International Journal of Organic Chemistry
Vol.06 No.02(2016), Article ID:66896,7 pages
10.4236/ijoc.2016.62011
Microwave Application and Anhydrous Cu(OAc)2 Mediated O-Arylation of Aliphatic Amino Alcohols
Mohammad Al-Masum*, Linda Quinones, Laurance T. Cain
Department of Chemistry, Tennessee State University, Nashville, TN, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 February 2016; accepted 23 May 2016; published 27 May 2016
ABSTRACT
Anhydrous Cu(OAc)2 mediated efficient protocol has been developed in the area of C-O coupling from potassium aryltrifluoroborates and aliphatic amino alcohols such as β-hydroxy, γ-hydroxy, and δ-hydroxy amines. The scope of this transformation focuses on direct O-arylation and O-sty- rylation. The reaction vial loaded with reactants under argon atmosphere is microwaved at 140°C for 30 min to furnish the corresponding cross-coupling product, amino ethers, in good yields.
Keywords:
Hydroxylamine, Amino Ether, O-Arylation, O-Styrylation, Microwave

1. Introduction
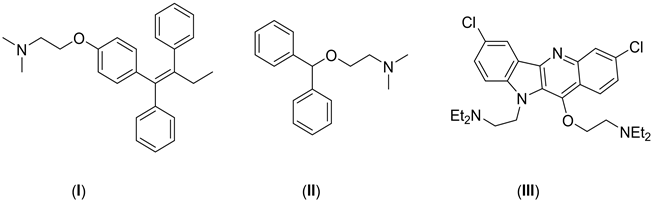
Amino ethers are important intermediates in organic synthesis and compounds of pharmaceutical interest such as tamoxifen (I), antihistamines (II), potent marine natural products such as quindolone (III), and also agricultural interest such as water-based organic coating amino ether surfactants [1] - [7] (Scheme 1).
Potassium organotrifluoroborates have already been proven as effective organoboron reagents in cross- coupling chemistry [8] - [10] . Recently, this reagent is used in copper-promoted carbon-oxygen cross-coupling reaction. Batey’s group has reported a protocol for the alkyl-aryl and alkyl-vinyl ethers via Cu (II)-catalyzed cross-coupling of organotrifluoroborates and aliphatic alcohols [11] - [17] . Chan [18] - [20] and Lam’s groups reported heteroatom arylation reaction for alkyl-aryl ether synthesis although this observation was limited to phenols only. Further development of copper-mediated C-O bond formation has explained by oxygen nucleophiles
Scheme 1. Amino ethers.
such as carboxylic acids, aliphatic alcohols, aryl oximes, silanols, N-hydroxypthalimides, water with boron reagents [21] - [23] .
But using aliphatic hydroxyl amine for similar cross-coupling reaction and making amino ether are rarely known. Very recently, Molander’s group [24] - [27] reported an effective protocol toward the O-arylation of β- hydroxy-α-amino acid substrates. Molander’s report of O-arylation of protected serines and threonines by introducing amino alcohols, such as β-hydroxy-α-amino acid derivatives with arylboronic acids and aryltrifluoroborates for the formation of C-O alkyl aryl ethers, is a new development of Chan-Lam cross-coupling process [24] .
In this work, we also wanted to see whether anhydrous Cu(OAc)2 would be able to provide similar transformation in minutes under microwave irradiation and in the absence of air. Interest in exploring various organic transformations by using potassium organotrifluoroborates led to investigate the cross-coupling reaction of β- hydroxy, γ-hydroxy, and δ-hydroxy amines with potassium aryltrifluoroborates in the presence of anhydrous Cu(OAc)2 under microwave irradiation (Scheme 2). The C-O cross-coupling initiated with the optimization of the reaction partners and conditions for the formation of O-arylated amino ether moiety. We first investigated the catalytic activities of anhydrous Cu(OAc)2 (10 mole%, 20 mol%, and 50 mol%). No significant improvement was observed. Longer reaction time for more than 30 minutes and conventional heating system has no effect on increasing the yield. Other catalyst system such as palladium-catalyst was also employed and showed no product. Then we promote the model reaction of β-hydroxyamine such as 2-dimethylaminoethanol, 2a (1 equivalent), potassium tolyltrifluoroborate, 1a (2.5 equivalent), K2CO3 (2.0 equivalent), and anhydrous Cu(OAc)2 (1 equivalent) in 2.0 mL 1,4-dioxane microwaved at 140˚C for 30 minutes (Entry 1, Table 1). After chromatography 76% isolated amino ether product, 3a was obtained. The product was characterized by GC/MS (Saturn 2200 Benchtop GC/MS) and NMR (Varian 300 MHz). GCMS: Calculated for C11H17NO M+ 180. Found: 180. 1H NMR (Acetone-d6, 300 MHz) δ 7.11 (d, J = 8.4 Hz, 2H, aromatic), 6.90 (d, J = 8.7 Hz, 2H, aromatic), 4.46 (t, J = 4.8 Hz, 2H, CH2), 3.85 (t, J = 4.8 Hz, 2H, CH2), 3.23 (s, 6H, 2 x CH3), 2.25 (s, 3H, CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 129.9, 114.5, 61.9, 56.8, 43.4, 19.5.
