Journal of Surface Engineered Materials and Advanced Technology
Vol.4 No.2(2014), Article ID:45005,9 pages DOI:10.4236/jsemat.2014.42010
Effect of Tubular Chiralities and Diameters of Single Carbon Nanotubes on Gas Sensing Behavior: A DFT Analysis
A. A. EL-Barbary1,2*, Kh. M. Eid1,3, M. A. Kamel1, H. M. Osman1, G. H. Ismail1,2
1Physics Department, Faculty of Education, Ain Shams University, Cairo, Egypt
2Physics Department, Faculty of Science, Jazan University, Jazan, KSA
3Bukairiayh for Science, Qassim University, Buraydah, KSA
Email: *ahla_eg@yahoo.co.uk
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 January 2014; revised 12 February 2014; accepted 11 March 2014
ABSTRACT
Using density functional theory, the adsorption of CO, CO2, NO and CO2 gas molecules on different chiralities and diameters of single carbon nanotubes is investigated in terms of energetic, electronic properties and surface reactivity. We found that the adsorption of CO and CO2 gas molecules is dependent on the chiralities and diameters of CNTs and it is vice versa for NO and NO2 gas molecules. Also, the electronic character of CNTs is not affected by the adsorption of CO and CO2 gas molecules while it is strongly affected by NO and NO2 gas molecules. In addition, it is found that the dipole moments of zig-zag CNTs are always higher than the arm-chair CNTs. Therefore, we conclude that the zig-zag carbon nanotubes are more preferred as gas sensors than the arm-chair carbon nanotubes, especially for detecting NO and NO2 gas molecules.
Keywords:Carbon Nanotubes, DFT, Gas Sensors

1. Introduction
Monitoring of combustible gas alarms, gas leak detection, and environmental pollution is of great concern in public security. Advances in nanotechnology give great promise for achieving new sensing materials. Since the discovery of carbon nanotubes in 1991, the single-walled carbon nanotubes (SWCNTs) have been intensively investigated as nanoscale gas sensors because of their great surface areas to bulk ratio and their abilities to modulate electrical properties upon adsorption of various kinds of gas molecules [1] -[17] . The emission of carbon and nitrogen oxides (CO, CO2, NO and NO2) results from the combustion of fossil fuels, contributing to both smog and acid precipitation, and affecting both terrestrial and aquatic ecosystems [18] . Although many efforts have been made to use catalysts to reduce the amount of carbon or nitrogen oxides in the air [19] -[25] , an efficient method of sensing and removing carbon and nitrogen oxides is still required.
Because carbon and nitrogen oxides are the most dangerous air pollutants, toxic and global warming gases, our work is concentrated on investigating the effect of tubular chiralities and diameters of single carbon nanotubes on gas sensing behavior for CO, CO2, NO and NO2 gas molecules, applying the first principle calculations.
2. Computational Methods
All calculations were performed with the density functional theory as implemented within G03W package [26] - [29] , using B3LYP exchange-functional and applying basis set 6 - 31g (d,p). Pure carbon nanotubes 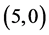 and
and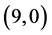 ,
, 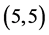 and
and 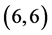 are fully optimized with spin average as well as the adsorption of CO, CO2, NO and NO2 gas molecules.
are fully optimized with spin average as well as the adsorption of CO, CO2, NO and NO2 gas molecules.
The obtained diameters [30] and the adsorption energies of gas molecules on CNTs (Eads) [31] are calculated from the following relations:
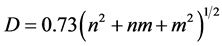
where n and m are integral numbers, the composition of chiral vector.
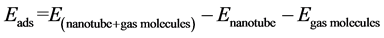
where 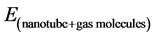 is the total energy of nanotube and gas molecules,
is the total energy of nanotube and gas molecules, 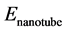 is the energy of the carbon nanotube, and
is the energy of the carbon nanotube, and 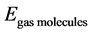 is the energy of gas molecules.
is the energy of gas molecules.
3. Results and Discussion
We will investigate the adsorption of gas molecules, CO, CO2, NO and NO2 on four carbon nanotubes with different charilities and diameters 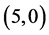 CNT,
CNT, 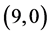 CNT,
CNT, 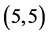 CNT and
CNT and 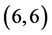 CNT as shown in Figure 1 and Table 1.
CNT as shown in Figure 1 and Table 1.
