International Journal of Organic Chemistry
Vol.05 No.03(2015), Article ID:59452,12 pages
10.4236/ijoc.2015.53017
Synthesis of New Fluorinated 1,2,4-Triazino [3,4-b][1,3,4]thiadiazolones as Antiviral Probes-Part II-Reactivities of Fluorinated 3-Aminophenyl-1,2,4-triazinothiadiazolone
Mohammed Saleh Tawfek Makki, Reda Mohammady Abdel Rahman, Ola Ahmad Abu Ali*
Chemistry Department, Faculty of Science, King Abdul Aziz University, Jeddah, Kingdom of Saudi Arabia
Email: *lolo_aa4@hotmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 June 2015; accepted 5 September 2015; published 8 September 2015
ABSTRACT
Some new fluorinated 3-N-acyl/3-N-alkylaminophenyl-1,2,4-triazino[3,4-b][1,3,4]thiadiazolones (2-12) have been obtained from treatment of 2-(4'-fluorophenyl)-6-(2'-amino-5'-fluorophenyl)- 1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one(1) with active functional oxygen, sulfur and halogen compounds in different conditions. Former structures of the products have been characterized from elemental and spectral data (UV, IR, NMR and Mass). The new products were evaluated as potential anthelmintic drugs.
Keywords:
Synthesis, Fluorinated 1,2,4-Triazinothiadiazolones, Anthelmintic Drugs

1. Introduction
The treatment of infectious diseases still remains an important and challenging problem because of a combination of many factors including emerging infectious diseases and the increasing number of multi-drug resistant microbial pathogens [1] -[4] . There is real perceived need for the discovery of new compounds endowed with biocidal activity. Through the various molecules designed and synthesized for this aim, it was demonstrated that fluorinated 1,2,4-triazine fused with 1,3,4-thiadiazole systems. The introduction of fluorine atom to the heterocyclic systems improves or enhances the medicinal properties [5] -[9] . On the other hand, most of heterocyclic nitrogen systems bearing an amino-groups exhibit a wide spectrum of biological activities [10] . And their use is as starting material.
Recently, synthesis and chemistry of 1,3,4-thiadiazoles as biocidal agents have been reviewed [11] [12] . Also, 1,2,4-triazine derivatives have been synthesized and evaluated as biological and pharmacological probes [13] -[15] .
Abdel-Rahman et al. [16] , reported that 1,2,4-triazino[3,4-b][1,3,4]thiadiazolones (Figure 1) used as anti HIV and anticancer drugs. In contamination of our work in these researches for new biocidal agents [17] , the present investigation reports the preparation of fluorinated 3-substitutedamino-1,2,4-triazino[3,4-b][1,3,4]thiadiazolones starting from the corresponding 3-amino analogus, as potential anthelmintic drugs.
2. Results and Discussion
3-Substituted-1,2,4-triazines and their azole-fused analogs reacts with bifunctional compounds give a more stable polycyclic systems depends on the triazine substrate nature. Search for new bioactive compounds, the main aim of the present work is preparation of fluorinated 3-substituted amino-1,2,4-triazino[3,4-b][1,3,4]thiadiazo- lones in view of their pharmacological properties.
Thus, addition of cyclohexyl isocyanate and/or 4-fluorophenyl-isothiocyanate to 3-(2'-amino-5'-fluoro-phenyl) -7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one (1) [17] , (Scheme 1) in warm DMF afforded N- (cyclohexyl)-N'-(4'-oxo-1,2,4-triazino[3,4-b][1,3,4] thiadiazol-7'-(4'-fluorophenyl)-3-(4'-fluorophenyl) urea (2) and/or N-(4'-fluorophenyl)-N'-(4'-oxo-1',2',4'-triazino[3,4-b][1,3,4]thiadiazole-7'-(4''-fluoro-phenyl)-3'-(4"-flu- orophenyl)thiourea (3) (Scheme 2). Formation of compound 3 may be tack’s place via a nucleophilic attack of NH2 group to a more electrophilic carbon of isothiocyanate.
Figure 1. Some 1,2,4-triazino[3,4-b][1,3,4]thiadiazolones as anti HIV and anti-cancer drugs.
