Optics and Photonics Journal
Vol.3 No.6A(2013), Article ID:38498,6 pages DOI:10.4236/opj.2013.36A003
A Highly Luminous LiCaPO4:Eu2+ Phosphor Synthesized by a Solution Method Employing a Water-Soluble Phosphate Ester
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Japan
Email: *kakihana@tagen.tohoku.ac.jp
Copyright © 2013 Minsung Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 19, 2013; revised July 23, 2013; accepted August 26, 2013
Keywords: Solution Method; Stable Phosphate Ester; Homogeneity; LiCaPO4:Eu2+
ABSTRACT
A LiCaPO4:Eu2+ phosphor with high photoluminescence was synthesized using a polymerizable complex (PC) method employing a water-soluble polyethylene glycol-conjugated phosphate ester (PEG-P). PEG-P could be obtained from a reaction among polyethylene glycol 300, phosphorus pentoxide, and pyrophosphoric acid. The PEG-P prepared was stable in an aqueous condition. A transparent solution and gel were obtained when the PEG-P was used as a source of P during the PC method, whereas the use of H3PO4 caused an undesirable precipitate. The LiCaPO4:Eu2+ obtained via the PC method employing the PEG-P showed higher emission intensity than those synthesized by a solid state reaction method and the PC method employing H3PO4. The high luminescence properties of the sample synthesized using the PEG-P may be attributed to high homogeneity of constituents in the sample.
1. Introduction
Phosphate phosphors have been well-known as one of the most important luminescence materials because of their excellent thermal stability and high emission intensity as well as lower temperature synthesis [1,2]. Among them, Eu2+-activated ABPO4 phosphors (A and B are monoand divalent cations, respectively) have been reported as blue-emitting phosphors excited by near UVLEDs. For instance, KSrPO4:Eu2+, KBaPO4:Eu2+, and LiCaPO4:Eu2+ have excellent luminescence properties including quantum efficiency and thermal quenching behavior. Therefore, they are considered to be potential application as phosphors for the white light emitting diodes [3-5].
Several kinds of methods were applied to synthesis of inorganic powders, such as solid state reaction (SSR), solution-based method, and combustion process. In the synthesis of phosphors, the SSR method is the most extensively used. However, the SSR method includes some drawbacks such as low homogeneity, and non-uniform particles morphology and size. Generally, solution-based synthesis is considered to be a desirable approach because it can produce highly homogeneous compounds in the atomic level [6,7]. Homogeneity of constituents and control of morphology and size of particles are requisites for highly efficient phosphors. One of limitations in a synthesis of phosphate phosphors using solution methods is requirement of appropriate P source, which is soluble and stable in an aqueous condition. The use of conventional phosphate reagents such as phosphoric acid or ammonium phosphate produce precipitates with metal ions in an aqueous condition [8]. Precipitates would lead to samples with inhomogeneous composition accompanying by secondary phases. In ion-activated type phosphors, distribution of an activator is closely connected to luminescence intensity in the view of concentration quenching. It indicates that inhomogeneity results in low performance of phosphor. Additionally, in a highly homogeneous sample, high amount of rare-earth can be doped, and as a result, high luminescence intensity would be achieved.
Solution-based synthesis highly requires the use of appropriate raw materials, which do not produce precipitate with any metals present in a given aqueous solution. Therefore, development of a new water-soluble P source that does not form precipitates with other metal ions is indispensable to realization of synthesis of extremely homogeneous phosphate compounds with high performance by aqueous solution-based methods. It is known that precipitates between phosphorus and metals could be prevented by introduction of condensed chain-structured phosphates [9,10]. Recently, we have succeeded in a synthesis of a phosphate phosphor with high luminescence by a solution method using such a phosphate oligomer [11]. The prepared oligomer had good solubility and stability in an aqueous condition, though approximately 50% H3PO4 unreacted remained. Therefore, it can be expected that if a phosphorus source with further less H3PO4 can be prepared, the range of synthesis of phosphate phosphors with even better photoluminescence properties can be greatly expanded. The condensed chain-structured phosphates are commercially available and are synthesized from a rather simple reaction among alcohol, phosphorus pentoxide, and polyphosphoric acid [12]. Especially, polyethylene glycol (PEG) is considered to be a good candidate for its condensation with the phosphate moiety because PEG is widely used as a cross-linking agent for promoting formation of “gel” in a variety of “sol-gel”-based solution methods. Polyethylene glycol-conjugated phosphate ester (PEG-P) is considered to be soluble and stable in an aqueous solution, and also PEG-P may have a role as a cross-linking agent. In this study, we report the synthesis of PEG-P whose chemical structures are deduced from 1H and 31P{1H} NMR measurements, and then we stress the great advantage of the use of PEG-P as a P source for the synthesis of Eu2+-doped LiCaPO4, chosen as a model among phosphate-based phosphors, by demonstrating the superior photoluminescence properties of the target phosphor synthesized by the polymerizable complex (PC) method employing the PEG-P instead of the conventional P source, that is H3PO4.
