Open Journal of Internal Medicine
Vol.1 No.2(2011), Article ID:7694,4 pages DOI:10.4236/ojim.2011.12006
Brief review: management of lupus nephritis—randomized controlled trials: an update
![]()
1Department of Medicine, The University of Western Ontario, London, Canada;
2Nephrology Division, London Health Sciences Centre, London, Canada.
Email: *william.clark@lhsc.on.ca
Received 31 May 2011; 7 July 2011; 20 July 2011.
Keywords: Lupus Nephritis; Immunosppressive Therapy; Randomized Controlled Trials; Lupus Treatment update; Induction Therapy; Maintenance Therapy; Evidence Based Comparison
ABSTRACT
Lupus nephritis leads to significant morbidity and mortality in patients with systemic lupus erythematous. Immunosuppressive agents are recommended in management of Class III, IV and V lupus nephritis. The goals of therapy are to control the disease and to prevent relapse while minimizing side-effects of therapy. Most of the evidences in managements of Class III and IV lupus nephritis comes from randomized controlled trials using intravenous cyclophosphamides, oral mycophenolate mofetil and oral azathioprine. In Class V lupus nephritis, there are few studies available and they have assessed the use of intravenous cyclophsophamide, oral mycophenolates mofetil and oral cyclosporine. In this review article, we have summarized the major randomized controlled trials in managements of Class III, IV and V lupus nephritis and offer an interpretation of the evidence to date.
1. INTRODUCTION
Systemic lupus erythematous (SLE) is a multi-organ disease in which renal involvement contributes to significant morbidity and mortality. In 2004, the International Society of Nephrology/Renal Pathology Society (ISN/RPS) Working Group modified the previous World Health Organization (WHO) classification [1] of lupus nephritis to emphasize clinically relevant lesions. However, immunosuppressive therapy remains the accepted treatment of Class III, IV and V lupus nephritis, regardless of which classification is used. Treatments are categorized into induction and maintenance therapies; the choice of which depends on the risk of relapse and longterm drug toxicities, including infection, malignancy and infertility. Over the last decade, numerous randomized control trials have been published, comparing different management strategies for Class III, IV and V lupus nephritis, most focusing on Classes III and IV. However, major limitations to these have been the varied classification systems used and limited long-term follow-up. This article summarizes the major randomized control trials prior to 2011, for treatment of Class III, IV and V lupus nephritis.
2. CLASS III AND IV LUPUS NEPHRITIS
2.1. Induction Therapy
Cyclophosphamide has the most evidence and long-term follow-up results for the management of Class III and IV lupus nephritis. One of the landmark trials was from the National Institutes of Health (NIH) by Illei et al. [2] This study compared intravenous pulse cyclophosphamide (1 g/m2 monthly for 6 months, and then every 3 months for 24 months), intravenous pulse methylprednisolone (1 g/m2 monthly for 12 - 36 months) and a combination of both, in 82 patients with extensive follow-up design. They used an intention-to-treat analysis to assess treatment failure, defined by doubling of serum creatinine, need for additional immunosuppressive therapy, or death. There were significantly lower rates of treatment failure in the cyclophosphamide alone (p = 0.04) and combination (p = 0.002) arms, compared to the methylprednisolone alone arm; and there was a trend towards lower rates of treatment failure in the combination arm as compared to the cyclophosphamide alone arm, though this was not statistically significant (p = 0.24). By contrast, there were increased side effects with the use of cyclophosphamide, including a higher risk of infection (26% - 32%) and pre-mature menopause (52% - 60%), compared to the methylprednisolone alone arm (8% and 33%). There were 5 deaths in each of the cyclophosphamide arms with only one in the methylprednisolone alone arm. As a result of this poor safety profile, the EuroLupus trial by Houssiau et al. [3] was designed to assess the efficacy of shorter duration and lower dosage of intravenous pulse cyclophosphamide as induction therapy (500 mg every 2 weeks for 3 months) and compared this to the modified NIH protocol with intravenous pulse cyclophosphamide (0.5 g/m2 monthly and up to 1.5 g per pulse for 6 months and then quarterly for 6 months). Both arms were treated with intravenous methylprednisolone (750 mg daily for 3 days), followed by oral prednisolone (0.5 mg/kg per day for 4 weeks and then tapered to 5 - 7.5 mg per day for 30 months). Both arms were followed by maintenance therapy with oral azathioprine 2 weeks after completion of the last cyclophosphamide dose (2 mg/kg per day until month 30). Treatment failure was defined as the absence of a primary response after 6 months of therapy, occurrence of glucocorticoid-resistant flare, or doubling of the serum creatinine level. There was no difference in treatment failure (16% vs. 20%; p = 0.64), however there was a non-statistically significant trend towards a decreased risk of severe infection with the lower dosage group than the higher dosage group (hazard ratio of 0.5; p = 0.20). There was no difference in the gonadal toxicity between the two arms in the initial publication. Long-term follow-up for this study was published in 2010 [4], and there remained no difference in the primary outcome at 10 years.