γ-hydroxy amine such as 3-diethylamino-1-propanol, 2b and δ-hydroxyamine such as 4-(dimethylamino)-1- butanol, 2c were used with tolyltrifluoroborate under the same reaction conditions afforded the corresponding amino ethers 3b and 3c in good yields (Entries 2 and 3, Table 1). In several other instances, amino alcohols 2a, 2b, 2c are microwaved with various aryltrifluoroborates such as phenyltrifluoroborate, 1b, 4-fluorophenyltrif- luoroborate, 1c, 4-trifluoromethylphenyltrifluoroborate, 1d, 4-trifluoromethoxyphenyltrifluoroborate, 1e, and 4- chlorophenyltrifluoroborate, 1f, in the presence of anhydrous Cu(OAc)2. In all cases, amino ether products were furnished (Products 3d-3k, Table 1).
To explore the generality and scope of the O-arylation of β-hydroxy and γ-hydroxy amines, we examined the reaction with styryltrifluoroborates under the same reaction conditions. It worked well as shown in Table 2. In all cases, reaction looked very clean with trans selectivity. When subjected to silica gel chromatography, product didn’t collect effectively and showed less than expected yield.
Cu(OAc)2 mediated cross-coupling reaction of O-arylation typically requires air in the system for REDOX process. But, O-arylation of amino alcohols in the presence of anhydrous Cu(OAc)2 reported herein is completed under argon atmosphere, not in air. Excess K2CO3 may favor the transmetallation followed by reductive coupling and form the amino ether product.
In addition to Molander’s effective protocol toward copper(II)-mediated O-arylation of protected serines and threonines via Chan-Lam cross-coupling, this work of anhydrous copper acetate mediated reaction O-arylation and O-styrylation of amino alcohols for new series of aminoethers synthesis is interesting development.

Scheme 2. O-Arylation of alcohols and amino alcohols.
2. Procedure
The product N, N-dimethyl-2-(p-tolyloxy) ethan-1-amine, 3a from the cross-coupling of potassium tolyltrifluoroborate, 1a and 2-dimethylaminoethanol, 2a is shown as a representative procedure. The reaction was performed on a 0.5 mmol scale. After purging with argon, a microwave reaction tube with a stirrer bar was loaded with 246.0 mg (1.25 mmol) of potassium tolyltrifluoroborate, 138.0 mg (1.0 mmol) of K2CO3, 90.8 mg (0.5 mmol) of anhydrous Cu(OAc)2, and 50 μL (0.5 mmol) of 2-dimethylaminoethanol. The reaction tube was capped and flushed with argon followed by adding 2.0 mL of 1,4-dioxane. The resulting reaction mixture was then inserted in the microwave vessel (CEM Explorer 24, Discover SP, and 300 W) and irradiated at 140˚C for 30 min. The crude reaction product was extracted from inorganic material using ethyl acetate followed by washing with brine and dried over anhydrous sodium sulphate. For purification the crude product was subjected to preparative TLC using hexane/ethyl acetate (2/1) as eluent and collected the 68.4 mg (76%) amino ether 3a. The product was characterized by GC/MS (Saturn 2200 Benchtop GC/MS) and NMR (Varian 300 MHz).
Compound 3a. GCMS: Calculated for C11H17NO M+ 180. Found: 180. 1H NMR (Acetone-d6, 300 MHz) δ 7.11 (d, J = 8.4 Hz, 2H, aromatic), 6.90 (d, J = 8.7 Hz, 2H, aromatic), 4.46 (t, J = 4.8 Hz, 2H, CH2), 3.85 (t, J = 4.8 Hz, 2H, CH2), 3.23 (s, 6H, 2 x CH3), 2.25 (s, 3H, CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 129.9, 114.5, 61.9, 56.8, 43.4, 19.5.
