Open Journal of Pediatrics
Vol.3 No.2(2013), Article ID:32443,4 pages DOI:10.4236/ojped.2013.32011
Influence of gender and race on presentation of eosinophilic esophagitis in children
![]()
Division of Pediatric Gastroenterology, University of Mississippi Health Center, Jackson, USA
Email: mnowicki@umc.edu
Copyright © 2013 Dangtue Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 7 March 2013; revised 6 April 2013; accepted 14 April 2013
Keywords: Eosinophilic Esophagitis; Gender; Race
ABSTRACT
Background: Eosinophilic esophagitis (EoE) is thought to be more common in males and Caucasians, yet little data exists regarding the presentation of EoE among children. Methods: A retrospective study of children undergoing esophagogastroduodenoscopy (EGD) was done to determine gender and racial differences in the prevalence and presenting symptoms of EoE. Data collected included gender, race, indication for EGD, and presence of EoE. Results: No gender or racial differences were found for indication for EGD. EoE was identified in 4.1% of children, more commonly in males than females (6% vs. 2.5%, p < 0.01). No racial difference was seen. Symptoms showing a racial/gender difference included dysphagia, vomiting, and foreign body impaction. Conclusions: Prevalence of EoE differs by gender, but not race. Gender/racial differences exist for EoE in children presenting with dysphagia, vomiting, and foreign body impaction. This data may help guide the clinician on when to refer for EGD.
1. INTRODUCTION
Eosinophilic esophagitis (EoE) is an inflammatory disorder of the esophagus with an increasing incidence both in children and adults [1-5]. A 2007 consensus report defined EoE as a clinicopatholgic disorder characterized by symptoms of esophageal dysfunction, eosinophilicpredominant inflammation limited to the esophagus, and response to topical steroids and/or dietary exclusion [6]. Although EoE can occur in any age group, gender, or racial group, a number of studies have documented gender and racial differences for EoE, with EoE being more common in males [7-16] and Caucasians [5,8,10,14]. All but one of these studies was retrospective in nature and most did not have a control group for comparisons [1-5, 7-16]. In pediatric studies, males made up 65% to 78% of patients with EoE, and >90% were Caucasian [1-5, 7-9,11-13]. A recent study addressing the influence of gender and race on the presentation of EoE in adults and children showed that there were no differences in symptoms between genders for children; failure to thrive was the only symptom with racial differences, being more common in African American children with EoE [14]. We conducted a retrospective review of our database of children having esophgaogastroduodenoscopy (EGD) to determine if there were gender and racial differences in the indication for EGD, prevalence of EoE, and symptoms of patients with EoE.
2. METHODS
A retrospective review was performed of a database of endoscopic procedures performed by the Division of Pediatric Gastroenterology at The University of Mississippi Health Center from September 1, 1998 through April 30, 2011. We identified all diagnostic esophagogastroduodenoscopy (EGD) procedures that were performed during this time period. Charts were reviewed and the following information retrieved: gender, race, indication for EGD, and presence of eosinophilic esophagitis (EoE). Groups consisted of African American females (AAF), Caucasian females (CF), African American males (AAM), and Caucasian males (CM). We excluded children that had EGD to evaluate for anemia, upper gastrointestinal bleeding, pancreatic insufficiency, celiac disease, inflammatory bowel disease, graft-versushost disease, and polyposis syndromes. The indication for EGD was compared to determine if racial or gender differences existed. The rate of EoE was determined for each group, stratified by indication for EGD. EoE was defined according to the 2007 consensus statement [6]. Descriptive analyses were done using frequency distribution and proportions. Chi-square or Fisher’s Exact tests were performed, as appropriate, to test the association between different categorical variables. Significance level was set 0.05, and SPSS 19.0 was used for all analyses.