In contrast, evidence for mycophenolate mofetil (MMF) as induction therapy for lupus nephritis is emerging. In a Chinese study, Chan et al. [5] reported a similar response rate for oral MMF (2 g per day for 6 months, then 1g per day for another 6 months, followed by oral azathioprine 1 mg/kg per day) and oral cyclophosphamide (2.5 mg/kg per day, followed by oral azathioprine 1.5 mg/kg per day for 6 months, then 1 mg per day) in a randomized control trial of 42 patients who had Class IV lupus proliferative lesions. All patients received oral prednisone (0.8 mg/kg per day, tapered to 10 mg per day over 6 months). The primary outcome was complete remission (urinary protein excretion < 0.3 g per day, normal urinary sediment, normal serum albumin concentration, and serum creatinine and creatinine clearance that were 15% or less above the baseline values). Although there was no statistically significant difference in the primary outcome (81% vs. 76%; p = 1.00), there were non-statistically significant trends of lower risk of infection rates (19% vs. 33%) and amenorrhea rates (0% vs. 23%) in the MMF arm as compared to cyclophosphamide arm. A further study by Ong et al. [6] assessed intravenous pulse cyclophosphamide (0.75 - 1 g/m2 monthly) versus oral MMF (2 g per day) plus prednisolone (60 mg per day for 4 - 6 weeks, tapered to a maintenance dose of 5 - 10 mg per day). There were similar results for the primary outcome, which was remission of lupus nephritis (stabilization or improvement in renal function, urinary red blood cell < 10 cells/high power field, and reduction of proteinuria < 3 g per day if baseline proteinuria was > 3 g per day and > 50% reduction or < 1 g per day if baseline proteinuria was in the sub-nephrotic range) at 6 months. Remission occurred in 52% of the cyclophosphamide group and 58% of the MMF group (p = 0.70). The MMF group had less proteinuria compared with the cyclophosphamide group (1.8 g per day vs. 3 g per day).
Ginzler et al. [7] assessed oral MMF (1 - 3 g per day for 24 weeks) and compared to intravenous pulse cyclophosphamide (NIH protocol) plus prednisone (1 mg/kg per day and tapered 10% - 20% every 1 - 2 weeks) in a randomized controlled non-inferiority trial. The study involved 140 patients who had Class III, IV and V lupus nephritis. Although this study demonstrated the superiority of oral MMF to intravenous cyclophosphamide (22.5% vs. 5.8%, p = 0.005) in the primary outcomes (within 10% of normal level of serum creatinine, proteinuria, and urine sediment). The result was mainly driven by the urine sediment variable, instead of the parameters that correlated highly with renal outcomes such as serum creatinine and proteinuria. This suggests that the mild chemical cystitis of cyclophosphamide at 6 months may be solely responsible for the positive MMF outcomes. It, however, demonstrated fewer severe infections (1 vs. 6) and reports of amenorrhea (0 vs. 2) with MMF arm as compared to the cyclophosphamide arm.