3.1. Adsorption of CO, CO2, NO and NO2 Gas Molecules on CNTs
We have adsorbed CO and CO2 gas molecules vertically on different three positions of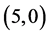 ,
, 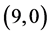 ,
, 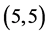 and
and 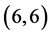 CNTs: above a carbon atom (carbon site), above a bond between two carbon atoms (bond site) and above a center of a hexagon ring (vacant site). The calculated adsorption energies of CO and CO2 gas molecules
CNTs: above a carbon atom (carbon site), above a bond between two carbon atoms (bond site) and above a center of a hexagon ring (vacant site). The calculated adsorption energies of CO and CO2 gas molecules
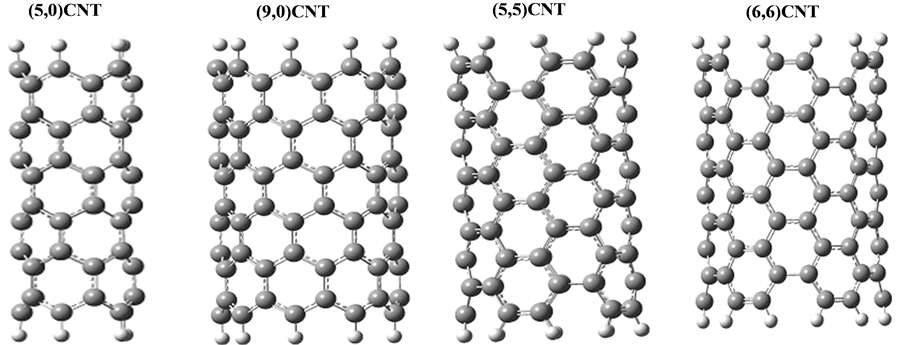
Figure 1. The fully optimized structures of ,
,  ,
, 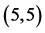 and
and 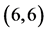 CNTs. Carbon atoms (gray) and hydrogen atoms (white).
CNTs. Carbon atoms (gray) and hydrogen atoms (white).
are listed in Table 2. It is found that the best position and adsorption energy for CO gas molecule is above the bond site on 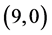 CNT with adsorption energy of −0.43 eV, however for CO2 gas molecule is above the vacant site on
CNT with adsorption energy of −0.43 eV, however for CO2 gas molecule is above the vacant site on 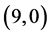 CNT with adsorption energy of −0.26 eV. Therefore, one can conclude that the best CNT gas sensor for CO and CO2 gas molecules is the
CNT with adsorption energy of −0.26 eV. Therefore, one can conclude that the best CNT gas sensor for CO and CO2 gas molecules is the 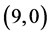 CNT.
CNT.
Also, we have adsorbed NO and NO2 gas molecules vertically on different three positions of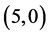 ,
, 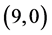 ,
, 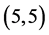 and
and 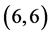 CNTs: above a carbon site, above a bond site and above a vacant site. The calculated adsorption energies of NO and NO2 gas molecules are listed in Table 3. It is found that the best adsorption energies of NO gas molecule are on the
CNTs: above a carbon site, above a bond site and above a vacant site. The calculated adsorption energies of NO and NO2 gas molecules are listed in Table 3. It is found that the best adsorption energies of NO gas molecule are on the 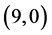 CNT above a bond site, then above a carbon site and after that above a vacant site with adsorption energies of −1.65 eV, −1.55 eV and −1.34 eV, respectively. However, for NO2 gas molecule is found to be above the bond site on
CNT above a bond site, then above a carbon site and after that above a vacant site with adsorption energies of −1.65 eV, −1.55 eV and −1.34 eV, respectively. However, for NO2 gas molecule is found to be above the bond site on 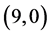 CNT with adsorption energy of −1.75 eV. Also, it is noticed that the vacant site is always preferred for NO2 gas adsorption on all the studied CNTs except for
CNT with adsorption energy of −1.75 eV. Also, it is noticed that the vacant site is always preferred for NO2 gas adsorption on all the studied CNTs except for 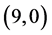 CNT. Therefore, one can conclude that all CNTs can be used as gas sensors for NO and NO2 gas molecules.
CNT. Therefore, one can conclude that all CNTs can be used as gas sensors for NO and NO2 gas molecules.
From Table 2, Table 3, one can investigate the effect of the chiralities and the diameters on the CNT gas sensors behavior. It is clear that the adsorption of CO and CO2 gas molecules is dependent on the chiralities and the diameters of CNTs. The adsorption of CO and CO2 gas molecules is enhanced with increasing the diameter of the zig-zag CNTs. However, the adsorption of NO and NO2 gas molecules is independent on the chiralities and the diameters of CNTs.
3.2. Energy Gaps of Adsorbed CO, CO2, NO and NO2 Gas Molecules on CNTs
From Table 4, it is clear that the adsorption of CO and CO2 gas molecules on CNTs does not affect the elec
Table 1. The configuration structures and diameters of the studied CNTs.
Table 2. The calculated adsorption energies (Eads) of CO and CO2 above a carbon site, a bond site and a vacant site of pristine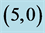 ,
, 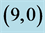 ,
, 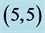 and
and 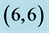 CNTs. All energies are given by eV.