2. Experimental Section
2.1. Synthesis of PEG-P
Pyrophosphoric acid (10 mmol phosphorus, Kanto Chemical) and polyethylene glycol 300 (PEG300, 10 mmol Kanto Chemical) were mixed at 323 K and kept for 2 h. Phosphorus pentoxide P4O10 (10 mmol phosphorus, Wako Chemical) was weighed in an inert atmosphere to prevent its hydrolysis, and then slowly added into the mixture. After P4O10 was dispersed homogeneously, temperature of the mixture increased to 358 K and kept for 5 h. Finally, 10 mmol of PEG300 was added, and the reaction was continued for 12 h at 358 K. The entire reaction was conducted in N2 atmosphere. Gel with high viscosity was formed and it was dissolved in distilled water. The gel was dissolved in D2O, and pH was adjusted to 13 using 10 M NaOH to prepare a solution for NMR analysis. 1H and 31P{1H} NMR spectra of the prepared PEG-P were recorded on a Bruker AVANCE 400 spectrometer (400 MHz for 1H resonance) to analyze the chemical structure of PEG-P. The phosphorus concentration of diluted PEG-P was determined using an inductively-coupled plasma (ICP) method (Perkin Elmer; Optima 3300XL) prior to its use for the synthesis of Eu2+-doped LiCaPO4 by the PC method described in the following section.
2.2. Preparation of Eu2+-Doped LiCaPO4
A Eu2+-doped LiCaPO4 phosphor was synthesized via the PC method employing the PEG-P as a P source. LiNO3 (99%, Kanto Chemical), Ca(NO3)2∙4H2O (99.5%, Kanto Chemical), and Eu(NO3)3, which was prepared by dissolution of Eu2O3 in HNO3, were dissolved in a citric acid (CA) solution at a ratio of Li:Ca:Eu:CA = 1:0.97:0.03:8. The mixture was firstly heated at 353 K to allow chelation for 2 h, and then, the PEG-P and propylene glycol (PG) were added into the solution at a molar ratio of 1:8. The temperature was subsequently increased to 423 K to promote gel formation. The formed gel was heated at 1123 K in air to remove the organic content, and then it was reduced at 1373 K under a flow of Ar containing 4% H2 for 3 h. To enhance the phase purity, post-heat treatment was conducted at 1073 K for 18 h in an Ar/4%H2 atmosphere. A Eu2+-doped LiCaPO4 phosphor was also synthesized by the PC method using H3PO4 as the P source. In addition, synthesis using the SSR method was carried out for comparison by stoichiometric mixing raw materials including Eu2O3 (Furuuchi Chemical), and Li2CO3 (Wako Chemical), CaCO3, and (NH4)H2PO4 (both from Kanto Chemical). X-ray diffraction analysis (XRD, Bruker AXS; D2 Phaser) was conducted. Excitation and emission spectra of phosphors were recorded using a fluorescence spectrometer (Hitachi; F-4500) at room temperature. Quantum efficiencies of the samples were evaluated using fluorescence spectrometer (Jasco; FP-6500).
3. Results and Discussion
Scheme 1 shows estimated chemical structures of the synthesized PEG-P; (a) monoand (b) di-esters, and Figure 1 shows 1H (a) and 31P{1H} (b) NMR spectra of the obtained PEG-P. In the 1H NMR spectra (Figure 1(a)), each peak was assigned to each hydrogen marked with “1 - 6” in Scheme 1(a). No peaks of “4” and “5” were observed in 1H NMR spectra of PEG and H3PO4 (not shown here), which in turn indicates that the observation of these peaks evidences formation of a conjugation between PEG300 and phosphate. The 31P{1H} NMR spectrum (Figure 1(b)) indicates that only one side OH group of PEG was conjugated with phosphate. The integration
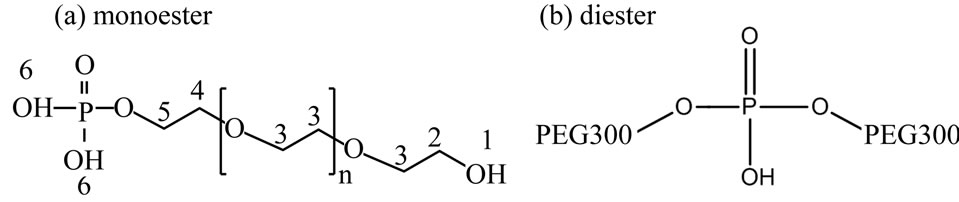
Scheme 1. Estimated chemical structures of synthesized PEG-P: (a) monoester, (b) diester.