Finally, the Aspreva Lupus Management Study (ALMS) trial [8] , which is the largest randomized control trial to date, was recently published assessing management of Class III, IV and V lupus nephritis. A total of 370 patients were included in the study. They compared oral MMF (1 - 3 g per day for 24 weeks) and intravenous pulse cyclophosphamide (0.5 - 1 g/m2 monthly) plus oral prednisone (60 mg per day, tapered over the 24 weeks). They used the composite end point of a decrease in proteinuria/creatinine ratio (< 3 with baseline nephrotic range and ≥ 50% in patients with sub-nephrotic baseline), and stabilization or improvement in serum creatinine level at 24 weeks. There was no statistically significant difference in the primary outcomes (56.2% vs. 53.0%, p = 0.58), and there was actually a non-statistically significant trend toward higher infectious rates (68.5% vs. 61.7%, p = 0.17) and death (4.9% vs. 2.8%, p = 0.29) with MMF compared to cyclophosphamide. All the studies are summarized in Table 1.
2.2. Maintenance Therapy
Again, the EuroLupus trial [3,4] compared high and low dose intravenous cyclophosphamide therapies. The low dose and high dose groups received maintenance oral

Table 1. Summary of the major randomized controlled trials for Lupus Nephritis Class III and IV ± V induction therapy.
azathioprine at 3 and 12 months respectively. At 10 years, there were no differences in the serum creatinine and proteinuria between the two groups. Contreras et al. [9] assessed the maintenance therapies in 59 patients with Class III and IV lupus nephritis using intravenous pulse cyclophosphamide (0.5 - 1 g/m2 every 3 months), oral azathioprine (1 - 3 mg/kg per day) and oral MMF (500 - 3000 mg per day) with prednisone (up to 0.5mg/kg per day for 1 - 3 years) for 25 - 30 months after induction therapy with intravenous pulse cyclophosphamide (0.5 - 1 g/m2 monthly) plus steroid for 6 months. The primary outcome was mortality and rate of chronic renal failure, defined by a sustained increase in serum creatinine to at least twice the lowest value reached during the induction phase. There was higher primary outcome rate in the cyclophosphamide group (8) as compare to the MMF (2) and azathioprine (1) groups. The 