3. RESULTS
Review of the database showed that 3247 children had undergone diagnostic EGD; sufficient data for review were found in 2878 (88.6%). The demographics for the children are shown in Table 1. There were slightly more females (52.3%) than males (47.7%). Most children were Caucasian (71.0%) or African American (27.7%), reflecting the racial make-up of the general population. No difference was seen for the age of African Americans (6.8 ± 4.8 years) and Caucasians (8.9 ± 4.7 years; p = ns) or for the age of females (9.3 ± 4.7 years) and males (8.2 ± 4.7 years; p = ns). Only African American and Caucasian children were included when comparing the rates of EoE; numbers in other races were too small for statistical analysis. We excluded 473 children for reasons mentioned in the Methods section. Thus, the final number of children studied was 2366.
The indication for upper endoscopy according to gender and race are outlined in Table 2. No differences were seen in the indication for EGD among the groups. We compared the finding of EoE for each diagnosis stratified by gender and race (Table 3). Overall, significant differences were seen in the prevalence of EoE according to gender and race being more commonly identified in CM (6.7%), followed by AAM (4.0%), CF (2.7%), and AAF (1.9%) (p < 0.001). When comparing the prevalence of EoE by gender, boys were more likely to have EoE than girls (6% vs 2.5% respectively, odds ratio 2.5, 95%C.I. 1.63 - 3.87; p < 0.001). When comparing the prevalence of EoE by race, African Americans were less likely to have EoE than Caucasians but the association was not statistically significant (2.8% vs 4.6% respectively, odds ratio 0.60, 95%C.I. 0.35 - 1.02; p = 0.058).
No difference was seen in the prevalence of EoE among the various racial/gender groups for children having EGD for abdominal pain, dyspepsia, or gastroesophageal reflux symptoms. Significant differences were
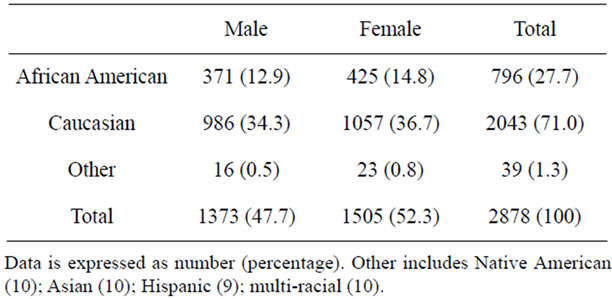
Table 1. Demographics of children having EGD during the study period.
seen in the prevalence of EoE for children having EGD for vomiting, dysphagia, and foreign body impaction with Caucasian males being most likely to have EoE (Table 3).
4. DISCUSSION
In this report we compare gender and racial differences in children for the symptom prompting EGD, prevalence of EoE, and symptoms of patients with EoE. Previous studies both in children and adults show EoE is more commonly identified in Caucasian males [5,8,10,14]. However, to date no studies have determined if there is a selection bias based on performing more EGDs on those at risk for EoE.
Symptoms of EoE include failure to thrive/feeding disorder, vomiting, gastroesophageal reflux, abdominal pain, dyspepsia, dysphagia, and food bolus impaction [3,5,7-9, 11,13,14]. Yet, most of this data derives from studies where the diagnosis of EoE was rendered and then the symptom leading to investigation was determined. We took a different approach, identifying the symptom prompting EGD and then determining the rate of EoE for each symptom. All children with EoE in this report presented with abdominal pain, dyspepsia, dysphagia, gastroesophageal reflux, foreign body impaction, or vomiting. We did not record any children with a complaint of failure to thrive as the reason for endoscopy. We do not routinely perform EGD for evaluation of failure to thrive without other gastrointestinal complaints, thus children with poor growth were included under symptoms such as vomiting, gastroesophageal reflux, and dysphagia. We found that indications for EGD in our population did not differ according to gender or race, negating any selection bias for choosing who to have EGD (Table 2). There are age-related differences in the presenting symptoms of EoE in pediatric patients [3]. It has not been determined if these differences are due to the common age-specific symptoms prompting EGD, improved communicative skills with increasing age, worsening esophageal fibrosis/ stricture formation with age, or other, as yet, undiscovered factors. We did not stratify by age as the purpose of this study was to determine gender and racial differences.