Compound 3b. GCMS: Calculated for C14H23NO M+ 222. Found: 222. 1H NMR (Acetone-d6, 300 MHz) δ 7.05 (d, J = 8.7 Hz, 2H, aromatic), 6.82 (d, J = 8.4 Hz, 2H, aromatic), 4.13 (t, J = 5.7 Hz, 2H, CH2), 3.52 (m, 4H, 2 x CH2), 3.4 (t, J = 7.5 Hz, 2H, CH2), 2.24 (m, 2H, CH2), 1.4 (t, J = 7.2 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 130.7, 115.2, 65.8, 50.7, 48.7, 24.9, 20.5, 9.4.
Compound 3c. GCMS: Calculated for C13H21NO M+ 208. Found: 208. 1H NMR (Acetone-d6, 300 MHz) δ 7.08 (d, J = 8.7 Hz, 2H, aromatic), 6.80 (d, J = 8.4 Hz, 2H, aromatic), 3.97 (t, J = 5.7 Hz, 2H, CH2), 2.60 (m, 2H,
Table 1. C-O bond by cross-coupling of potassium aryltrifluorobotates and hydroxyaminesa.
aCu(OAc)2 (1.0 eq), ArBF3K 1 (2.5 eq), Hydroxylamine 2 (1.0 eq), K2CO3 (2.0 eq), 1,4-dioxane 2.0 mL MW, 140˚C, 30 min.
CH2), 2.42 (s, 6H, 2 x CH3), 2.23 (s, 2H, CH2), 1.78 (m, 4H, 2 x CH2); 13C NMR (Acetone-d6, 75.5 MHz) δ 130.6, 115.1, 68.2, 45.1, 27.6, 24.2, 20.5.
Compound 3d. GCMS: Calculated for C10H15NO M+ 166. Found: 166. 1H NMR (Acetone-d6, 300 MHz) δ 7.27 (m, 2H, aromatic), 6.92 (m, 3H, aromatic), 4.07 (t, J = 6.0 Hz, 2H, CH2), 2.67 (t, J = 6.0 Hz, 2H, CH2), 2.26 (s, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 159.9, 130.3, 121.3, 115.3, 67.0, 59.0, 46.2.
Compound 3e. GCMS: Calculated for C13H21NO M+ 208. Found: 208. 1H NMR (Acetone-d6, 300 MHz) δ 7.26 (m, 2H, aromatic), 6.91 (m, 3H, aromatic), 4.05 (t, J = 6.3 Hz, 2H, CH2), 2.67 (t, J = 6.6 Hz, 2H, CH2), 2.57 (q, J = 7.2 Hz, 4H, 2 x CH2), 1.92 (m, 2H, CH2), a.03 (t, J = 7.2 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 160.1, 130.3, 121.2 115.3, 66.5, 50.0, 47.8, 27.7, 12.1.
Compound 3f. GCMS: Calculated for C10H14NOF M+ 184. Found: 184. 1H NMR (Acetone-d6, 300 MHz) δ 7.0 (m, 4H, aromatic), 4.07 (m, 2H, CH2), 2.73 (t, J = 5.86, 2H, CH2), 2.31 (s, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 116.6, 116.3, 67.6, 60.6, 58.8, 46.0, 20.8, 14.5.; 19F NMR (Acetone-d6, 300 MHz) δ −125.8.
Table 2. C-O bond by cross-coupling of potassium stryltrifluoroborates and hydroxya- minesa.
aCu(OAc)2 (1.0 eq), StyrylBF3K 4 (2.5 eq), Hydroxylamine 2 (1.0 eq), K2CO3 (2.0 eq), 1,4-dioxane 2.0 mL MW, 140˚C, 30 min.
Compound 3g. GCMS: Calculated for C13H20NOF M+ 225. Found: 225. 1H NMR (Acetone-d6, 300 MHz) δ 6.98 (m, 4H, aromatic), 4.0 (t, J = 6.0 Hz, 2H, CH2), 2.60 (d, J = 6.9 Hz, 2H, CH2), 4H, CH2), 2.53 (q, J = 7.2 Hz, 4H, 2 x CH2), 1.87 (m, 2H, CH2), 0.99 (t, J = 6.9 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 116.6, 116.3, 67.3, 49.9, 47.7, 27.8, 12.3.