area corresponding to the peak “2” was greatly decreased after the reaction, and finally, it could be estimated that more than 90% of PEG 300 was bonded with phosphate. From the 31P{1H} NMR spectrum, it could be confirmed that the PEG-P contained H3PO4, monoester, and diester with ratio of 17.4:72.5:10.1 (Figure 1(b)). Previously, we reported preparation of a phosphate oligomer starting from ethylene glycol and phosphoric acid by promoting their esterification reaction under reflux [11]. The yield of the phosphate oligomer was about 55.4% with 44.6% of unreacted H3PO4. This indicates that the present method can produce the condensed chain-structured phosphate with less H3PO4 than the reported method. The use of such a P source having a large proportion of esters containing a small amount of H3PO4 is considered to be suitable for solution-based synthesis of phosphate-based phosphors because of less opportunity that precipitates form resulting from interaction between H3PO4 and metal ions present in a given solution. Another important characteristic of PEG-P is its stability in H2O, which was confirmed by the fact that the initial small proportion of H3PO4 remained unchanged over 2 months.
To demonstrate the advantages of the use of PEG-P in a solution-based method, a LiCaPO4 doped with Eu2+ phosphor was synthesized by the PC method employing the PEG-P. Figure 2 shows XRD patterns of LiCaPO4: Eu0.03 synthesized by the PC method employing the PEG-P under various conditions; (a) 1123 K for 3 h in air, (b) 1373 K for 3 h in Ar/4%H2 atmosphere, and (c) postheating of (b) at 1073 K for 18 h. No precipitate was formed when the PEG-P was added into an aqueous solution containing LiNO3, Ca(NO3)2·4H2O, and Eu(NO3)3. When the sample obtained after the heat-treatment at 1123 K in air, a single phase LiCaPO4 was formed without any impurity phases as shown in Figure 2(a). Synthesis of a single phase LiCaPO4:Eu2+ phosphor was rarely achieved in previous studies, and they suffered from significant contamination by impurities, such as Li3PO4 and Ca3(PO4)2 [13-15]. It should therefore be stressed here that it was possible to obtain a single phase of LiCaPO4 resulting from the achievement of highly homogeneous distribution of constituents. Reduction at 1373 K for 3 h gave the sample exhibiting the highest emission intensity among various reduction temperatures (1073 - 1473 K). However, at this relatively higher temperature, strong reflections due to impurity phases such as Li3PO4 and Ca3(PO4)2 showed up (Figure 2(b)).