Overall, EoE was found in 4.2% of all children undergoing EGD in this retrospective review, comparable to previous rates of 3% and 5% [7,11]. The rate of EoE by symptom in decreasing order was dysphagia (18.1%), food bolus impaction (8.1%), vomiting (5.0%), dyspepsia (4.3%), gastroesophageal reflux (4.0%), and abdominal pain (2.2%). EoE was more commonly found in Caucasian males (6.7%) than African American males (4.0%), Caucasian females (2.7%), and African American females (1.9%) in keeping with previous studies.

Table 2. Indication for EGD according to gender and race.

Table 3. Percentage of children with eosinophilic esophagitis according to indication for EGD.
In previous studies, both in adults and children, the study population was derived from individuals with a known diagnosis of EoE allowing for selection bias. Most studies fail to mention a control group for comparing gender and racial differences. Other reports used control groups such as patients with atopy, non-EoE patients, or census data [2,10,16]. We included all children having diagnostic EGD then determined the rate of EoE for each gender and race, thus eliminating any selection bias. In the present study if only children with EoE are included, males were over-represented (68.6%) similar to previous reports (65% to 80%) [2-5,7-16]. More significant is the higher prevalence of EoE in males (6.0%) than females (2.5%) when including all study subjects.
Similarly, if only children with EoE in the present study are included, similar to previous studies, Caucasians account for 83% of the study population. This percentage is within the range of previous studies, where Caucasians accounted for 82% to 94% of the study population [8,10,14]. However, if one looks at the prevalence of EoE in the population described, Caucasians (4.6%) were more likely to have EoE than African Americans (2.8%), but statistical significance was not reached. Thus, our data does not support a racial predilection for EoE in children.
There was no gender or racial differences seen for the reason EGD was performed in this study (Table 2). This data suggests that the symptoms prompting EGD were similar among gender and races. Overall, EoE was more commonly found in children with dysphagia, followed in order by food bolus impaction, vomiting, dyspepsia, gastroesophageal reflux, and abdominal pain. These are the same symptoms reported in previous studies.
A recent study that included both adults and children with EoE showed that Caucasians were significantly older at diagnosis that African Americans [14]. We found no difference in the age at diagnosis between Caucasians and African Americans. Sperry, et al., showed that males with EoE were more likely to present with dysphagia and food bolus impaction, and less likely to present with abdominal pain, then females [14].
Racial differences were seen for dysphagia in adults, which were more common in Caucasian than African Americans. For children, failure to thrive was a more common presentation for EoE in African Americans; there was a trend toward dysphagia being more common in Caucasian children, although statistical significance was not reached [14]. In the present study, dysphagia, food bolus impaction, and vomiting were all more common in Caucasian males with EoE than in other groups. No racial or gender differences were seen for dyspepsia, abdominal pain, or gastroesophageal reflux.
In summary, there were no gender or race differences in the presenting symptoms for children having EGD in our patient population. We found an overall incidence of EoE in children having EGD of 4.2%, with Caucasian males being most commonly affected (6.7%). A gender difference was found in the prevalence of EoE, with a male predominance (6%, compared to 2.5% for females); we did not find a racial difference in the prevalence of EoE. Symptoms associated with EoE with gender and racial differences included dysphagia, vomiting, and foreign body impaction.