CNTs. All energies are given by eV.
Table 3. The calculated adsorption energies (Eads) of NO and NO2 above a carbon site, a bond site and a vacant site of pristine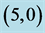 ,
, 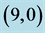 ,
, 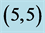 and
and 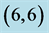 CNTs. All energies are given by eV.
CNTs. All energies are given by eV.
tronic character of the CNTs. Also, the band gaps of pristine CNTs and the adsorbed CO and CO2 gas molecules on CNTs are so close.
From Table 5, the adsorption of NO and NO2 gas molecules on CNTs is strongly affected the electronic character of the 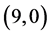 and
and 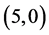 CNTs. However, there is not any change of the electronic character for
CNTs. However, there is not any change of the electronic character for 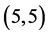 and
and 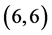 CNTs. The band gap of pristine
CNTs. The band gap of pristine 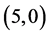 CNT is increased from 0.70 eV to 1.61 eV and to 1.37 eV when NO and NO2 gas molecules are adsorbed on it, respectively. Also, The band gap of pristine
CNT is increased from 0.70 eV to 1.61 eV and to 1.37 eV when NO and NO2 gas molecules are adsorbed on it, respectively. Also, The band gap of pristine 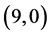 CNT is increased from 0.25 eV to 1.34 eV and to 1.25 eV when NO and NO2 gas molecules are adsorbed on it, respectively. One can conclude that the electronic character of
CNT is increased from 0.25 eV to 1.34 eV and to 1.25 eV when NO and NO2 gas molecules are adsorbed on it, respectively. One can conclude that the electronic character of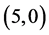 ,
, 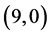 ,
, 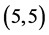 and
and 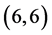 CNTs is not affected by the adsorption of CO and CO2 gas molecules. The adsorption of NO and NO2 gas molecules on CNTs is only strongly affected the electronic character of the
CNTs is not affected by the adsorption of CO and CO2 gas molecules. The adsorption of NO and NO2 gas molecules on CNTs is only strongly affected the electronic character of the 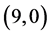 and
and 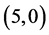 CNTs, however the
CNTs, however the 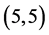 and
and 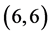 CNTs are not affected at all.
CNTs are not affected at all.
3.3. HOMO-LUMO Orbitals of Adsorbing CO, CO2, NO and NO2 Gas Molecules on CNTs
Our calculated band gaps show that the adsorption of CO and CO2 gas molecules on CNTs is not affected the band gaps of the pristine CNTs, however the adsorption of NO and NO2 gas molecules is strongly affected the band gaps. To explain that the molecular orbitals of adsorbing CO, CO2, NO and NO2 gas molecules on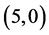 ,
, 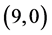 ,
, 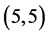 and
and 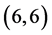 CNTs are investigated, see Figure 2, Figure 3. The band gaps of the pristine CNTs are calculated and are listed in Table 4. The HOMO and LUMO energy orbitals for pristine
CNTs are investigated, see Figure 2, Figure 3. The band gaps of the pristine CNTs are calculated and are listed in Table 4. The HOMO and LUMO energy orbitals for pristine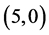 ,
, 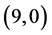 ,
, 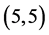 and
and 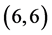 CNTs are found to be (−4.13 eV, −3.42 eV), (−3.93 eV, −3.68 eV), (−4.62 eV, −2.82 eV) and (−4.70 eV, −2.82 eV), respectively. Comparing the HOMO-LUMO energies of the pristine CNTs with ones after the adsorption of CO and CO2 gas molecules, it is clear that the energy values are so close. Also, it is noticed that there is not any contribution from the gas molecules at the molecular orbitals and the electron density of HOMO and LUMO is distributed over all the carbon atoms of CNTs except for
CNTs are found to be (−4.13 eV, −3.42 eV), (−3.93 eV, −3.68 eV), (−4.62 eV, −2.82 eV) and (−4.70 eV, −2.82 eV), respectively. Comparing the HOMO-LUMO energies of the pristine CNTs with ones after the adsorption of CO and CO2 gas molecules, it is clear that the energy values are so close. Also, it is noticed that there is not any contribution from the gas molecules at the molecular orbitals and the electron density of HOMO and LUMO is distributed over all the carbon atoms of CNTs except for 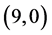 CNT is located at the terminals of the tube, see Figure 2. Comparing the HOMO-LUMO energies of the pristine CNTs with ones after the adsorption of NO and NO2 gas molecules, it is clear that the energy values are so close in case of
CNT is located at the terminals of the tube, see Figure 2. Comparing the HOMO-LUMO energies of the pristine CNTs with ones after the adsorption of NO and NO2 gas molecules, it is clear that the energy values are so close in case of 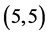 and
and  CNTs and are quite far in case of
CNTs and are quite far in case of  and
and  CNTs. The HOMO energy levels in case of
CNTs. The HOMO energy levels in case of  and
and  CNTs after adsorbing NO and NO2 gas molecular are getting deep (lower) in energy however the LUMO energy levels are getting higher in energy. Results in increasing the band gap from 0.70 eV to 1.81 eV in
CNTs after adsorbing NO and NO2 gas molecular are getting deep (lower) in energy however the LUMO energy levels are getting higher in energy. Results in increasing the band gap from 0.70 eV to 1.81 eV in
Table 4. The calculated energy gaps (Eg) of CO and CO2 above a carbon site, a bond site and a vacant site of pristine ,
,  ,
,  and
and 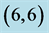 CNTs. All energies are given by eV.