72-month event free survival rate was significantly lower with the azathioprine group (p = 0.009) and MMF group (p = 0.05) as compared to the cyclophosphamide group (p = 0.009), and there was no statistically significant difference between MMF and azathioprine (p = 0.50). Both the MMF and the azathioprine groups had significant lower rates of hospitalization, amenorrhea, and infections as compared to the cyclophosphamide group. The studies are summarized in Table 2.
2.3. Other Therapies
There have been studies assessing tacrolimus as induction therapy for lupus nephritis. Although a double blind randomized trial by Miyasaka et al. [10] showed a decrease in lupus nephritis disease activity with tacrolimus and glucocorticoid, their control group was glucocorticoid therapy alone. A more recent non-inferiority randomized controlled trial by Chen et al. [11] compared oral prednisone (1 mg/kg per day and taper to 10 mg per
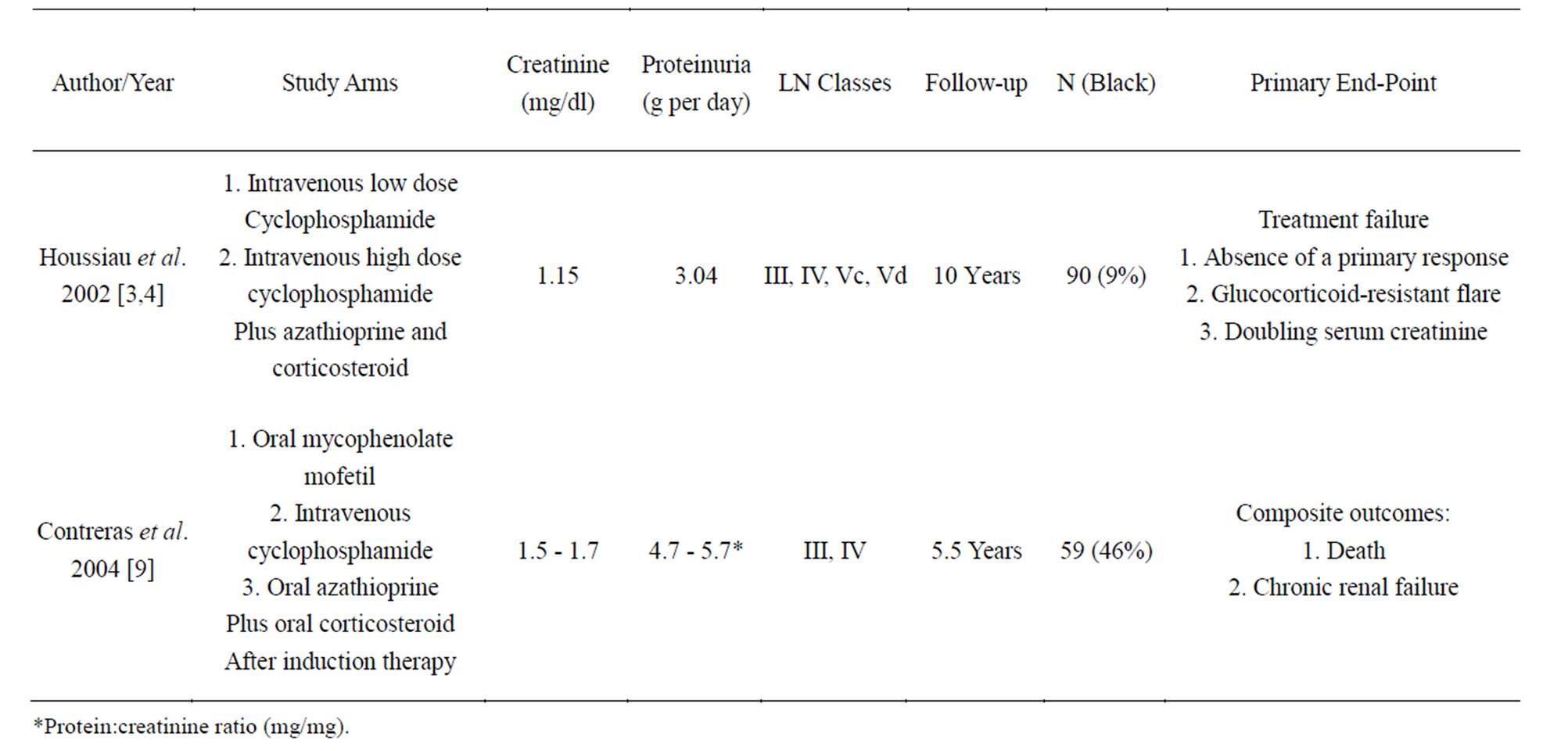
Table 2. Summary of the major randomize control trials for Lupus Nephritis Class III and IV ± V maintenance therapy.
day until month 6) plus either tacrolimus (0.05 mg/kg per day) or intravenous pulse cyclophosphamide (0.5 - 1 g/m2 every 4 weeks) for 6 months as induction therapy in 81 patients with ISN/RPS class III and IV lupus nephritis. The primary outcome was complete remission (stable kidney function, normal urinary sediment, serum albumin ≥ 3.5g/dL and proteinuria < 0.3 g per day). There was a non-statistically significant increase in response rate with tacrolimus (52%) as compared to cyclophosphamide (39%; p = 0.2). There were less adverse effects, such as leucopenia and gastrointestinal symptoms. However, the patients in this study had mean proteinuria of only 0.34 - 0.47 g per day, and more than 67% of patients had serum creatinine < 1.0 mg/dL. Furthermore, 13% of patients had a Class V nephropathy. Although this study showed some promising results with tacrolimus, it is significantly under-powered for a non-inferiority trial. An expected 160 patients were required, but only 81 patients were recruited after 2 years. Therefore, more evidence with long-term follow-up is required before it becomes a standard induction therapy.
