograft tumor models [34]. Therefore, our results provide a new insight into the clinical use of Vitex for colon cancer besides those cancer mentioned above.
Our results suggested that the activation of JNK and caspase-9 and -3 resulted from ER stress contributed to the apoptosis induction in Vitex-treated COLO 201 cells. Furthermore, we recently reported for the first time that 5-FU in combination with Vitex achieved an enhanced cytocidal effect on the cells, but lesser cytotoxic effect on human peripheral blood mononuclear cells [13]. Therefore, these results leave open a possibility of a new regimen as an alternative medicine approach for such devastating colon cancer treatment.
5. ACKNOWLEDGEMENTS
We thank Makoto Origuchi, Hirokazu Ishikawa and Tetsuya Kimata for their technical assistance. We are also grateful to Dr. Yoshinori Nozawa for excellent advises. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology and by the Promotion and Mutual Aid Corporation for Private School of Japan.
REFERENCES
- Yiu, H.-Y., Whittemore, A.S. and Shibata, A. (2004) Increasing colorectal cancer incidence rates in Japan. International Journal of cancer, 109, 777-781. doi:10.1002/ijc.20030
- Jemal, A., Siegel, R., Ward, E., Murray, T., Xu, J. and Thun, M.J. (2007) Cancer statistics, 2007. CA: A Cancer Journal for Clinicians, 57, 43-66. doi:10.3322/canjclin.57.1.43
- Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., Berlin, J., Baron, A., Griffing, S., Holmgren, E., Ferrara, N., Fyfe, G., Rogers, B., Ross, R. and Kabbinavar, F. (2004) Bevacizmub plus irinotechan, fluorouracil, and leucovorin for metastatic colorecatal cancer. The New England Journal of Medicine, 350, 2335-2342. doi:10.1056/NEJMoa032691
- Meyerhardt, J.A. and Mayer, R.J. (2005) Systemic therapy for colorectal cancer. The New England Journal of Medicine, 352, 476-487. doi:10.1056/NEJMra040958
- Cassileth, B., Yeung, K.S. and Gubili, J. (2008) Herbs and other botanicals in cancer patient care. Current Treatment Options in Oncology, 9, 109-116. doi:10.1007/s11864-008-0061-5
- Surh, Y.J. (2003) Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer, 3, 768-780. doi:10.1038/nrc1189
- Berger, D., Schaffner, W., Schrader, E., Meier, B. and Brattström, A. (2000) Efficacy of Vitex agnus-castus L extract Ze 440 in patients with pre-menstrual syndrome (PMS). Archives of Gynecology and Obstetrics, 264, 150- 153. doi:10.1007/s004040000123
- Schellenberg, R. (2001) Treatment for the premenstrual syndrome with agnus castus fruit extract: Prospective, randomized, placebo controlled study. British Medical Journal, 322, 134-137. doi:10.1136/bmj.322.7279.134
- Hirobe, C., Qiao, Z-S., Takeya, K. and Itokawa, H. (1997) Cytotoxic flavonoids from Vitex agnus-castus. Phytochemistry, 46, 521-524. doi:10.1016/S0031-9422(97)00127-1
- Ohyama, K., Akaike, T., Imai, M., Toyoda, H., Hirobe, C. and Bessho, T. (2005) Human gastric signet ring carcinoma (KATO-III) cell apoptosis induced by Vitex agnus-castus fruit extract through intracellular oxidative stress. The International Journal of Biochemistry & Cell Biology, 37, 1496-1510. doi:10.1016/j.biocel.2005.02.016
- Ohyama, K., Akaike, T., Hirobe, C. and Yamakawa, T. (2003) Cytotoxicity and apoptotic inducibility of Vitex agnus-castus fruits extract in cultured human normal and cancer cells and effect on growth. Biological & Pharmaceutical Bulletin, 26, 10-18. doi:10.1248/bpb.26.10
- Imai, M., Kikuchi, H., Denda, T., Ohyama, K., Hirobe, C. and Toyoda, H. (2009) Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: A potential natural anti-cancer substance. Cancer Letters, 276, 74-80. doi:10.1016/j.canlet.2008.10.036
- Imai, M., Kikuchi, H., Yuan, B., Aihara, Y., Mizokuchi, A., Ohyama, K., Hirobe, C. and Toyoda, H. (2011) Enhanced growth inhibitory effect of 5-fluorouracil in combination with Vitex agnus-castus fruits extract against a human colon adenocarcinoma cell line, COLO 201. Journal of Chinese Clinical Medicine, 6, 14-19. http://www.cjmed.net/index.php/cjmed/article/view/109
- Semple, T.U., Quinn, L.A., Woods, L.K. and Moore, G.E. (1978) Tomor and lymphoid cell lines from a patient with carcinoma of the colon for a cytotoxicity model. Cancer Research, 38, 1345-1355. http://cancerres.aacrjournals.org/content/38/5/1345
- Takeuchi, R., Tsutsumi, H., Osaki, M., Haseyama, K., Mizue, N. and Chiba, S. (1998) Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1β-converting enzyme gene expression but does not cause apoptosis. The Journal of Virology, 72, 4498-4502. http://jvi.asm.org/content/72/5/4498.full