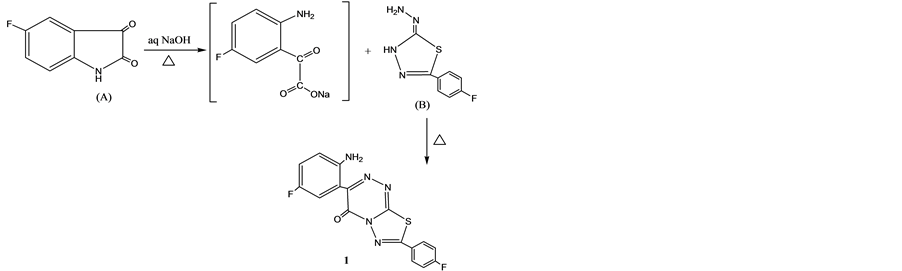
Scheme 1. Synthesis of compound 1.
Acylation of compound 1 using acetyl chloride (DMF) [18] and /orethyltrifluoroacetate in reflux (THF) [19] yielded 3-[(2'-acetylamino)-5'-fluorophenyl]-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one (4) and/or 3-(2'-trifluoroacetylamino-5'-fluoro-phenyl-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one (5) (Scheme 3). Also, self cyclo-condensation of compound 1 via boiling with DMF furnished thiadiazolo-1,2,4- triazinoindole derivative 6 (Scheme 3).
Bonded phosphorus atoms with S, O, N and C-atoms of heterocyclic system enhance their biocidal properties as herbicides, pesticides, insecticides and molluscicidal agents [20] -[22] . With this observations, the present work aims to synthesize of new fused heterobicyclicbearing fluorine and phosphorus atoms through phosphorylation of compound 1 with diphenyl phosphoryl chloride in warm DMF to give 3-(2'-diphenylphosphatoamino-

Scheme 2. Synthesis of compounds 2 and 3.

Scheme 3. Synthesis of compounds 4-6.
5'-flu-orophenyl)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one (7) (Scheme 4). Due to a highly with drown of P of phosphate moiety, the chlorine atom is very labile. Thus, simple Nu− attack of NH2 to P atom afforded the aminophosphate derivative.
Full fluorinated 3-[5'-fluoro-2'-(4"-fluorobenzoylamino)phenyl]-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4] thiadiazole-4-one (8) was obtained from treatment of compound 1 with 4-fluorobenzoylchloride in warm DMF (Scheme 4).
Due to a higher nucleophilicity of amino-group and the better displacement of labile chlorine atom of halo acids the interested point in this investigation is a simple nucleophilic attack of amino-group of compound 1 to a higher electrophilic carbons of α-haloacids as monochloroacetic acid and/or 1,1-dichloroacetic acid in warm DMF, yielded [23] 3-(5"-fluoro-2'-carboxymethylanilino)-7-(4'-fluorophenyl)-1,2,4,-triazino[3,4-b][1,3,4]thia-diazol-4- one (9) and/or 3-(5'-fluoro-2'-carboxymithinicanilino)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4] thiadiazol- 4-one (10) (Scheme 5).

Scheme 4. Synthesis of compounds 7 and 8.

Scheme 5. Synthesis of compounds 9 and 10.
Alkylation, reaction of 3-amino-1,2,4-triazinothiadiazolone 1with chloroacetonitrile in warm DMF produced [24] the 3-(5'-fluoro-2'-(cyanomethylanilido)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-4-one (11) (Scheme 6). Acidic hydrolysis of 11 by reflux with dil. HCl afforded compound 9. Decarboxylation of 9 via warm with sodium bicarbonate solution, 3-(5'-fluoro-2'-(methylanilido)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b] [1,3,4] thiadiazol-4-one (12) was isolated. The compound 12 was also, obtained from stirring of compound 1 with MeI in 1% KOH solution (Scheme 6).
The adducts formed by reactions of nitrogen containing aromatic heterocycles with various electrophilic carbon, may be stable systems, or they can undergo further transformation, such as aromatization.
Abdel-Rahmanetal reported [1] [25] , fluorine substituted thiobarbituric acid derivatives use as anti HIV-1 and cyclin dependent kinase 2 (CDK2) for cell tumor division, thus ring closure reaction of compound 3 with malonic acid in boiling with glacial acetic acid afforded the fluorine substituted N,N'-disubstitutedthiobarbituric acid 13 (Scheme 7). Formation of compound 13 was deduced from ring closure reaction of substituted thiourea 3 with malonic acid (Figure 2).

Scheme 6. Synthesis of compounds 11 and 12.