There has been one randomized controlled trial comparing standard therapy (prednisone and cyclophosphamide) with or without plasmapheresis in 86 patients with severe lupus nephritis [12]. This study had a mean follow-up of 136 weeks. There was no difference in mortality or renal outcome. As a result, plasmapheresis is not used as a standard therapy for lupus nephritis, unless there are other indications. Rituximab was initially thought to be another promising emerging therapy for Lupus Nephritis. Melander et al. [13] had demonstrated a 60% response rate to rituximab in patients with relapsing or refractory severe lupus nephritis. Although there are no randomized controlled trials that have been completed and published in detail, the 1 year follow-up of the Genentech sponsored Lunar Trial [14] is discouraging. This double blind phase III placebo controlled randomized controlled trial of 144 lupus nephritis patients with class III & IV disease were randomly assigned to receive placebo or 1gram of rituximab on days 1, 15, 168, 182 in conjunction with MMF and corticosteroids. No significant differences were observed in complete or partial response to therapy at week 52.
Stem cell transplantation has also been investigated in treatment of SLE. One retrospective study showed some efficacy of autologous stem cell transplantation in the treatment of SLE in which 65% of cases had renal involvement [15]. However, mortality rate was high (23%). Another single arm prospective trial looked at autologous nonmyeloablative hematopoietic stem cell transplantation in treatment of 50 patients with organor life-threatening visceral involvement [16]. The study showed a treatment related mortality of 4% with overall survival of 84% after 5 years and disease-free survival of 50% after 5 years. Large randomized controlled trials to assess the role of rituximab and autologous stem cell transplantation in severe SLE or lupus nephritis are needed.
3. CLASS V LUPUS NEPHRITIS
Ten to twenty percent of patients with lupus nephritis have Class V lupus membranous nephropathy [17]. Austin et al. [18] published a randomized controlled trial evaluating the efficacy of prednisone (40 mg/m2 every other days for 8 weeks and then tapered to 10 mg/m2every other day for remainder of the 1-year protocol) alone and with addition of intravenous pulse cyclophosphamide (0.5 - 1 g/m2 monthly for 6 months), or cyclosporine (200 mg/m2 per day for 11 months) in 42 patients with Class V lupus nephritis. In the study, the primary outcome was remission (complete remission: < 0.3 g per day proteinuria and partial remission: < 2.0 g/d proteinuria with a > 50% reduction from baseline proteinuria). Both the cyclosporine (83%, p = 0.002) and cyclophosphamide (60%, p = 0.04) groups were superior to the steroid alone arm (27%). There was no difference between cyclosporin and cyclophosphamide groups. By contrast, cyclosporine had a higher relapse rate than the cyclophosphamide arm (p = 0.02). Patients who relapsed after prednisone alone or cyclosporine were treated with cyclophosphamide and achieved an 80% response rate in 36 months.
Radhakrishnan et al. [19] assessed oral MMF (1 g per day increased to maximum 3 g per day) and intravenous pulse cyclophosphamide (NIH protocol) plus oral prednisone (1 mg/kg per day taper to 10 mg per day) as induction therapy in 84 patients with class V lupus nephritis over 24 weeks. The change of urine protein at 24 weeks was the primary end point. There was no difference between the MMF and cyclophosphamide groups in percent change of urine protein (95% confidence interval: –26.9 - 19.9) and percent change of serum creatinine (95% confidence interval: –18.5 to 13.4). The studies are summarized in Table 3.
4. CONCLUSIONS
In conclusion, for Class III and IV ± V lupus nephritis, there is no evidence that oral MMF is superior to cyclophosphamide. However, there are more long-term outcome data available with intravenous cyclophosphamide therapy at this point. As a result, we routinely use the EuroLupus protocol in patients with Class III and IV ± V lupus nephritis as induction therapy. MMF should be considered if fertility is a major concern (only 2 women in low dose IV cyclophosphamide protocol had premature menopause at age 44) or if there are other contraindications to cyclophosphamide. In terms of maintenance therapy, we prefer azathioprine as first line for Class III and IV ± V lupus nephritis due to its proven efficacy, low cost and safety record during pregnancy, although MMF may be considered as an alternative. We do hope that future studies on newer therapies will employ the best proven treatment in the control arm (at present low dose EuroLupus protocol) with a meaningful follow-up time of at least 24 - 36 months and objective end points that have prognostic significance [20].