Compound 3h. GCMS: Calculated for C11H14NOF3 M+ 234. Found: 234. 1H NMR (Acetone-d6, 300 MHz) δ 7.62 (d, J = 8.4 Hz, 2H, aromatic), 7.12 (d, J = 8.7 Hz, 2H, aromatic), 4.17 (t, J = 5.7 Hz, 2H, CH2), 2.70 (t, J = 6.0 Hz, 2H, CH2), 2.27 (s, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 127.8, 127.7, 115.7, 67.5, 58.7, 46.1; 19F NMR (Acetone-d6, 300 MHz) δ −61.8.
Compound 3i. GCMS: Calculated for C11H14NO2F3 M+ 250. Found: 250. 1H NMR (Acetone-d6, 300 MHz) δ 7.23 (d, J = 9.2 Hz, 2H, aromatic), 7.03 (d, J = 9.3 Hz, 2H, aromatic), 4.12 (t, J = 6.0 Hz, 2H, CH2), 2.73 (m, 2H, CH2), 2.30 (s, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 158.8, 129.7, 123.4, 116.4, 115.4, 67.5, 58.8,46.1; 19F NMR (Acetone-d6, 300 MHz) δ 58.0.
Compound 3j. GCMS: Calculated for C11H14NO2F3 M+ 250. Found: 250. 1H NMR (Acetone-d6, 300 MHz) δ 7.02 (m, 4H, aromatic), 4.07 (m, 2H, CH2), 2.61 (m, 2H, CH2), 2.51 (m, 4H, 2 x CH2), 1.9 (m, 2H, CH2), 0.99 (m, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 158.1, 122.4, 116.1, 115.3, 66.3, 48.9, 46.8, 26.9, 11.5.
Compound 3k. GCMS: Calculated for C14H20NOCl M+ 242. Found: 242. 1H NMR (Acetone-d6, 300 MHz) δ 7.28 (d, J = 7.2 Hz, 2H, aromatic), 6.94 (d, J = 6.9 Hz, 2H, aromatic), 4.07 (t, J = 6.6 Hz, 2H, CH2), 2.66 (m, 8H, 4 x CH2), 1.07 (m, 6H, CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 129.1, 115.9, 46.7.
Compound 5a. GCMS: Calculated for C15H23NO M+ 234. Found: 234. 1H NMR (Acetone-d6, 300 MHz) δ 7.22 (m, 5 H, aromatic), 7.16 (d, J = 13.2 Hz, 1H), 5.86 (d, J = 12.9 Hz, 1H), 3.91 (t, J = 6.3 Hz, 2H, CH2), 2.49 (m, 6H, CH2), 1.79 (q, J = 7.2 Hz, 2H, CH2), 0.98 (t, J = 6.9 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 148.4, 136.9, 132.7, 128.4, 124.8, 105.4, 67.9, 49.0, 46.7, 27.3, 11.6.
Compound 5b. GCMS: Calculated for C15H22NOF M+ 252. Found: 252. 1H NMR (Acetone-d6, 300 MHz) δ 7.62 ? 7.01 (m, 5H, aromatic), 6.8 (d, 1H, CH), 5.90 (d, J = 12.9 Hz, 1H, CH), 3.94 (t, J = 6.6 Hz, 2H, CH2), 2.54 (m, 6H, 3 x CH2), 2.5 (t, 2H, CH2), 1.82 (m, 2H, CH2), 1.0 (t, J = 6.9 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6,75.5 MHz) δ 149.3, 132.2, 129.0, 127.2, 116.2, 105.3, 68.9, 49.9, 47.7, 28.2, 12.5 19F NMR (Acetone-d6, 300 MHz) δ −115.8, −119.5.
Compound 5c. GCMS: Calculated for C16H25NO M+ 247. Found: 247. 1H NMR (Acetone-d6, 300 MHz) δ 7.08 (m, 5H), 5.81 (d, J = 13.2 Hz, 1H, CH), 3.89 (t, J = 6.0 Hz, 2H, CH2), 2.48 (m, 6H, 3 x CH2), 2.25 (s, 3H, CH3), 1.78 (m, 2H, CH2), 0.98 (t, J = 6.9 Hz, 6H, 2 x CH3); 13C NMR (Acetone-d6, 75.5 MHz) δ 148.7, 135.5, 130.2, 127.1, 125.7, 106.3, 68.8, 50.0, 47.7, 28.2, 21.0, 12.5.