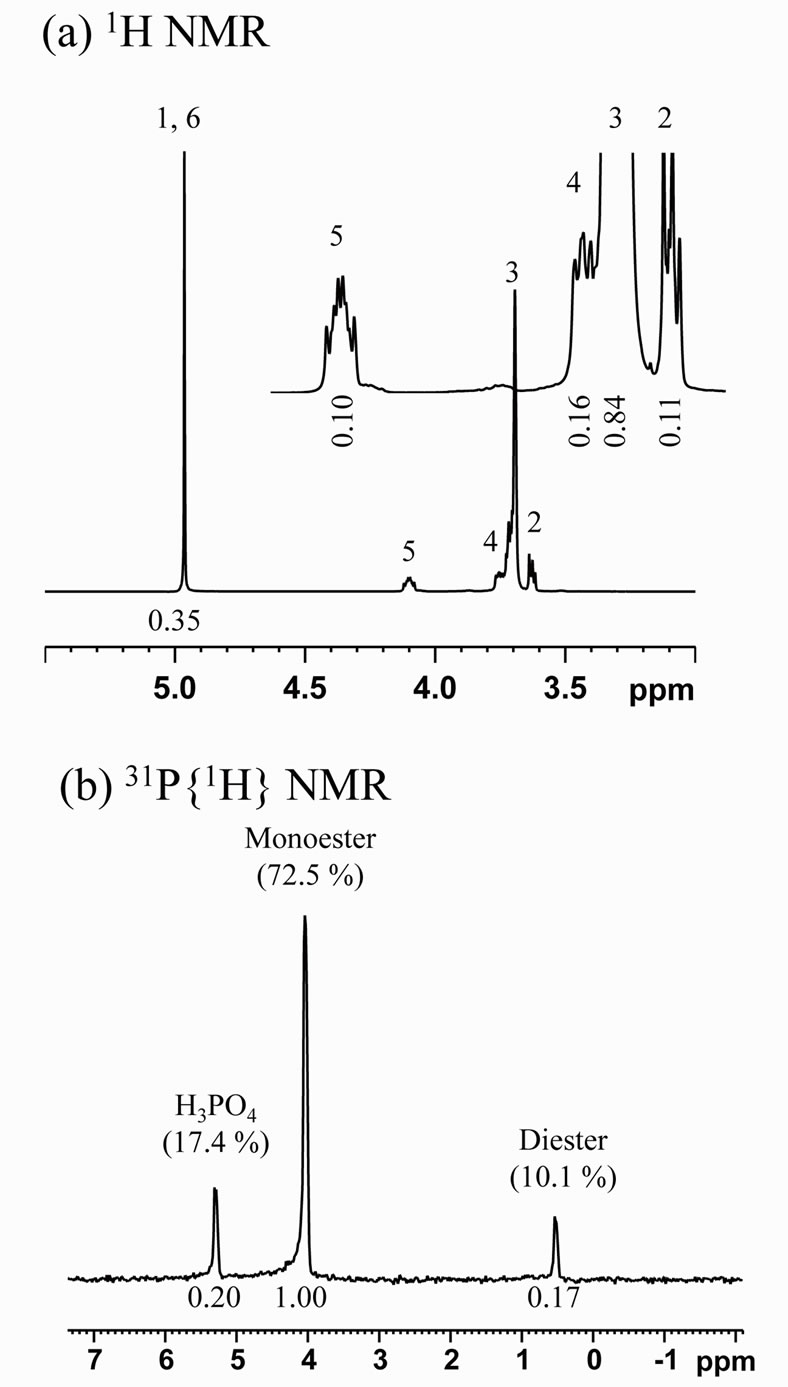
Figure 1. 1H and 31P{1H} NMR spectra of PEG-P in D2O at pH 13.
This is due to decomposition of LiCaPO4 at such a high temperature. Reaction between Li3PO4 and Ca3(PO4)2 appears to be possible when the corresponding phase diagram for these two compounds is taken into account [15,16]. The post-heat treatment of the above-mentioned sample at 1073 K for 18 h (Figure 2(c)) resulted in almost complete elimination of Li3PO4 and Ca3(PO4)2, which accompanied formation of LiCaPO4 resulting from a back reaction between the two impurities, and consequently an almost single phase of LiCaPO4 was formed with a very tiny amount of Ca3(PO4)2.
Figure 3 shows XRD patterns of LiCaPO4: synthesized by the PC method employing PEG-P or H3PO4 and the SSR method after post-heat treatment at 1073 K for 18 h. All the samples mainly consisted of LiCaPO4 as assigned to the JCPDS Card (LiCaPO4, No. 79-1396). The sample synthesized by the PC method employing PEG-P was the almost single phase LiCaPO4 with negligible extent of impurity phase, whereas the samples synthesized by the PC method using phosphoric acid and SSR method contained a large amount of impurities, which were Li3PO4 and Ca3(PO4)2. During the PC method employing H3PO4, precipitate was observed in
synthesized by the PC method employing PEG-P or H3PO4 and the SSR method after post-heat treatment at 1073 K for 18 h. All the samples mainly consisted of LiCaPO4 as assigned to the JCPDS Card (LiCaPO4, No. 79-1396). The sample synthesized by the PC method employing PEG-P was the almost single phase LiCaPO4 with negligible extent of impurity phase, whereas the samples synthesized by the PC method using phosphoric acid and SSR method contained a large amount of impurities, which were Li3PO4 and Ca3(PO4)2. During the PC method employing H3PO4, precipitate was observed in

Figure 2. XRD patterns of LiCaPO4:Eu0.03 phosphors synthesized by the PC method; (a) calcination at 1123 K for 3 h, (b) reduction at 1373 K for 3 h, and (c) post-heating at 1073 K for 18 h.
the mixture solution while PEG-P didn’t make any precipitate. It can be expected that low homogeneity led to formation of a large amount of Li3PO4 and Ca3(PO4)2 and these phases remained even after post-heat treatment. As mentioned above, phase purity is closely related to photoluminescence properties. The use of a novel PEG-P as a P source in the PC method could improve phase purity of LiCaPO4, and it is one of advantages of the PEG-P in the PC method.