- Dubrovskaya, V.A. and Wetterhahn, K.E. (1998) Effects of Cr (VI) on the expression of the oxidative stress genes in human lung cells. Carcinogenesis, 19, 1401-1407. doi:10.1093/carcin/19.8.1401
- Tajiri, S., Oyadomari, S., Yano, S., Morioka, M., Gotoh, T., Hamada, J.I., Ushio, Y. and Mori, M. (2004) Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death & Differentiation, 11, 403-415. doi:10.1038/sj.cdd.4401365
- Inoue, J. and Aramaki, Y. (2007) Cyclooxygenase-2 inhibitor promotes enhancement of antitumor responses by transcutaneous vaccination with cytosine-phosphate-guanosine-oligodeoxynucleotides and model tumor antigen. Journal of Investigative Dermatology, 127, 614-621. doi:10.1038/sj.jid.5700656
- Wada, T. and Penninger, J.M. (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene, 23, 2838- 2849. doi:10.1038/sj.onc.1207556
- Li, J. and Holbrook, N.J. (2004) Elevated gadd153/chop expression and enhanced c-Jun N-terminal protein kinase activation sensitizes aged cells to ER stress. Experimental Gerontology, 39, 735-744. doi:10.1016/j.exger.2004.02.008
- Lee, H.J., Wang, C.J., Kuo, H.C., Chou, F.P., Jean, L.F. and Tseng, T.H. (2005) Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicology and Applied Pharmacology, 203, 124-131. doi:10.1016/j.taap.2004.08.004
- Yang, S.-H., Chien, C.-M., Chang, L.-S. and Lin, S.-R. (2007) Involvement of c-jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by cardiotoxin III (Naja naja atra) in K562 leukemia cells. Toxicon, 49, 966-974. doi:10.1016/j.toxicon.2007.01.005
- Feng, Q., Cao, H.L., Xu, W., Li, X.R., Ren, Y.Q. and Du, L.F. (2011) Apoptosis induced by genipin in human leukemia K562 cells: Involvement of c-Jun N-terminal kinase in G2/M arrest. Acta Pharmacologica Sinica, 32, 519-527. doi:10.1038/aps.2010.158
- Kang, S.J., Kim, B.M., Lee, Y.J., Hong, S.H. and Chung, H.W. (2009) Titanium dioxide nanoparticles induce apoptosis through the JNK/p38-caspase-8-Bid pathway in phytohemagglutinin-stimulated human lymphocytes. Biochemical and Biophysical Research Communications, 386, 682- 687. doi:10.1016/j.bbrc.2009.06.097
- Boisvieux-Ulrich, E., Sourdeval, M. and Marano, F. (2005) CD437, a synthetic retinoid, induces apoptosis in human respiratory epithelial cells via caspase-independent mitochondrial and caspase-8-dependent pathways both up-regulated by JNK signaling pathway. Experimental Cell Research, 307, 76-90. doi:10.1016/j.yexcr.2005.02.005
- Deng, Y., Ren, X., Yang, L., Lin, Y. and Wu, X. (2003) A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell, 115, 61-70. doi:10.1016/S0092-8674(03)00757-8
- Butt, A.J., Dickson, K.A., Jambazov, S. and Baxter, R.C. (2005) Enhancement of tumor necrosis factor-alpha-induced growth inhibition by insulin-like growth factorbinding protein-5 (IGFBP-5), but not IGFBP-3 in human breast cancer cells. Endocrinology, 146, 3113-3122. doi:10.1210/en.2004-1408
- Kim, H.P., Pae, H.O., Back, S.H., Chung, S.W., Woo, J.M., Son, Y. and Chung, H.T. (2011) Heme oxygenase-1 comes back to endoplasmic reticulum. Biochemical and Biophysical Research Communications, 404, 1-5. doi:10.1016/j.bbrc.2010.11.067
- Liu, X.M., Peyton, K.J., Ensenat, D., Wang, H., Schafer, A.I., Alam, J. and Durante, W. (2005) Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. The Journal of Biological Chemistry, 280, 872-877. doi:10.1074/jbc.M410413200
- Berridge, M.J. (2002) The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium, 32, 235- 249. doi:10.1016/S0143416002001823
- Urano, F., Wang, X., Bertolotti, A., Zhang, Y., Chung, P., Harding, H.P. and Ron, D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science, 287, 664-666. doi:10.1126/science.287.5453.664
- Yoneda, T., Imaizumi, K., Oono, K., Yui, D., Gomi, F., Katayama, T. and Tohyama, M. (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. The Journal of Biological Chemistry, 276, 13935-13940. doi:10.1074/jbc.M010677200
- Kurisu, J., Honma, A., Miyajima, H., Kondo, S., Okumura, M. and Imaizumi, K. (2003) MDG1/ERdj4, an ERresident DnaJ family member, suppresses cell death induced by ER stress. Genes to Cells, 8, 189-202. doi:10.1046/j.1365-2443.2003.00625.x
- Zhou, Y., Liu, Y.E., Cao, J., Zeng, G., Shen, C., Li, Y., Zhou, M., Chen, Y., Pu, W., Potters, L. and Shi, Y.E. (2009) Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clinical Cancer Research, 15, 5161-5169. doi:10.1158/1078-0432.CCR-09-0661
NOTES
*Corresponding author.