Acknowledgements
Linda Quinones gratefully acknowledges the receipt of a MARC fellowship from NIH’s MARC program for her undergraduate research study (National Institutes of Health 2T34GM007663-32).
Cite this paper
Mohammad Al-Masum,Linda Quinones,Laurance T. Cain, (2016) Microwave Application and Anhydrous Cu(OAc)2 Mediated O-Arylation of Aliphatic Amino Alcohols. International Journal of Organic Chemistry,06,100-106. doi: 10.4236/ijoc.2016.62011
References
- 1. Kangas, L. (1990) Review of the Pharmacological Properties of Toremifene. Journal of Steroid Biochemistry, 36, 191- 195.
http://dx.doi.org/10.1016/0022-4731(90)90003-B - 2. Mass, B. (2012) Extending Tamoxifen Saves Lives, Reduces Breast Cancer Recurrences.
http://abcnews.go.com/ - 3. Krakowiak, K.E., Bradshaw, J.S., Izatt, R.M. and Za-mecka-Krakowiak, D.J. (1989) A New Building Block Method to Synthesize Symmetrical and Asymmetrical Per-N-alkyl-substituted Polyaza-Crown Compounds. The Journal of Organic Chemistry, 54, 4061-4067.
http://dx.doi.org/10.1021/jo00278a016 - 4. Anelli, P.L., Lunazzi, L., Montanari, F. and Quici, S. (1984) Doubly and Triply Bridged Polyoxapolyazaheterophanes Derived from 2,4,6-Trichloro-s-triazine. The Journal of Organic Chemistry, 49, 4197-4203.
http://dx.doi.org/10.1021/jo00196a019 - 5. Duriez, M.-C., Pigot, T., Picard, C., Cazaux, L. and Tisnes, P. (1992) Macrocyclic Polyether Tetralactams I: Synthesis and Cyclization Studies. Tetrahedron, 48, 4347-4358.
http://dx.doi.org/10.1016/S0040-4020(01)80444-0 - 6. Petranek, J. and Ryba, O. (1977) A New Type of Macrocyclic Polyether-Diamide Ligand-Binding Properties for Alkaline Earth Ions. Tetrahedron Letters, 18, 4249-4250.
http://dx.doi.org/10.1016/S0040-4039(01)83477-8 - 7. Rogers, G.A., Parsons, S.M., Anderson, D.C., Nilson, L.M., Bahr, B.A., Kornreich, W.D., Kaufman, R., Jacobs, R.S. and Kirtman, B. (1989) Synthesis, in Vitro Acetylcho-line-Storage-Blocking Activities, and Biological Properties of Derivatives and Analogs of Trans-2-(4-phenylpiperidino)cyclohexanol (Vesamicol). Journal of Medicinal Chemistry, 32, 1217-1230.
http://dx.doi.org/10.1021/jm00126a013 - 8. Molander, G.A. and Figueroa, R. (2005) Organotrifluoroborates: Expanding Organoboron Chemistry. Aldrichimica Acta, 38, 49-56.
- 9. Molander, G.A. and Ellis, N. (2007) Organotrifluoroborates: Protected Boronic Acids that Expand the Versatility of the Suzuki Coupling Reaction. Accounts of Chemical Research, 40, 275-286.
http://dx.doi.org/10.1021/ar050199q - 10. Darses, S. and Genet, J.-P. (2008) Potassium Organotrifluoroborates: New Perspectives in Organic Synthesis. Chemical Reviews, 108, 288-325.
http://dx.doi.org/10.1021/cr0509758 - 11. Quach, T.D. and Batey, R.A. (2003) Copper(II)-Catalyzed Ether Synthesis from Aliphatic Alcohols and Potassium Organotrifluoroborate Salts. Organic Letters, 5, 1381-1384.
http://dx.doi.org/10.1021/ol034454n - 12. Antilla, J.C. and Buchwald, S.L. (2001) Copper-Catalyzed Coupling of Aryl-boronic Acids and Amines. Organic Letters, 3, 2077-2079.
http://dx.doi.org/10.1021/ol0160396 - 13. Petrassi, H.M., Sharpless, K.B. and Kelly, J.W. (2001) The Copper-Mediated Cross-Coupling of Phenylboronic Acids and N-Hydroxyphthalimide at Room Temperature: Synthesis of Aryloxyamines. Organic Letters, 3, 139-142.