For Class V lupus nephritis, there is limited evidence available. Cyclosporin, MMF and cyclophosphamide all show benefit compared to steroids alone for reducing proteinuria. Cyclosporin has higher and faster rates of inducing remission, but higher relapse rates compared to cyclophosphamide. The choice of therapy will need to be tailored to the patients’ co-morbidities.
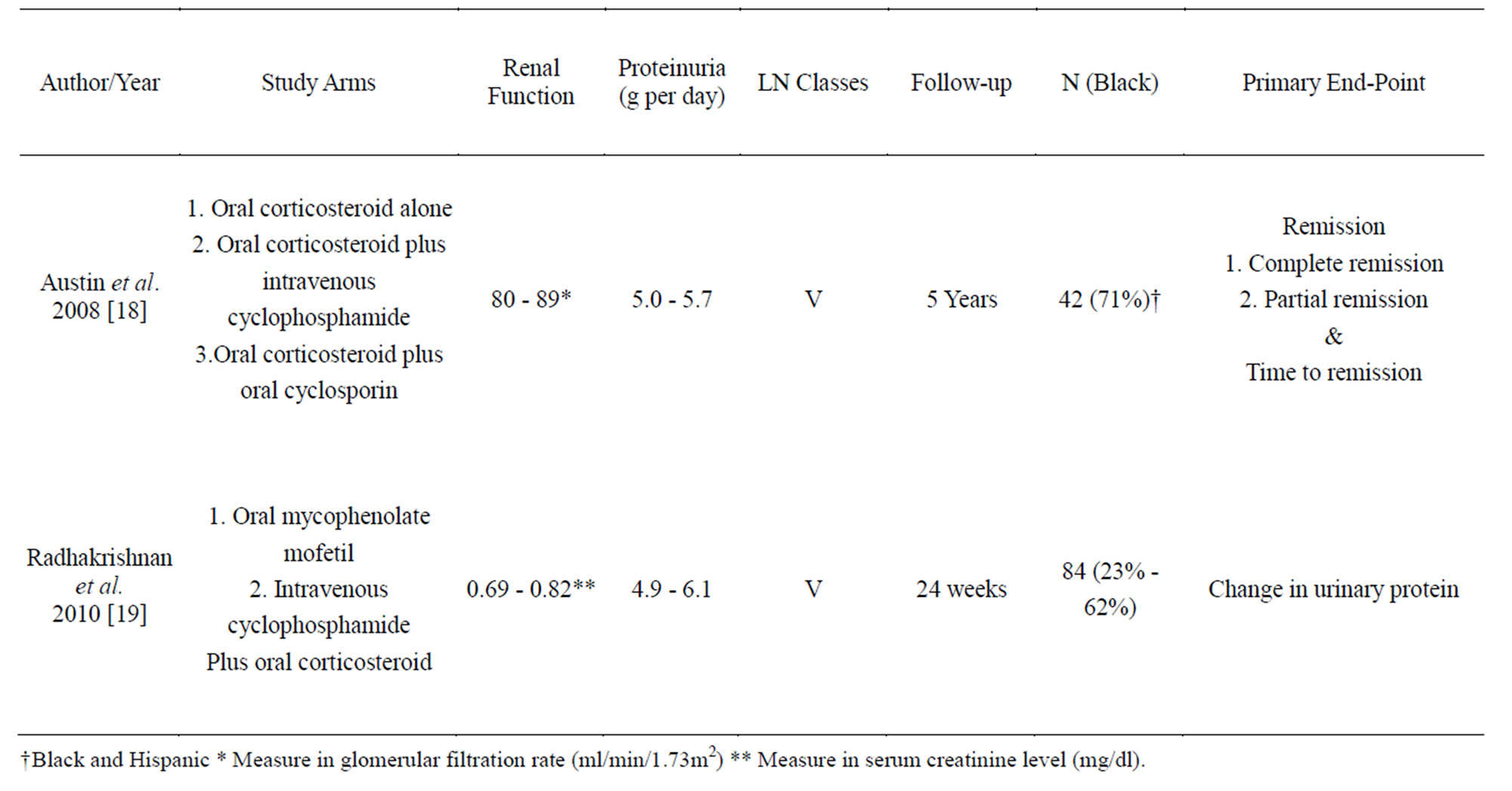
Table 3. Summary of the major randomize control trials for Lupus Nephritis Class V maintenance therapy.