CNTs. All energies are given by eV.
Table 5. The calculated energy gaps (Eg) of NO and NO2 above a carbon site, a bond site and a vacant site of pristine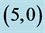 ,
, 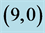 ,
, 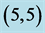 and
and  CNTs. All energies are given by eV.
CNTs. All energies are given by eV.
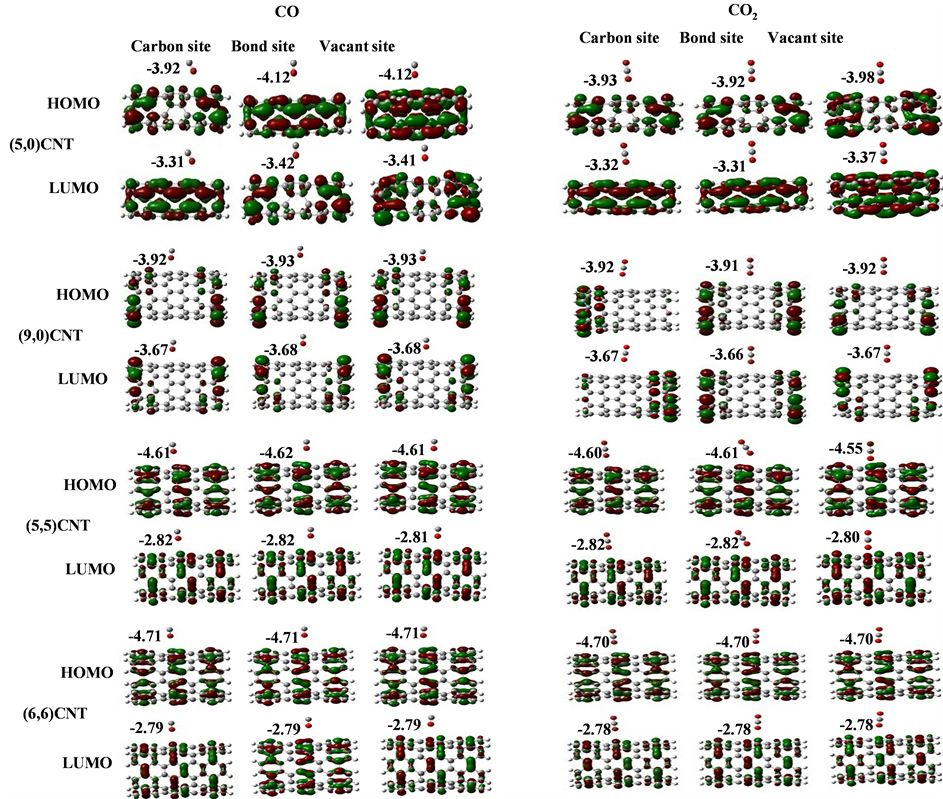
Figure 2. HOMO and LUMO molecular orbitals of adsorbing CO and CO2 gas molecules on the pristine ,
,  ,
, 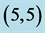 and
and 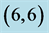 CNTs. Energies of HOMO and LUMO are listed above the molecular orbitals and are given by eV.
CNTs. Energies of HOMO and LUMO are listed above the molecular orbitals and are given by eV.
case of 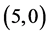 CNT and from 0.25 eV to 1.34 eV in case of
CNT and from 0.25 eV to 1.34 eV in case of 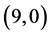 CNT. Also, it is noticed that there is representation from the NO gas molecule at LUMO of
CNT. Also, it is noticed that there is representation from the NO gas molecule at LUMO of 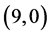 and
and 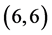 CNTs, see Figure 3.
CNTs, see Figure 3.