http://dx.doi.org/10.1021/ol0003533 - 14. Decci, C.P., Song, Y. and Evans, D.A. (2001) Intramolecular O-Arylation of Phenols with Phenylboronic Acids: Application to the Synthesis of Macrocyclic Metalloproteinase Inhibitors. Organic Letters, 3, 1029-1032.
http://dx.doi.org/10.1021/ol015572i - 15. Collman, J.P. and Zhong, M. (200) An Efficient Diamine Cop-per Complex-Catalyzed Coupling of Arylboronic Acids with Imidazoles. Organic Letters, 2, 1233-1236.
http://dx.doi.org/10.1021/ol000033j - 16. Evans, D.A., Katz, J.L. and West, T.R. (1998) Synthesis of Diaryl Ethers through the Copper-Promoted Arylation of Phenols with Arylboronic Acids: An Expedient Synthesis of Thyroxine. Tetrahe-dron Letters, 39, 2937-2940.
http://dx.doi.org/10.1016/S0040-4039(98)00502-4 - 17. Chan D.G., Winternheimer, D.J. and Merlic, C.A. (2011) Enol Silyl Ethers via Copper(II)-Catalyzed C-O Bond Formation. Organic Letters, 13, 2778-2781.
http://dx.doi.org/10.1021/ol2009297 - 18. Chan, D.M.T., Monaco, K.L., Wang, R.-P. and Winters, M.P. (1989) New N- and O-Arylations with Phenylboronic Acids and Cupric Acetate. Tetrahedron Letters, 39, 2933-2936.
http://dx.doi.org/10.1016/S0040-4039(98)00503-6 - 19. Lam, P.Y.S., Vincent, G., Clark, C.G., Deudon, S. and Jadhab, P.K. (2001) Copper-Catalyzed General C-N and C-O Bond Cross-Coupling with Arylboronic Acid. Tetrahedron Letters, 42, 3415-3418.
http://dx.doi.org/10.1016/S0040-4039(01)00510-X - 20. Herradura, P.S., Pendola, K.A. and Guy, R.K. (2000) Copper-Mediated Cross-Coupling of Aryl Boronic Acids and Alkyl Thiols. Organic Letters, 2, 2019-2022.
http://dx.doi.org/10.1021/ol005832g - 21. Zhang, L., Zhang, G., Xhang, M. and Cheng, J. (2010) Cu(OTf)2-Mediated Chan-Lam Reaction of Carboxylic Acids to Access Phenolic Esters. The Journal of Organic Chemistry, 75, 7472-7474.
http://dx.doi.org/10.1021/jo101558s - 22. Villalobes, J.M., Srogl, J. and Liebeskind, L.S. (2007) A New Paradigm for Carbon-Carbon Bond Formation:? Aerobic, Copper-Templated Cross-Coupling. Journal of the American Chemical Society, 129, 15734-15735.
http://dx.doi.org/10.1021/ja074931n - 23. Xu, J., Wang, X., Shao, C., Su, D., Cheng, G. and Hu, Y. (2010) Highly Efficient Synthesis of Phenols by Copper- Catalyzed Oxidative Hydroxylation of Arylboronic Acids at Room Temperature in Water. Organic Letters, 12, 1964-1967.
http://dx.doi.org/10.1021/ol1003884 - 24. Khatib, M.E. and Molander, G.A. (2014) Copper(II)-Mediated O-Arylation of Protected Serines and Threonines. Organic Letters, 16, 4944-4947.
http://dx.doi.org/10.1021/ol5024689 - 25. Simon, M.-O., Girard, S.A. and Li, C.-J. (2012) Catalytic Aerobic Synthesis of Aromatic Ethers from Non-Aromatic Precursors. Angewandte Chemie International Edition, 51, 7537-7540.
http://dx.doi.org/10.1002/anie.201200698 - 26. Job, G.E. and Buchwald, S.L. (2002) Copper-Catalyzed Arylation of β-Amino Alcohols. Organic Letters, 4, 3703-3706.
http://dx.doi.org/10.1021/ol026655h - 27. Shafir, A., Lichtor, P.A. and Buchwald, S.L. (2007) N- versus O-Arylation of Aminoalcohols:? Orthogonal Selectivity in Copper-Based Catalysts. Journal of the American Chemical Society, 129, 3490-3491.
http://dx.doi.org/10.1021/ja068926f
NOTES
*Corresponding author.



