International Journal of Organic Chemistry
Vol.06 No.04(2016), Article ID:72505,13 pages
10.4236/ijoc.2016.64021
Synthesis and Evaluation of 4-Hydroxy Quinolinone Derivatives as Antioxidants of Lubricating Grease
Modather F. Hussein1, Mostafa A. Ismail2, Refaat A. El-Adly3*
1Faculty of Science, Al-Azhar University, Assiut, Egypt
2Faculty of Education, Ain Shams University, Cairo, Egypt
3Egyptian Petroleum Research Institute, Cairo, Egypt

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 27, 2016; Accepted: December 2, 2016; Published: December 5, 2016
ABSTRACT
3-(5-(2.4-dichlorophyenyl)-4.5-dihydro-1H-pyrazol-3-yl)-4hydroxy-1-methylquinolin(1H)-one, 5-((4-hydroxy-8-methyl-2-oxo-1.2-dihydroquinolin-3-yl)methylene)-1- phenyl-2-hioxodihydropyrimidine-4.6(1H.5H)-dione, and 1-butyl-4-hydroxy-3-(5- styryl-4.5-dihydro-1H-pyrazol-3yl)quinolin-2(1H)-one were synthesized and charac- terized by spectroscopy analysis. These compounds are designated I, II and III, respectively. The antioxidants efficiency of the synthesized compounds in lubricating greases had been investigated using ASTM d-942 and ASTM d-664. The obtained data showed that the total acid number and oxygen pressure drop of these compounds in lubricating greases decrease in the order: Comp.III. < Comp.I. < Comp.II. The antioxidant efficiency of the prepared quinolinones derivatives was discussed. Acceptable correlations were obtained between the obtained oxidation inhibition and the calculated quantum chemical parameters.
Keywords:
Lubricating Greases, Antioxidants, 4-Hydroxy Quinolinone, Quantum Chemical Calculation

1. Introduction
Oxidative stability and consistency of the grease matrix control a wide variety of performance properties in grease lubrication [1] . In particular, phenolic antioxidants boast greater benefits when added into grease, since they themselves make stable radicals that help to terminate chain reactions while they donate hydrogen atoms [2] . Most phenolic antioxidants used in lubricating greases are phenolic derivatives having a tertiary butyl group. Recently, bearings have been increasingly used in applications where high temperature environments are present [3] . El-Adly et al. [4] prepared some azine and azole derivatives and they were evaluated as antioxidant additives for lithium lubricating grease.. Antioxidant efficiency of 4-hydroxyquinoline derivatives on free-radical-initi- ated hemolysis of erythrocytes was studied [4] .
This fact motivates us to study the relationship between the structure of 4-hydroxy- quinoline with various substituents and its antioxidant effect against free-radical-in- itiated peroxidation.
The 4-hydroxyquinoline-3-carboxylic acid ester with the S-alkyl subsistent was invented by Stharfeldt et al. [5] to use as stabilizer for organic material against the harmful effect of light, oxygen and heat. 8-carbonyl quinoline and other components were invented by Richard J. Lee et al. [6] to provide lubricant compositions which are resistant to oxidative deterioration, and which act as catalysts to increase the rate of non- radical producing reactions relative to the rate of radical producing reactions. It was found that all of the lubricant compositions of the invention contain effective new antioxidants.
2,2,4-trialkyl-1,2-dihydroquinoline and other compounds as lubricant antioxidants were invented by Cyril A. Migdal [7] . Antioxidant 2, 2, 4-Trimethyl-1,2-dihydroquinoline had been investigated by Yu Liu et al. [8] .
Recently, antioxidant publications contain substantial chemical calculations [9] [10] . Such calculations are usually used to explore the relationship between the antioxidants molecular properties and their inhibition efficiency.
The aim of this paper is a study on the preparation of three 4-hydroxy quinolinone derivatives. The ultimate objective was to explore the efficiency of these derivatives as antioxidant additives to the prepared lubricating grease. Also, the correlation between quantum chemical calculations for the quinolone compounds and their oxidation stability data was investigated.
2. Experimental
2.1. Raw Material
2.1.1. Synthesis of Compound I
3-(5-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)-4-hydroxy-1-methylquinolin-2(1H)-one was prepared in the following steps.