3.4. The Reactivity of CNT Surfaces before and after Adsorbing Gas Molecules
Our calculated band gaps and molecular orbitals show that the adsorption of CO and CO2 gas molecules on CNTs is not affected neither the band gaps nor the molecular orbitals of the pristine CNTs but the adsorption of NO and NO2 gas molecules is strongly affected both of the band gaps and the molecular orbitals of 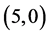 and
and 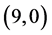 CNTs. To clear that the reactivity of CNT surfaces before and after adsorbing CO, CO2, NO and NO2 gas molecules on
CNTs. To clear that the reactivity of CNT surfaces before and after adsorbing CO, CO2, NO and NO2 gas molecules on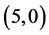 ,
, 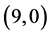 ,
, 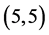 and
and 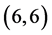 CNTs are studied, see Table 6, Table 7. The surface reactivity of the pristine CNTs is calculated and is listed in Table 6. The dipole moments of pristine
CNTs are studied, see Table 6, Table 7. The surface reactivity of the pristine CNTs is calculated and is listed in Table 6. The dipole moments of pristine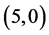 ,
, 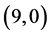 ,
, 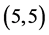 and
and 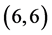 CNTs are found to be 0.54 Debye, 0.20 Debye 0.00 Debye and 0.00 Debye, respectively.
CNTs are found to be 0.54 Debye, 0.20 Debye 0.00 Debye and 0.00 Debye, respectively.
Comparing the dipole moments of the pristine CNTs with ones that are adsorbed the CO and CO2 gas molecules, it is clear that the dipole moment values are so close in case of the adsorption of the CO gas molecule but they are higher in case of the adsorption of the CO2 gas molecule, see Table 6. Also, it is noticed that the highest dipole moments after the adsorption of the CO2 gas molecule are 0.74 Debye (when CO2 is adsorbed above the bond site of 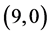 CNT) and 0.77 Debye (when CO2 is adsorbed above the vacant site of
CNT) and 0.77 Debye (when CO2 is adsorbed above the vacant site of 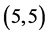 CNT), re-
CNT), re-
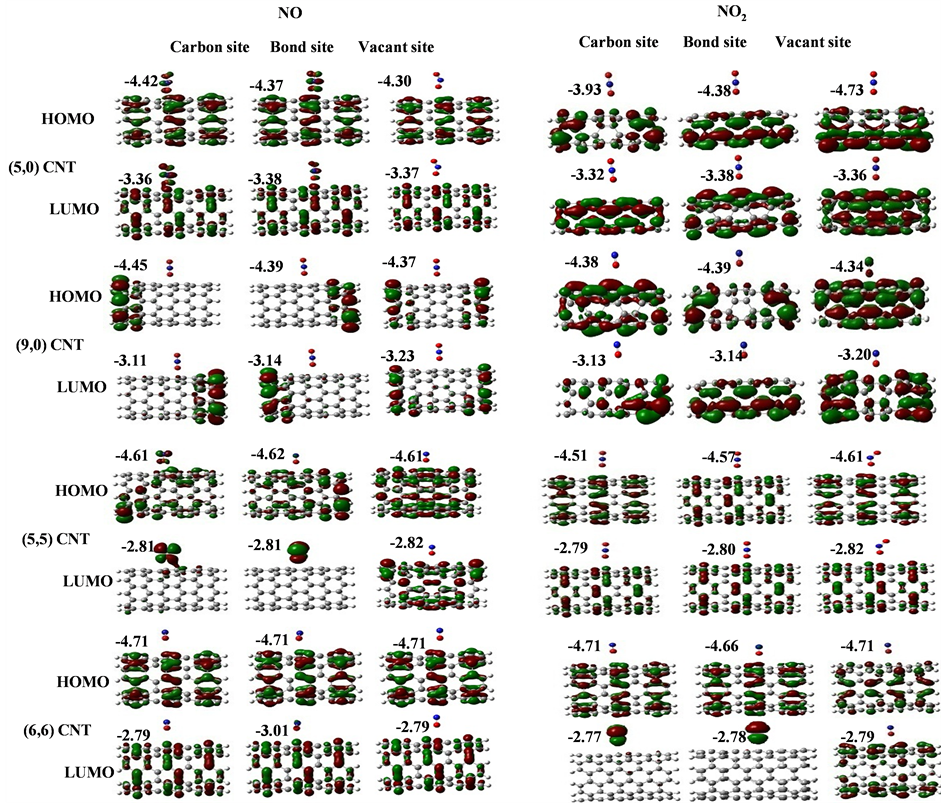
Figure 3. HOMO and LUMO molecular orbitals of adsorbing NO and NO2 gas molecules on the pristine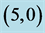 ,
, 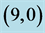 ,
, 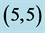 and
and 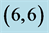 CNTs. Energies of HOMO and LUMO are listed above their molecular orbitals and are given by eV.
CNTs. Energies of HOMO and LUMO are listed above their molecular orbitals and are given by eV.