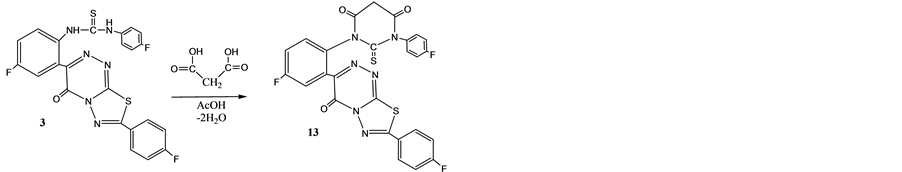
Scheme 7. Synthesis of compounds 13.
Figure 2. Formation of compound 13 from 3.
Former structures of the fluorinated 1,2,4-triazino[3,4-b][1,3,4]thiadiazolonederivatives have been established by help of their correct elemental analysis and spectral measurements:
1) UV absorption spectra of most N-acyl/phosphoryl derivatives for example 4, 6 and 7 recorded λmax 304 nm as parent amino-derivatives 1, which is may be that electronic inhibition over NH2 by high electronic acceptor acyl, and/or phosphoryl. On the other hand, UV absorption spectra of compound 5 and 8 showed λmax at 311, and 375 nm respectively, which is may be the introduction of COCF3 and/or COC6H4F-p to an amino group of 1. Thus NH proton of these compounds is highly acidic character. In addition, UV absorption spectrum of 13 showed an additive λmax at 410 nm, which attribute to formation of fluorinated thiobarbituric acid bearing of 1,2,4-triazino-1,3,4-thiadiazinone moiety.
2) IR-spectra of all the obtained compounds (expected 6, 10, and13) recorded the absorption bands at 3200 - 3100 cm for NH functional group, while, that of compounds showed an two C=O of NH acyl and 1,2,4-triazinone at 1690 - 1650 cm−1. All the synthesized showed a charactic bands of stretching and bending of C-F at γ 1250 and 720 cm−1. Only the compounds 7, exhibited the presence of P=O and C-O-Ar at 1097 and 1016 cm−1, while the compounds 4, 9, 11, and 12 showed the absorption bands of aliphatic groups at 2880 and 1440 cm−1. Some compounds as 5, 7, 8, and 11recorded a lack’s of NH functional group, which is may be formation a type of H-bonding (Figure 3).
3) NMR spectra of the new synthesized compounds was confired that structures.
a) 1H NMR spectra of all the obtained systems exhibited the presence of a resonated signals at δ 8.73 ppm for NH proton (s), in addition, δ at 7.97 - 7.94 and 7.38 - 7.35 ppm for 7 aromatic protons (m). On the other hand, compounds 2, 4, 9, 11, 12, 13 showed signals at 2.51 ppm for an aliphatic protons (COCH3, CH2CN, CH2- COOH, CH2CO) (J = 8.5). Only the compound 10 recorded δ at 8.8 ppm for N=CH proton (s).
4) 13C NMR spectra of all the prepared compounds showed a resonated signals of fluorinated 1,2,4-triazi- no[3,4-b][1,3,4]thiadiazinone carbons at δ 164-163 (C=O), 135 (C-F), 130 - 120 (aromatic C), 116 (C=N) ppm. Only the compound 3 showed a resonated signals at 180 ppm attribute to C=S. On the other hand most
Figure 3. A possible structural formula of compounds 5, 7.
of the new compounds showed an additional δ 160 ppm for new NHCO carbons.
5) Mass fragmentation study of some new systems 5 showed the molecular ion peak, with a base peak at m/e 95 and 190 (100%). The first base peak is 4-fluorophenylradical while the other is 4,4'-difluorobiphenyl radical.. Also present M+2 is attributed to S and F isotopic (Figure 4).
On the other hand, mass fragmentation study of compound 7 recorded a molecular ion peak at m/e 590 with a base peak at m/e 248 as C12H11NPO3iminophosphato ion radical with 4-fluorophenyl cation at 95%. Stability of a base peak is may be due to a higher stability of N = P group, which supported by donation and back-donation between N and P atoms (Figure 5).
3. Conclusion
The present work describes a facile and simple nucleophilic attack of amino group bearing fluorine substituted 1,3,4-thiadiazolo[2,3-c][1,2,4]triazine(1) to active electrophilic reagents via addition, fluorinated acylation/ aroylation, phosphorylation and/or alkylation reactions. Substituted amino derivatives were obtained coupling with electronic modifications overall the molecule which led to potential anthelmintic activity. Among the new compounds 5 > 7 > 1 exhibit a higher activity (55% - 60%), while other 13 > 9 > 6 > 4 (45% - 48%) showed a moderate activity towards N. brasiliensis virus. A higher activity of compounds 5 and 7 may be attributed to bourdation of COCF3 and/or phosphoryl groups with amino groups of start 1.