REFERENCES
- Liacouras, C.A., Spergel, J.M., Ruchelli, E., Verma, R., Mascarenhas, M., Semeao, E., Flick, J., Kelly, J., BrownWhitehorn, T., Mamula, P. and Markowitz, J.E. (2005) Eosinophilic esophagitis: A 10-year experience in 381 children. Clinical Gastroenterology and Hepatology, 3, 1198- 1206. doi:10.1016/S1542-3565(05)00885-2
- Kapel, R.C., Miller, J.K., Torres, C., Aksoy, S., Lash, R. and Katzka, D.A. (2008) Eosinophilic esophagitis: A prevalent disease in the United States that affects all age groups. Gastroenterology, 134, 1316-1321. doi:10.1053/j.gastro.2008.02.016
- Noel, R.J., Putnam, P.E. and Rothenberg, M.E. (2004) Eosinophilic esophagitis. New England Journal of Medicine, 351, 940-941. doi:10.1056/NEJM200408263510924
- Prasad, G.A., Alexander, J.A., Schleck, C.D., Zinsmeister, A.R., Smyrk, T.C., Elias, R.M., Locke III, G.R. and Talley, N.J. (2009) Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clinical Gastroenterology and Hepatology, 7, 1055-1061. doi:10.1016/j.cgh.2009.06.023
- Spergel, J.M., Brown-Whitehorn, T.F., Beausoleil, J.L., Franciosi, J., Shuker, M., Verma, R. and Liacouras, C.A. (2009) 14 years of eosinophilic esophagitis: Clinical features and prognosis. Journal of Pediatric Gastroenterology and Nutrition, 48, 30-36. doi:10.1097/MPG.0b013e3181788282
- Furuta, G.T., Liacouras, C.A., Collins, M.H., Gupta, S.K., Justinich, C., Putnam, P.E., Bonis, P., Hassall, E., Straumann, A. and Rothenberg, M.E. (First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees) (2007) Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology, 133, 1342-1363. doi:10.1053/j.gastro.2007.08.017
- Aceves, S.S., Newbury, R.O., Dohil, R., Schwimmer, J. and Bastian, J.F. (2007) Distinguishing eosinophilic esophagitis in pediatric patients: Clinical, endoscopic, and histologic features of an emerging disorder. Journal of Clinical Gastroenterology, 41, 252-256. doi:10.1097/01.mcg.0000212639.52359.f1
- Assa’ad, A.H., Putnam, P.E., Collins, M.H., Akers, R.M., Jameson, S.C., Kirby, C.L., Buckmeier, B.K., Bullock, J.Z., Collier, A.R., Konikoff, M.R., Noel, R.J., Guajardo, J.R. and Rothenberg, M.E. (2007) Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. Journal of Allergy and Clinical Immunology, 119, 731-738. doi:10.1016/j.jaci.2006.10.044
- Dauer, E.H., Freese, D.K., El-Youssef, M. and Thompson, D.M. (2005) Clinical characteristics of eosinophilic esophagitis in children. Annals of Otology, Rhinology, and Laryngology, 114, 827-833.
- Franciosi, J.P., Tam, V., Liacouras, C.A. and Spergel, J.M. (2009) A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clinical Gastroenterology and Hepatology, 7, 415-419. doi:10.1016/j.cgh.2008.10.006
- Gill, R., Durst, P., Rewalt, M. and Elitsur, Y. (2007) Eosinophilic esophagitis disease in children from West Virginia: A review of the last decade (1995-2004). American Journal of Gastroenterology, 102, 2281-2285. doi:10.1111/j.1572-0241.2007.01352.x
- Pentiuk, S.P., Miller, C.K. and Kaul, A. (2007) Eosinophilic esophagitis in infants and toddlers. Dysphagia, 22, 44-48. doi:10.1007/s00455-006-9040-9
- Sant’Anna, A.M., Rolland, S., Fournet, J.C., Yazbeck, S. and Drouin, E. (2004) Eosinophilic esophagitis in children: Symptoms, histology and pH probe results. Journal of Pediatric Gastroenterology and Nutrition, 39, 373-377. doi:10.1097/00005176-200410000-00013
- Sperry, S.L., Woosley, J.T., Shaheen, N.J. and Dellon, E.S. (2012) Influence of race and gender on the presentation of eosinophilic esophagitis. American Journal of Gastroenterology, 107, 215-221. doi:10.1038/ajg.2011.342
- Straumann, A., Spichtin, H.P., Grize, L., Bucher, K.A., Beglinger, C. and Simon, H.U. (2003) Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for up to 11.5 years. Gastroenterology, 125, 1660- 1669. doi:10.1053/j.gastro.2003.09.024
- Veerappan, G.R., Perry, J.L., Duncan, T.J., Baker, T.P., Maydonovitch, C., Lake, J.M., Wong, R.K. and Osgard, E.M. (2009) Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: A prospective study. Clinical Gastroenterology and Hepatology, 7, 420-426.