Table 6. The calculated dipole moments of pristine and after adsorbing CO and CO2 gas molecules above a carbon site, a bond site and a vacant site of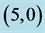 ,
, 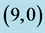 ,
, 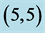 and
and 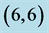 CNTs. All dipole moments are given by Debye.
CNTs. All dipole moments are given by Debye.
spectively. Comparing the dipole moments of the pristine CNTs with ones that are adsorbed the NO and NO2 gas molecules, it is found that the dipole moments are getting higher. When the NO and NO2 gas molecules are adsorbed on the vacant sites of CNTs, their dipole moments are either quite close to or are lower than the dipole moments of pristine CNTs, except in case of adsorbing NO2 on 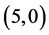 CNT, the dipole moment is increased. Also, all the calculated dipole moments of adsorbing NO and NO2 gas molecules on the carbon sites of CNTs
CNT, the dipole moment is increased. Also, all the calculated dipole moments of adsorbing NO and NO2 gas molecules on the carbon sites of CNTs
Table 7. The calculated dipole moments of pristine and after adsorbing NO and NO2 gas molecules above a carbon site, a bond site and a vacant site of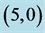 ,
, 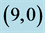 ,
, 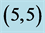 and
and 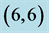 CNTs. All dipole moments are given by Debye.
CNTs. All dipole moments are given by Debye.
are increased, except in case of adsorbing NO2 on 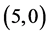 CNT, the dipole moment is decreased. In case of adsorbing NO and NO2 gas molecules on the bond sites of CNTs the dipole moments are also increased, except in case of adsorbing NO2 on
CNT, the dipole moment is decreased. In case of adsorbing NO and NO2 gas molecules on the bond sites of CNTs the dipole moments are also increased, except in case of adsorbing NO2 on 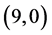 CNT is decreased, see Table 7.
CNT is decreased, see Table 7.
From Table 6, Table 7, it is clear that the dipole moments of zig-zag 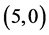 and
and 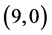 CNTs are always higher than the arm-chair
CNTs are always higher than the arm-chair 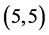 and
and 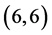 CNTs. Also, it is noticed that the dipole moment of adsorbing NO gas molecule on the bond site of
CNTs. Also, it is noticed that the dipole moment of adsorbing NO gas molecule on the bond site of 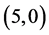 CNT is increased by ten times comparing with the dipole moment of pristine
CNT is increased by ten times comparing with the dipole moment of pristine 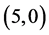 CNT.
CNT.
4. Conclusion
The gas sensing behavior of CNTs, considering a range of different nanotube diameters and chiralities, as well as different adsorption sites is reported. The adsorption of CO, CO2, NO, and NO2 gas molecules on the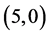 ,
, 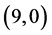 ,
, 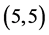 and
and 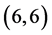 CNTs are studied using B3LYP/6-31 g(d, p). Three different adsorption sites (above a carbon site, a bond site and a vacant site) are applied on CNTs. It is found that the adsorption of CO and CO2 gas molecules is dependent on the chiralities and the diameters of CNTs and it is enhanced with increasing the diameter of the zig-zag CNTs. However, the adsorption of NO and NO2 gas molecules is independent on the chiralities and the diameters of CNTs. Also, the electronic character of
CNTs are studied using B3LYP/6-31 g(d, p). Three different adsorption sites (above a carbon site, a bond site and a vacant site) are applied on CNTs. It is found that the adsorption of CO and CO2 gas molecules is dependent on the chiralities and the diameters of CNTs and it is enhanced with increasing the diameter of the zig-zag CNTs. However, the adsorption of NO and NO2 gas molecules is independent on the chiralities and the diameters of CNTs. Also, the electronic character of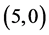 ,
, 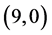 ,
, 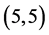 and
and 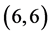 CNTs is not affected by the adsorption of CO and CO2 gas molecules. While, the adsorption of NO and NO2 gas molecules on CNTs is only strongly affected by the electronic character of the
CNTs is not affected by the adsorption of CO and CO2 gas molecules. While, the adsorption of NO and NO2 gas molecules on CNTs is only strongly affected by the electronic character of the 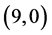 and
and 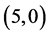 CNTs but the
CNTs but the 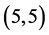 and
and 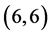 CNTs are not affected at all. It is found that the dipole moments of zig-zag
CNTs are not affected at all. It is found that the dipole moments of zig-zag 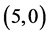 and
and 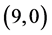 CNTs are always higher than the arm-chair
CNTs are always higher than the arm-chair 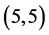 and
and 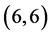 CNTs. Also, it is noticed that the dipole moment of adsorbing NO gas molecule on the bond site of
CNTs. Also, it is noticed that the dipole moment of adsorbing NO gas molecule on the bond site of  CNT is increased by ten times compared with the dipole moment of pristine
CNT is increased by ten times compared with the dipole moment of pristine  CNT. Therefore, these findings prove that the zig-zag carbon nanotubes are better than the arm-chair carbon nanotubes as gas sensors, especially for NO and NO2 gas molecules.