REFERENCES
- Weening, J.J., D’Agati, V.D., Schwartz, M.M., Seshan, S.V., Alpers, C.E., Appel, G.B., Balow, J.E., Bruijn, J.A., Cook, T., Ferrario, F., Fogo, A.B., Ginzler, E.M., Hebert, L., Hill, G., Hill, P., Jennette, J.C., Kong, N.C., Lesavre, P., Lockshin, M., Looi, L.M., Makino, H., Moura, L.A. and Nagata, M. (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology, 15, 241-250. doi:10.1097/01.ASN.0000108969.21691.5D
- Illei, G.G., Austin, H.A., Crane, M., Collins, L., Gourley, M.F., Yarboro, C.H., Vaughan, E.M., Kuroiwa, T., Danning, C.L., Steinberg, A.D., Klippel, J.H., Balow, J.E. and Boumpas, D.T. (2001) Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Annals of Internal Medicine, 135, 248-257.
- Houssiau, F.A., Vasconcelos, C., D’Cruz, D., Sebastiani, G.D., Garrido Ed, E.R., Danieli, M.G., Abramovicz, D., Blockmans, D., Mathieu, A., Direskeneli, H., Galeazzi, M., et al. (2002) Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis & Rheumatism, 46, 2121-2131. doi:10.1002/art.10461
- Houssiau, F.A., Vasconcelos, C., D’Cruz, D., Sebastiani, G.D., de Ramon, G.E., Danieli, M.G., Abramovicz, D., Blockmans, D., Cauli, A., Direskeneli, H., Galeazzi, M., Gul, A., Levy, Y., Petera, P., Popovic, R., Petrovic, R., Sinico, R.A., Cattaneo, R., Font, J., Depresseux, G., Cosyns, J.P. and Cervera, R. (2010) The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing lowdose and high-dose intravenous cyclophosphamide. Annals of the Rheumatic Diseases, 69, 61-64. doi:10.1136/ard.2008.102533
- Chan, T.M., Tse, K.C., Tang, C.S., Mok, M.Y. and Li, F.K. (2005) Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. Journal of the American Society of Nephrology, 16, 1076-1084. doi:10.1681/ASN.2004080686
- Ong, L.M., Hooi, L.S., Lim, T.O., Goh, B.L., Ahmad, G., Ghazalli, R., Teo, S.M., Wong, H.S., Tan, S.Y., Shaariah, W., Tan, C.C. and Morad, Z. (2005) Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology, 10, 504-510. doi:10.1111/j.1440-1797.2005.00444.x
- Ginzler, E.M., Dooley, M.A., Aranow, C., Kim, M.Y., Buyon, J., Merrill, J.T., Petri, M., Gilkeson, G.S., Wallace, D.J., Weisman, M.H. and Appel, G.B. (2005) Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. The New England Journal of Medicine, 353, 2219-2228. doi:10.1056/NEJMoa043731
- Appel, G.B., Contreras, G., Dooley, M.A., Ginzler, E.M., Isenberg, D., Jayne, D., Li, L.S., Mysler, E., SanchezGuerrero, J., Solomons, N. and Wofsy, D. (2009) Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. Journal of the American Society of Nephrology , 20, 1103-1112. doi:10.1681/ASN.2008101028
- Contreras, G., Pardo, V., Leclercq, B., Lenz, O., Tozman, E., O’Nan, P. and Roth, D. (2004) Sequential therapies for proliferative lupus nephritis. The New England Journal of Medicine, 350, 971-980. doi:10.1056/NEJMoa031855