Figure 4 shows the SEM and the corresponding EDS maps of Eu in LiCaPO4: phosphors prepared by the SSR method (a, b) and the PC method employing PEG-P (c, d) after the post-heat treatment. The state of Eu distribution is closely related to the photoluminescence properties of given phosphors [7]. 7 mol% Eudoped samples were analyzed to obtain clearer images of Eu distribution. As to the sample synthesized using the SSR method, inhomogeneity, especially, deficient Eu area as marked with the white arrow in Figure 4(b) corresponding to the black arrow in Figure 4(a) could be observed. On the other hand, as to the sample prepared using the PC method, the EDS mapping image showed uniform distribution of Eu without observation of deficient and localization of Eu ions. These results suggest that the PC method allowed highly homogeneous distribution of each element throughout the Eu2+-doped LiCaPO4 phosphor, which may result in improvement of the corresponding photoluminescence properties.
phosphors prepared by the SSR method (a, b) and the PC method employing PEG-P (c, d) after the post-heat treatment. The state of Eu distribution is closely related to the photoluminescence properties of given phosphors [7]. 7 mol% Eudoped samples were analyzed to obtain clearer images of Eu distribution. As to the sample synthesized using the SSR method, inhomogeneity, especially, deficient Eu area as marked with the white arrow in Figure 4(b) corresponding to the black arrow in Figure 4(a) could be observed. On the other hand, as to the sample prepared using the PC method, the EDS mapping image showed uniform distribution of Eu without observation of deficient and localization of Eu ions. These results suggest that the PC method allowed highly homogeneous distribution of each element throughout the Eu2+-doped LiCaPO4 phosphor, which may result in improvement of the corresponding photoluminescence properties.
Figure 5 shows excitation and emission spectra of the LiCaPO4: phosphors synthesized using the SSR method and the PC method employing the PEG-P or H3PO4 after post-heat treatment at 1073 K for 18 h. The sample synthesized by the PC method using H3PO4 showed the lowest emission intensity. As stated above, the emission intensity of materials is closely related to the homogeneity of each element. The formation of pre-
phosphors synthesized using the SSR method and the PC method employing the PEG-P or H3PO4 after post-heat treatment at 1073 K for 18 h. The sample synthesized by the PC method using H3PO4 showed the lowest emission intensity. As stated above, the emission intensity of materials is closely related to the homogeneity of each element. The formation of pre-
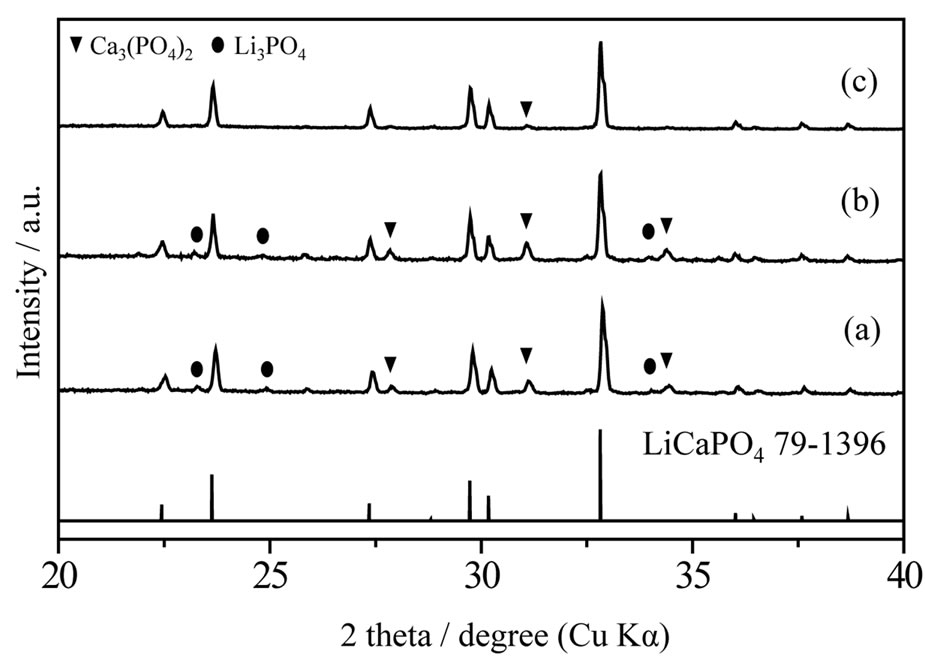
Figure 3. XRD profiles of LiCaPO4: phosphors after post-heating synthesized by the SSR method (a) and PC method employing H3PO4 (b) and PEG-P(c).