CNT. Therefore, these findings prove that the zig-zag carbon nanotubes are better than the arm-chair carbon nanotubes as gas sensors, especially for NO and NO2 gas molecules.
References
- Kong, J., Franklin, N.R., Zhou, C., Chapline, M.G., Peng, S. and Cho, K. (2000) Nanotube Molecular Wires as Chemical Sensors. Science, 287, 622. http://dx.doi.org/10.1126/science.287.5453.622
- Snow, E.S., Perkins, F.K., Houser, E.J., Badescu, S.C. and Reinecke, T.L. (2005) Chemical Detection with a Single-Walled Carbon Nanotube Capacitor. Science, 307, 1942-1945. http://dx.doi.org/10.1126/science.1109128
- Baei, M.T., Soltani, A.R., Moradi, A.V. and Lemeski, E.T. (2011) Adsorption Properties of NO on (6, 0), (7, 0), and (8, 0) Zigzag Single-Walled Boron Nitride Nanotubes: A Computational Study. Computational and Theoretical Chemistry, 970, 30-35. http://dx.doi.org/10.1016/j.comptc.2011.05.021
- Breza, M. (2006) Model Studies of SOCl2 Adsorption on Carbon Nanotubes. Journal of Molecular Structure: THEOCHEM, 767, 159-163. http://dx.doi.org/10.1016/j.theochem.2006.06.006
- Zhao, J., Buldum, A., Han, J. and Lu, J.P. (2002) Gas Molecule Adsorption in Carbon Nanotubes and Nanotube Bundles. Nanotechnology, 13, 195-200. http://dx.doi.org/10.1088/0957-4484/13/2/312
- Ricca, A. and Bauschlicher Jr., C.W. (2006) The Adsorption of NO on (9, 0) and (10, 0) Carbon Nanotubes. Chemical Physics, 323, 511-518. http://dx.doi.org/10.1016/j.chemphys.2005.10.019
- Abbas Rafati, A., Majid Hashemianzadeh, S. and Bolboli Nojini, Z. (2008) Electronic Properties of Adsorption Nitrogen Monoxide on Inside and Outside of the Armchair Single Wall Carbon Nanotubes: A Density Functional Theory Calculations. The Journal of Physical Chemistry C, 112, 3597-3604. http://dx.doi.org/10.1021/jp709955g
- Azizi, K., Majid Hashemianzadeh, S. and Bahramifar, Sh. (2011) Density Functional Theory Study of Carbon Monoxide Adsorption on the Inside and Outside of the Armchair Single-Walled Carbon Nanotubes. Current Applied Physics, 11, 776-782. http://dx.doi.org/10.1016/j.cap.2010.11.071
- Ricca, A., Bauschlicher Jr., C.W. (2006) The Physisorption of CH4 on Graphite and on a (9, 0) Carbon Nanotube. Chemical Physics, 324, 455-458. http://dx.doi.org/10.1016/j.chemphys.2005.11.010
- Santucci, S., Picozzi, S., Di Gregorio, F., Lozzi, L., Cantalini, C., Valentini, L., Kenny, J.M. and Delley, B. (2003) NO2 and CO Gas Adsorption on Carbon Nanotubes: Experiment and Theory. The Journal of Chemical Physics, 119, 10904-10910. http://dx.doi.org/10.1063/1.1619948
- Zanolli, Z. and Charlier, J.C. (2009) Defective Carbon Nanotubes for Single-Molecule Sensing. Physical Review B, 80, 155447. http://dx.doi.org/10.1103/PhysRevB.80.155447
- Tang, S. and Cao, Z. (2009) Defect-Induced Chemisorption of Nitrogen Oxides on (10, 0) Single-Walled Carbon Nanotubes: Insights from Density Functional Calculations. The Journal of Chemical Physics, 131, 114706. http://dx.doi.org/10.1063/1.3226572
- García-Lastra, J.M., Mowbray, D.J., Thygesen, K.S., Rubio, A. and Jacobsen, K.W. (2010) Modeling Nanoscale Gas Sensors under Realistic Conditions: Computational Screening of Metal-Doped Carbon Nanotubes. Physical Review B, 81, 245429. http://dx.doi.org/10.1103/PhysRevB.81.245429
- Denis, P.A. (2008) Methane Adsorption Inside and Outside Pristine and N-Doped Single Wall Carbon Nanotubes. Chemical Physics, 353, 79-86. http://dx.doi.org/10.1016/j.chemphys.2008.07.024
- Yeung, C.S., Liu, L.V. and Wang, Y.A. (2008) Adsorption of Small Gas Molecules onto Pt-Doped Single-Walled Carbon Nanotubes. The Journal of Physical Chemistry C, 112, 7401-7411. http://dx.doi.org/10.1021/jp0753981
- Zhao, J.X. and Ding, Y.H. (2008) Theoretical Study of the Interactions of Carbon Monoxide with Rh-Decorated (8, 0) Single-Walled Carbon Nanotubes, Materials Chemistry and Physics, 110, 411-416. http://dx.doi.org/10.1016/j.matchemphys.2008.02.036
- An, W. and Turner, C.H. (2009) Electronic Structure Calculations of Gas Adsorption on Boron Doped Carbon Nanotubes Sensitized with Tungsten. Chemical Physics, 482, 274-280.