- Miyasaka, N., Kawai, S. and Hashimoto, H. (2009) Efficacy and safety of tacrolimus for lupus nephritis: A placebo-controlled double-blind multicenter study. Modern Rheumatology, 19, 606-615. doi:10.1007/s10165-009-0218-5
- Chen, W., Tang, X., Liu, Q., Chen, W., Fu, P., Liu, F., Liao, Y., Yang, Z., Zhang, J., Chen, J., Lou, T., Fu, J., Kong, Y., Liu, Z., Fan, A., Rao, S., Li, Z. and Yu, X. (2011) Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: A multicenter randomized clinical trial. American Journal of Kidney Diseases, 57, 235-244. doi:10.1053/j.ajkd.2010.08.036
- Lewis, E.J., Hunsicker, L.G., Lan, S.P., Rohde, R.D. and Lachin, J.M. (1992) A controlled trial of plasmapheresis therapy in severe lupus nephritis. New England Journal of Medicine, 326, 1373-1379. doi:10.1056/NEJM199205213262101
- Melander, C., Sallee, M., Trolliet, P., Candon, S., Belenfant, X., Daugas, E., Remy, P., Zarrouk, V., Pillebout, E., Jacquot, C., Boffa, J.J., Karras, A., Masse, V., Lesavre, P., Elie, C., Brocheriou, I., Knebelmann, B., Noel, L.H. and Fakhouri, F. (2009) Rituximab in severe lupus nephritis: Early B-cell depletion affects long-term renal outcome. Clinical Journal of the American Society of Nephrology, 4, 579-587. doi:10.2215/CJN.04030808
- Bosch, X. (2010) Inflammation: Rituximab in ANCA vasculitis and lupus: Bittersweet results. Nature Reviews Nephrology, 6, 137-139. doi:10.1038/nrneph.2010.13
- Jayne, D., Passweg, J., Marmont, A., Farge, D., Zhao, X., Arnold, R., Hiepe, F., Lisukov, I., Musso, M., Ou-Yang, J., Marsh, J., Wulffraat, N., Besalduch, J., Bingham, S.J., Emery, P., Brune, M., Fassas, A., Faulkner, L., Ferster, A., Fiehn, C., Fouillard, L., Geromin, A., Greinix, H., Rabusin, M., Saccardi, R., Schneider, P., Zintl, F., Gratwohl, A. and Tyndall, A. (2004) Autologous stem cell transplantation for systemic lupus erythematosus. Lupus, 13, 168-176. doi:10.1191/0961203304lu525oa
- Burt, R.K., Traynor, A., Statkute, L., Barr, W.G., Rosa, R., Schroeder, J., Verda, L., Krosnjar, N., Quigley, K., Yaung, K., Villa, B.M., Takahashi, M., Jovanovic, B., Oyama, Y. (2006) Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. Journal of the American Medical Association, 295, 527-535. doi:10.1001/jama.295.5.527
- Korbet, S.M. (1999) Membranous lupus glomerulonephritis. Oxford University Press, Oxford, 219-240.
- Austin III, H.A., Illei, G.G., Braun, M.J., Balow, J.E. (2009) Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. Journal of the American Society of Nephrology, 20, 901-911. doi:10.1681/ASN.2008060665
- Radhakrishnan, J., Moutzouris, D.A., Ginzler, E.M., Solomons, N., Siempos, I.I. and Appel, G.B. (2010) Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney International, 77, 152-160. doi:10.1038/ki.2009.412
- Clark, W.F. and Sontrop, J.M. (2008) What have we learned about optimal induction therapy for lupus nephritis (III through V) from randomized, controlled trials? Clinical Journal of the American Society of Nephrology, 3, 895-898. doi:10.2215/CJN.00170108