phosphors after post-heating synthesized by the SSR method (a) and PC method employing H3PO4 (b) and PEG-P(c).
cipitate between H3PO4 and metals during the mixing step in the PC method implies poor homogeneity despite of a solution-based method. The SSR method could produce better condition of sample compared to that of the PC method employing H3PO4, because it exhibited higher emission intensity. Formation of undesirable precipitate in the PC method is thought to be a critical factor for homogeneity. The strongest emission intensity was observed from the sample synthesized via the PC method using the PEG-P as a P source. The internal quantum efficiencies of LiCaPO4: prepared using the SSR method and the PC method employing PEG-P under excitation at 375 nm were 53.7% and 67.6%, respectively, although the corresponding absorption rate (81.3%) of the sample prepared by the PC method using PEG-P was slightly smaller than that of the one prepared by the SSR method (82.7%). The PEG-P was stable in the aqueous condition and the use of PEG-P didn’t produce any precipitates with coexisting metal ions, and it allowed us to obtaining a highly homogeneous phosphor sample. As a result, despite of a lower absorption rate, the sample obtained from the PC method using PEG-P showed the high emission intensity and enhanced quantum efficiency.
prepared using the SSR method and the PC method employing PEG-P under excitation at 375 nm were 53.7% and 67.6%, respectively, although the corresponding absorption rate (81.3%) of the sample prepared by the PC method using PEG-P was slightly smaller than that of the one prepared by the SSR method (82.7%). The PEG-P was stable in the aqueous condition and the use of PEG-P didn’t produce any precipitates with coexisting metal ions, and it allowed us to obtaining a highly homogeneous phosphor sample. As a result, despite of a lower absorption rate, the sample obtained from the PC method using PEG-P showed the high emission intensity and enhanced quantum efficiency.
4. Conclusion
A stable and water soluble phosphate ester was prepared by the reaction using pyrophosphoric acid, phosphorus pentoxide, and PEG300. The method could produce P source with less H3PO4. It was confirmed that the synthesized PEG-P didn’t make any precipitate in a mixture of metal salts while the use of H3PO4 resulted in formation of a precipitate. An almost single phase of LiCaPO4 was obtained using the PEG-P, while SSR and PC method using H3PO4 led to formation of impurity phases, which were Li3PO4 and Ca3(PO4)2. As a result, the phosphor prepared by the PC method using the synthesized
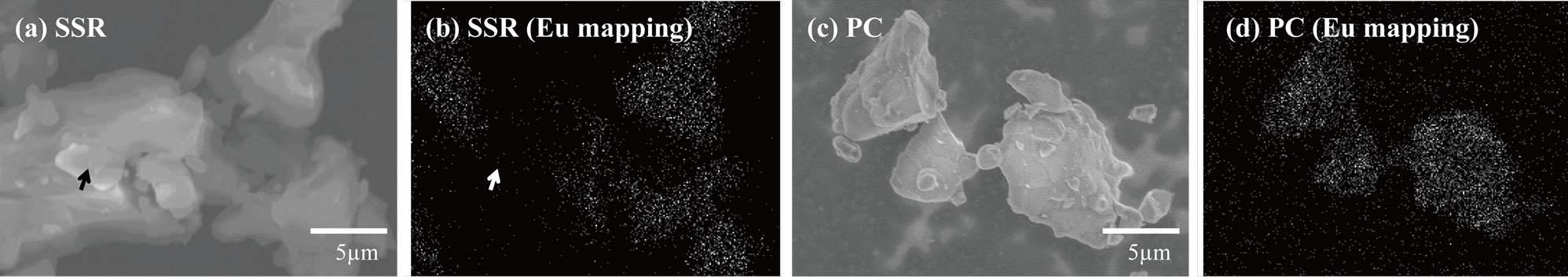
Figure 4. SEM micrographs and corresponding EDS mapping images of Eu ion in LiCaPO4: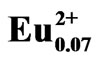 phosphors prepared by (a), (b) SSR method, and (c), (d) PC method using PEG-P.
phosphors prepared by (a), (b) SSR method, and (c), (d) PC method using PEG-P.