- Sayago, I., Santos, H., Horrillo, M.C., Aleixandre, M., Fernández, M.J., Terrado, E., Tacchini, I., Aroz, R., Maser, W.K., Benito, A.M., Martínez, M.T., Gutiérrez, J. and Munoz, E. (2008) Carbon Nanotube Networks as Gas Sensors for NO2 Detection. Talanta, 77, 758-764. http://dx.doi.org/10.1016/j.talanta.2008.07.025
- Li, X.M., Tian, W.Q., Dong, Q., Huang, X.R., Sun, C.C. and Jiang, L. (2011) Substitutional Doping of BN Nanotube by Transition Metal: A Density Functional Theory Simulation. Computational and Theoretical Chemistry, 964, 199-206. http://dx.doi.org/10.1016/j.comptc.2010.12.026
- Chen, H.L., Wu, S.Y., Chen, H.T., Chang, J.G., Ju, S.P., Tsai, C. and Hsu, L.C. (2010) Theoretical Study on Adsorption and Dissociation of NO2 Molecule on Fe(1 1 1) Surface. Langmuir, 26, 7157-7164. http://dx.doi.org/10.1021/la904233b
- Wickham, D.T., Banse, B.A. and Koel, B.E. (1991) Adsorption of Nitrogen Dioxide and Nitric Oxide on Pd(1 1 1). Surface Science, 243, 83-95. http://dx.doi.org/10.1016/0039-6028(91)90347-U
- Jirsak, T., Kuhn, M. and Rodriguez, J.A. (2000) Chemistry of NO2 on Mo(1 1 0): Decomposition Reactions and Formation of MoO2. Surface Science, 457, 254-266. http://dx.doi.org/10.1016/S0039-6028(00)00381-2
- Huang, W., Jiang, Z., Jiao, J., Tan, D., Zhai, R. and Bao, X. (2002) Decomposition of NO on Pt(1 1 0): Formation of a New Oxygen Adsorption State. Surface Science, 506, 287-292. http://dx.doi.org/10.1016/S0039-6028(02)01381-X
- Hellman, A., Panas, I. and Grönbeck, H. (2008) NO2 Dissociation on Ag(1 1 1) Revisited by Theory. Journal of Chemical Physics, 128, 104704-104709. http://dx.doi.org/10.1063/1.2832303
- Yen, M.Y. and Ho, J.J. (2010) Density-Functional Study for the NOx (x = 1, 2) Dissociation Mechanism on the Cu(1 1 1) Surface. Chemical Physics, 373, 300-306. http://dx.doi.org/10.1016/j.chemphys.2010.06.005
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Lamham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B.G., Chen, W., Wong, M.W., Andres, J.L., Head-Gordon, M., Replogle, E.S. and Pople, J.A. (2004) Gaussian. Wallingford CT, Inc., Wallingford.
- EL-Barbary, A.A., Lebda, H.I. and Kamel, M.A. (2009) The High Conductivity of Defect Fullerene C40 Cage. Computational Materials Science, 46, 128. http://dx.doi.org/10.1016/j.commatsci.2009.02.034
- El-Barbary, A.A., Eid, K.M., Kamel, M.A. and Hassan, M.M. (2013) Band Gap Engineering in Short Heteronanotube Segments via Monovacancy Defects. Computational Materials Science, 69, 87-94. http://dx.doi.org/10.1016/j.commatsci.2012.10.035
- EL-Barbary, A.A., Ismail, G.H. and Babeer, A.M. (2013) Effect of Monovacancy Defects on Adsorbing of CO, CO2, NO and NO2 on Carbon Nanotubes: First Principle Calculations. Journal of Surface Engineered Materials and Advanced Technology, 3, 287-294. http://dx.doi.org/10.4236/jsemat.2013.34039
- Nalwa, H. (2002) Nanostructured Materials and Nanotechnology. Academic Press, San Diego.
NOTES

*Corresponding author.