Step 1
A mixture of 3-acetyl-4-hydroxy-N-methyl-2 (1H) quinoline (0.01 mol), 2-4-dichlorobenzal (0.01 mol) and one drop of pipridine was heated on a boiling water bath for 4 hr. The reaction mixture was triturated with ethanol and the solid so obtained was filtered off, washed with diethyl ether and crystallized from acetic.
Step 2
To a solution of the compound a (0.01 mol) in DMF (10 cm3) hydrazine hydrate (0.01 mol) was added. The reaction mixture was refluxed for 5 h, then cooled and poured into water; the solid that deposited was filtered off and crystallized from DMF (Scheme 1).
2.1.2. Synthesis of Compound II
2-hydrazono-5-((4-hydroxy-8-methyl-2-oxo-1,2-dihydroquinolin-3yl) methylene)-1-ph- enyldihydropyrimidine-4,6(1H.5H)-dione (II). It was prepared by a mixture of pyrimidinethione (0.01 mol) and hydrazine hydrate (0.01 mol) in absolute ethanol (20 cm3) and then the mixture was refluxed for 4 h. The solid product that obtained was filtered off and crystallized from DMF: (Scheme 2).
2.1.3. Synthesis of Compound III
1-butyl-4-hydroxy-3-95-styryl-4,5-dihydro-1H-pyrazol-3-yl)quinoline-2(1H) (Scheme 3).
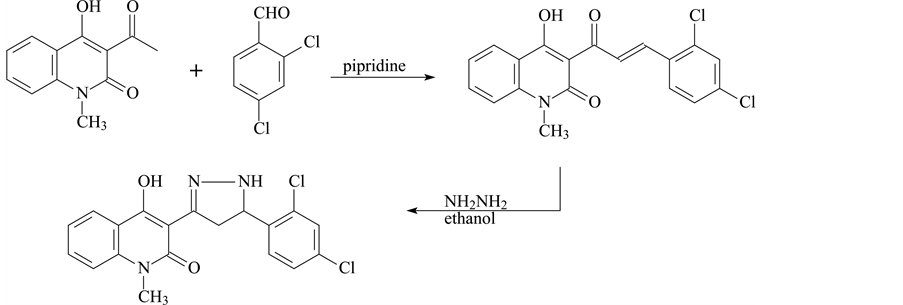
Scheme 1. Synthesis of Compound I.
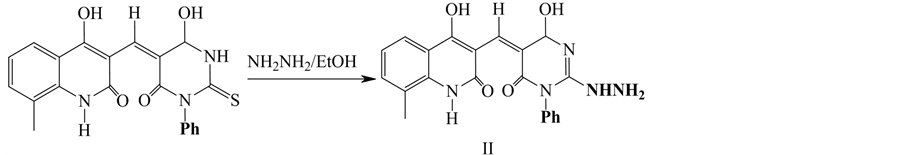
Scheme 2. Synthesis of Compound II.
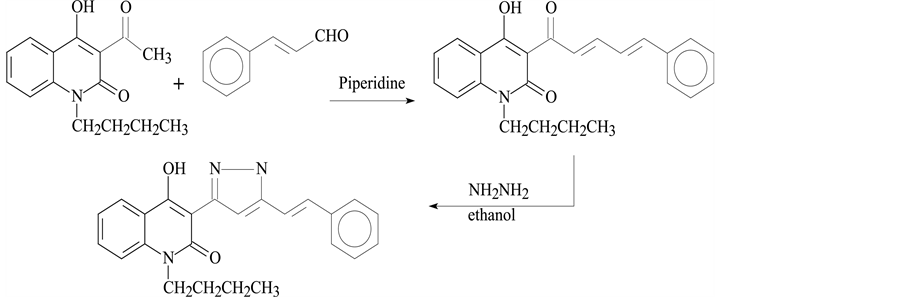
Scheme 3. Synthesis of Compound III.
Using the same method as for preparation of 1, 4, we used cinnamaldehyde with the some acetyl instead of thiophene-2-carboxaldehyde to produce III.