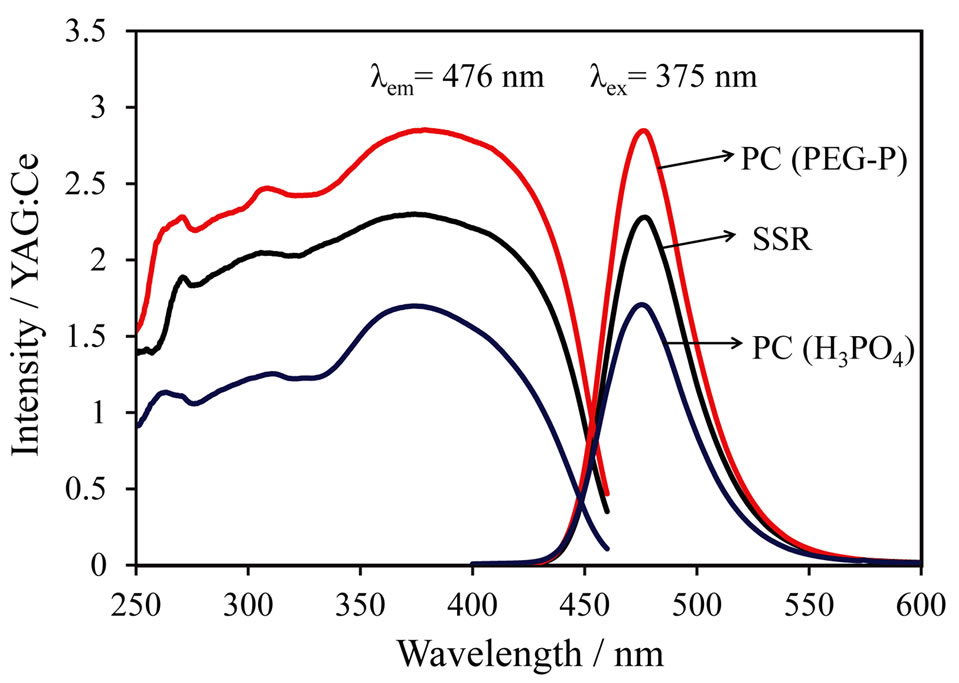
Figure 5. Excitation and Emission spectra of LiCaPO4: 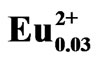 phosphors synthesized by SSR and PC method employing H3PO4 or PEG-P.
phosphors synthesized by SSR and PC method employing H3PO4 or PEG-P.
PEG-P exhibited enhanced photoluminescence properties, such as the highest emission intensity and quantum efficiency compared to other methods. Enhanced photoluminescence properties seemed to be attributed to homogeneous distribution of constituents in the atomic level. Therefore, the PEG-P is expected to be applicable in the solution-based synthesis of various kinds of phosphate-based ceramic compositions with enhanced material properties.
5. Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas of “Fusion Materials: Creative Development of Materials and Exploration of Their Function through Molecular Control” (no. 2206) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and a Grantin-Aid for JSPS Fellows (24∙9285) from Japan Society for the Promotion of Science (JSPS).