2.2. Analysis and Techniques
All melting points for the prepared compounds were determined using Gallenkamp electric melting point apparatus. The compounds I, II and III were identified and confirmed by microanalysis of carbon, hydrogen, oxygen and nitrogen. The IR spectra were recorded on Perkin-Elmer infrared spectrophotometer model 157, Grating. Also, the 1H NMR spectra were recorded on a Varian Spectrophotometer at 200 MHz using TMS as an internal reference and DMSO-das solvent. The mass spectra (EI) were recorded on 70 ev with Kratos MS equipment and/or a Varian MAT 311 Spectrometer.
2.3. Grease Preparation
The prepared grease under investigation was prepared, according to the procedure previously described [11] ; Table 1 presented the composition of G1, G2, G3 and G4. The Compound I, II and III with concentration 0.3 wt% were added to grease G2, G3 and G4, respectively. The physicochemical properties of the prepared greases were determined according National lubricating grease institute (NLGI) and Egyptian standard. The oxidation stability of the lithium greases with and without prepared quinolone derivatives was determined using oxygen pressure drop test ASTM D-942 and Total acid number ASTM D-664.
3. Results and Discussions
3.1. Structure Confirmation of the Synthesized Compounds
The micro analysis of carbon, hydrogen, nitrogen and oxygen of prepared compound I, II and III are shown in Table 1. It can be seen from Table 2 that the calculated data given agree to a large extent with the found data. The molecular weight, melting point and yield for compounds I, II and III are given in Table 3. The obtained data from Infrared and Mass spectra of Compounds I, II and III are Tabulated in Table 4. These data are confirmed the chemical structure of the prepared compounds. It was well known that the efficiency of quinolinone derivatives is increased by the presence of hydroxyl and alkyl groups in such system. Also, the presence of an extended conjugated system in the skeleton of quinolinones facilitates the delocalization of free radicals throughout the compound. For these reasons, the Compounds I, II and III were prepared and used as antioxidant for lubricating grease.
3.2. Physicochemical Properties of Prepared Grease
Table 5 shows the physicochemical properties for prepared greases such as consistency, dropping point, penetration and oil separation. These data indicate good interaction between Li-soap and oils to form good three dimensional networks and good texture. The prepared grease had satisfactory performance in most automotive applications and they have standard code NLGI 2. It was concluded that the weight percent of the ingre-
Table 1. Composition pf the prepared grease.
Table 2. Elemental analysis (wt%), calc./found of Quinolinones compounds (I - III).
Table 3. Characterization of the prepared organic compounds.
Table 4. Spectral data of quinolinone compounds (I - III).
Table 5. Physico-chemical properties of the prepared grease.
dients that’s needed to prepare obtained greases (G1, G2, G3 and G4) is a good selection.
3.3. Evaluation of the Prepared Compounds as Antioxidants for Lubricating Greases
The prepared compounds ( I, II and III) were evaluated as antioxidants for lubricating greases using ASTM D-942 and ASTM d 664 to determine the oxygen pressure drop and total acid number, respectively (Figure 1 & Figure 2). The obtained result in these figures showed that the oxygen pressure drop and total acid number with time remarkable increased with increasing time in case G1 (blank grease). While this trend is reduced with compounds I, II and III when added to blank grease to give G2, G3 and G4 respectively. The experimental data obtained are shown graphically in Figure 1 & Figure 2 which gives an overview on the efficiency of the 4-hydroxy quinolinones derivatives in controlling the oxidized components compared with the blank grease. It can be seen, from Figure 1 and Figure 2, that the total acid number and oxygen pressure drop for grease G1, without quinolinones increases considerably with time compared with slight increase after addition of the quinolinones (I, II and III). Comparatively, compound III possess the lowest overall acid number and oxygen drop. Based on these results, the prepared quinolinones investigated proved to be successful in controlling the oxidative deterioration of lubricating greases.
Comparison of the antioxidant activity of the compounds I, II and III reveals the following order Comp. III. < Comp. I < Comp. II. This indicates that the oxidation inhibition efficiency is dependent on the structure of the quinolinone compound. It appears, therefore, that the introduction of hydroxyl group with butyl group in quinolinones (compound III) leads to increasing the efficiency of inhibiting the oxidation chain reactions.
3.4. Correlation of Antioxidant Character of the Prepared Additives with Their Structures
Quantum chemical calculations were performed to investigate the relationships between the molecular structure of the prepared compounds and their oxidation stabilities. Quantitative structure activity relationship (QSAR) has been performed on the activity of antioxidants [12] .
Figure 1. Oxygen pressure drop against time for greases with and without quinolinones derivatives.
Figure 2. Total acid number against time for greases with and without quinolinones derivatives.
The full geometry-optimized structures with Mulliken charges of three prepared quinolone derivatives are shown in Figures 3-8. Also, the theoretical value of the prepared compounds was tabulated in Table 6 using the Gaussian 05 (HF/3-21G). These data demonstrated that neither E HOMO, nor ΔE (E LUMO - E HOMO) are correlated to data in Figure 9 & Figure 10.
E HOMO (the highest occupied molecular orbital) and E LUMO (the lowest unoccupied molecular orbital) values were calculated. The charges representative atoms and other relevant quantum parameters were listed in Table 6. The optimized structure of
Figure 3. The frontier molecule orbital density distribution of compound I.
Figure 4. Optimized structure with Mulliken charges of compound I.
Figure 5. The frontier molecule orbital density distribution of compound III.
Figure 6. Optimized structure with Mulliken charges of compound II.
the molecules and the electric/orbital density distributions of HOMO and LUMO illustrated in Figures 3-8. Mulliken population analysis is mostly used for the calculation of the charge distribution in a molecule. These numerical quantities are easy to obtain and they provide at least a qualitative understanding of the structure and reactivity of molecules [13] .
Highest occupied molecular orbital energy (E HOMO) and lowest unoccupied molecular orbital energy (
The HOMO is the orbital that could act as an electron donor, since it is the outermost (highest energy) orbital containing electrons. The LUMO is the orbital that could act as the electron acceptor, since it is the innermost (lowest energy) orbital that has room to accept electrons. According to the frontier molecular orbital theory, the formation of a transition state is due to an interaction between the frontier orbitals (HOMO and LUMO) of reactants [14] .
From Table 6, it was found that the E HOMO and the E LUMO changed, while the energy gap E LUMO E HOMO and E LUMO-E HOMO (the difference in energy between the) decreased with increasing the inhibition efficiency. The energy gap (ΔE) is an important stability index. A large E HOMO ? E LUMO gap implies high stability for the molecule in chemical reactions [15] , which means less inhibition efficiency. The values of (ΔE) indicate remarkably that the smaller energy gap results in a high oxidation inhibition efficiency, reflecting the stronger interaction between the inhibitors and grease ingredients. The interactions are probably physical adsorption [16] . These theoretical data adapted with obvious experimental data.
Figure 7. The frontier molecule orbital density distribution of compound III.
Figure 8. Optimized structure with Mulliken charges of compound III.
Figure 9. Correlation between experimental and theoretical data concerning TAN.
Figure 10. Correlation between experimental and theoretical data concerning pressure drop.
Table 6. Theoretical data of quantum chemical calculation using Gaussian 05 (HF/3-21G).
4. Conclusions
1) There may be concluded that, the optimum structure for the maximum efficiency of the 4-hydroxy quinolinones derivatives as antioxidants requires the following items:
a) The presence of butyl group (donating group) and hydroxyl group in quinolone to give compounds III. Such structure stabilized radical resulting from the oxidation reactions, therefore inhibiting the deterioration of the lithium lubricating grease G4.
b) The presence of hydroxyl group and imide group in quinolone to give compounds II. It also inhibits the deterioration of the lithium lubricating grease G3.
c) The Presence of extensive conjugation through the 4-hydroxy quinolinones derivatives (I, II, and III).
2) The obtained quantum chemical calculations using Gaussian model showed good agreement with experimental data.
Cite this paper
Hussein, M.F., Ismail, M.A. and El-Adly, R.A. (2016) Synthesis and Evaluation of 4-Hydroxy Quinolinone Derivatives as Antioxidants of Lubricating Grease. International Journal of Organic Chemistry, 6, 207-219. http://dx.doi.org/10.4236/ijoc.2016.64021
References
- 1. Odi-Owei, S. (1989) Tribological Properties of Some Vegetable Oils and Fats. Lubrication Engineering, 11, 685-690.