REFERENCES
- S. H. M. Poort, W. Janssen and G. Blasse, “Optical Properties of Eu2+-Activated Orthosilicates and Orthophosphates,” Journal of Alloys and Compound, Vol. 260, No. 1-2, 1997, pp. 93-97. http://dx.doi.org/10.1016/S0925-8388(97)00140-0
- Y. S. Tang, S. F. Hu, C. C. Lin, N. C. Bagkar and R. S. Liu, “Thermally Stable Luminescence of KSrPO4:Eu2+ Phosphor for White Light UV Light-Emitting Diodes,” Applied Physics Letter, Vol. 90, No. 15, 2007, Article ID. 151108. http://dx.doi.org/ 10.1063/1.2721846
- M. E. Hannah, A. P. Piquette, M. Anc, J. McKittrick, J. Talbot, J. Han and K. C. Mishra, “A Study of Blue Emitting Phosphors, ABPO4,” ECS Transaction, Vol. 41, No. 37, 2012, pp. 19-25. http://dx.doi.org/10.1149/1.3697441
- S. Zhang, Y. Huang and H. J. Seo, “The Spectroscopy and Structural Sites of Eu2+ Ions Doped KCaPO4 Phosphor,” Journal of Electrochemical Society, Vol. 157, No. 7, 2010, pp. J261-J266. http://dx.doi.org/10.1149/1.3429887
- S. D. More, M. N. Meshram, S. P. Wankhede, P. L. Muthal, S. M. Dhopte and S. V. Moharil, “Luminescence in LiCaPO4,” Physica B: Condensed Matter, Vol. 406, No. 5, 2011, pp. 1178-1181. http://dx.doi.org/10.1016/j.physb.2010.12.077
- M. Kakihana, “‘sol-gel’ Preparation of High Temperature Superconducting Oxides,” Journal of Sol-Gel Science and Technology, Vol. 6, No. 1, 1996, pp. 7-55. http://dx.doi.org/10.1007/BF00402588
- M. Kakihana, “Synthesis of High-Performance Ceramics Based on Polymerizable Complex Method,” Journal of Ceramic Society of Japan, Vol. 117, No. 1368, 2009, pp. 857-862. http://dx.doi.org/10.2109/jcersj2.117.857
- W. Weng, L. Huang and G. Han, “The Alkoxide Sol-Gel Process in the Calcium Phosphate System and Its Applications,” Applied Organometallic Chemistry, Vol. 13, No. 8, 1999, pp. 555-564. http://dx.doi.org/10.1002/(SICI)1099-0739(199908)13:8<555::AID-AOC913>3.0.CO;2-C
- J. R. Van Wazer and C. F. Callis, “Metal Complexing By Phosphates,” Chemical Reviews, Vol. 58, No. 6, 1958, pp. 1011-1046. http://dx.doi.org/10.1021/cr50024a001
- F. Rashchi and J. A. Finch, “Polyphosphates: A review Their Chemistry and Application with Particular Reference to Mineral Processing,” Minerals Engineering, Vol. 13, No. 10-11, 2000, pp. 1019-1035. http://dx.doi.org/0.1016/S0892-6875(00)00087-X
- M. Kim, M. Kobayashi, H. Kato and M. Kakihana, “Enhancement of Luminescence Properties of a KSrPO4:Eu2+ Phosphor Prepared Using a Solution Method with a Water-Soluble Phosphate Oligomer,” Journal of Materials Chemistry C, Vol. 1, No. 36, 2013, pp. 5741-5746. http://dx.doi.org/10.1039/c3tc31121j
- D. J. Tracy and R. L. Reierson, “Commercial Synthesis of Monoalkyl Phosphates,” Journal of Surfactants and Detergents, Vol. 5, No. 2, 2002, pp. 169-172. http://dx.doi.org/10.1007/s11743-002-0218-9
- P. Lightfoot, M. C. Pienkowski, P. G. Brucea, I. Abrahamd and E. Eh, “Synthesis and Structure of LiCaPO4, by Combined X-Ray and Neutron Powder Diffraction,” Journal of Materials Chemistry, Vol. 1, No. 6, 1991, pp. 1061-1063. http://dx.doi.org/10.1039/jm9910101061
- C. Wan, J. Meng, F. Zhang, X. Deng and C. Yang, “An Efficient Blue-Emitting Phosphor LiCaPO4:Eu2+ for White LEDs,” Solid State Communications, Vol. 150, No. 31-32, 2010, pp. 1493-1495. http://dx.doi.org/10.1016/j.ssc.2010.05.037
- J. K. Han, M. E. Hannah, A. Piquette, J. B. Talbot, K. C. Mishra and J. McKittrick, “Sol-Gel Synthesis of Single Phase, High Quantum Efficiency LiCaPO4:Eu2+ Phosphors,” ECS Journal of Solid State Science and Technology, Vol. 1, No. 1, 2012, pp. R37-R40. http://dx.doi.org/10.1149/2.026201jss
- T. Goto, N. Wakamatsu, H. Kamemizu, M. Iijima, Y. Doi and Y. Moriwaki, “Sintering Mechanism of Hydroxyapatite by Addition of Lithium Phosphate,” Journal of Materials Science: Materials in Medicine, Vol. 2, No. 3, 1991, pp. 149-152. http://dx.doi.org/10.1007/BF00692973
NOTES
*Corresponding author.