- 2. Mikami, H. (2004) Development of Long Life Grease for High-Speed Application “ME-1” Grease for Motor Bearings. NTN Technical Review. No. 72, 20-25.
http://www.ntnglobal.com/en/products/review/pdf/NTN_TR72_en_P020.pdf - 3. Chao, M.R., Li, W.M., Chen, L.F. and Wang, X.B. (2015) Industrial & Engineering Chemistry Research, 54, 6605-6610.
https:/doi.org/10.1021/acs.iecr.5b00374 - 4. Fikry, R.M., El-Adly, R.A., Ismail, N.A., El-Tabei, A.S. and Al-Aidy, H. (2013) Some Azine and Azole Derivatives as Antioxidant Additives for Lithium Lubricating Grease. Egyptian Journal of Petroleum, 22, 61-71.
- 5. Stahrfeldt, T., et al. (2001) 4-Hydroxyquinoline-3-Carboxylic Acid Derivatives as Light Stabilizers. US Patent No. US 6,194,493 B1.
- 6. Lee, R.J., et al. (1984) Antioxidants and Lubricating Oil Containing Same. US Patent 4,426,-306 A.
- 7. Migdal, C.A., et al. (1998) Lubricant Compositions Comprising Multiple Antioxidants. US Patent No. US 6,726,855 B1.
- 8. Liu, Y., Gao, Q.Y., Liu, L.X. and Li, S.T. (2013) Investigated on the Rubber Antioxidant 2,2,4-Trimethyl-1,2-Dihydroquinoline Polymer. Asian Journal of Chemistry, 25, 2956-2958.
- 9. Nessim, M.I., Ahmed, M.H.M., Bassoussi, A.A., Salem, A.A. and Attia, S.K. (2013) The Effect of Some Benzothiazol Dervatives as Antioxidants for Base Stock. International Journal of Current Research, 5, 1111-1117.
http://www.journalcra.com/sites/default/files/Download%203334.pdf - 10. El Ashry, El S.H., EL-Rafey, E., Rezki, N., Abou-Elnaga, H.H., Bakry, W.M.A. and Boghdadi, Y.M. (2014) Evaluation of Some Functionalized Imidazoles and 1,2,4-Triazoles as Antioxidant Additives for Industrial Lubricating Oils and Correlating the Results with the Structures of Additives Using Empirical AM1 Calculations. Journal of Saudi Chemical Society, 18, 443-449.
https:/doi.org/10.1016/j.jscs.2011.09.010 - 11. El-Adly, A.R. and Enas, A.I. (2011) Tribology—Lubricants and Lubrication. Chapter 8, Intech Publisher, ISBN 978-953-307-371-2.
- 12. Farkas, O., Jakus, J. and Héberger, K. (2004) Quantitative Structure—Antioxidant Activity Relationships of Flavonoid Compounds. Molecules, 9, 1079-1088.
https:/doi.org/10.3390/91201079 - 13. Suzuki, A., Ulfiati, R. and Masuko, M. (2009) Evaluation of Antioxidants in Rapeseed Oils for Railway Application. Tribology International, 24, 987-994.
https:/doi.org/10.1016/j.triboint.2009.02.001 - 14. Mikami, H. (2004) Development of Long Life Grease for High Speed Application—ME-1 Grease for Motor Bearings. NTN Technical Review, No. 72, 20-25.
- 15. Zhou, Z.X. and Parr, R.G. (1990) Activation Hardness: New Index for Describing the Orientation of Electrophilic Aromatic Substitution. Journal of the American Chemical Society, 112, 5720-5724.
https:/doi.org/10.1021/ja00171a007 - 16. Ozcan, M. and Dehri, I. (2004) Electrochemical and Quantum Chemical Studies of Some Sulphur-Containing Organic Compounds as Inhibitors for the Acid Corrosion of Mild Steel. Progress in Organic Coatings, 51, 181-187.