4. Experimental
Melting points of the products were determined on Stuart SMP3 (UK) and uncorrected. UV absorption spectra (λmax nm) were recorded in DMF on Shimadzu UV and visable 310 IPC-spectro-photometer. A Perkins Elmer Model RXI-FT IR system 55529 used for recording IR spectra of the prepared compounds γ cm−1. A Brucker advanced D P X 400 MHz model using TMS as internal standard used for recording the 1H and 13C NMR spectra of the compounds on deuterated (CDCl3, d6, δ ppm). Mass spectrum was measured on GCMS Q 1000 Ex at 70 eV. Elemental microanalysis were performed by the microanalytical at Cairo-University, Egypt.
4.1. 3-(2'-Amino-5'-fluorophenyl)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b][1,3,4] thiadi-azin-4-one (1)
Equimolar amounts of 5-fluoroisatin (in 5% aqueous NaOH), and 2-hydrazino-2-(4'-fluorophenyl)-4H-1,3,4-
Figure 4. Mass fragmentation pattern of compound 5.
Figure 5. Mass fragmentation pattern of compound 7.
thiadiazole and reflux for 3 h, cooled then poured on to ice-HCl. The solid thus obtained filtered off and crystallized from dioxan to give 1 faint yellow crystals, yield 77%, m.p. 189˚C - 195˚C. UV (EtOH) λmax 303nm. IR (γ cm−1): 3264 and 1631 (NH2), 1681 (C=O), 1601, 1507 (C=N), 1320 (cyclic NCSN), 1225 (C-F), 1156 (C-S), 826 (p-substituted phenyl), 618 (C-F). 1H NMR (DCCl3-d6) δ (ppm): 8.61 - 7.84, 7.84 - 7.83, 7.82 - 7.81, 7.818 - 7.812, 7.24,7.15,7.14, 7.13, 7.115, 7.110 (aromatic CH), 3.5 (s, 2H, NH2). 13C NMR (DCCl3-d6) δ (ppm): 165, 163, 160, 130.58, 130.49, 130.37, 116.15, 115.93, 77.34, 77.03, 76.71. M/Z: (Int.%): 357 (57% M+ H2O) 244 (1.0), 206 (15.0), 178 (100), 134 (45), 95 (25.0). CHNSF analysis for C16H9N5SF2O (357), Calcd: C, 53.78; H, 2.52; N, 19.60; S, 8.63; F, 10.64%. Found: C, 53.58; H, 2.32; N, 19.55; S, 8.33; F, 10.43%.
4.2. N-(Cyclohexyl)-N'-[4'-oxo-1,2,4-triazino[3,4-b][1,3,4]thiadiazol-7'- (4"-fluorophenyl)-3'-(4"-fluorophenyl)]-urea (2).
A mixture of compound 1 (0.01 mol) and cyclohexyl isocyanate (0.01 mol) in DMF (50 ml) warmed for 2h, cooled then poured onto ice. The solid produced filtered off and crystallized from dioxan to give 2 as faint yellow. Yield 60%, m.p. 178˚C - 180˚C. IR (γ) cm−1: 3325 (NH), 2928, 2851 (aliphatic CH, CH2) 1700 (C=O), 1629 (CONH), 1602 (C=N), 1413 (deformation CH2), 1320 (cyclic NCSN), 1225 (C-F), 1155 (C-S), 826, 796 (p-substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s, 2H, NH, NH), 7.97, 7.96, 7.95, 7.94 (4H, aryl protons), 7.38, 7.37, 7.35 (3H, aryl protons), 2.511, 1.8, 1.7, 1.6, 1.2, (each s, 11H, aliphatic protons). 13C NMR (DMSO-d6) δ (ppm): 168.4, 163.4 (2 C=O), 160 (C-S), 130.71, 130.65, 130.38, 130.36 (aromatic carbons), 116, 116.1 (C-F), 33.32, 25.28, 24.45 (aliphatic carbons). CHNSF analysis for C23H20N6SF2O2 (482). Calcd: C, 57.26; H, 4.14; N, 17.42; S, 6.6; F, 7.88%. Found: C, 56.96; H, 4.05; N, 17.11; S, 6.33; F, 7.75%.
4.3. N-(4'-Fluorophenyl)-N'-[(4'-oxo-1',2',4'-triazino[3,4-b][1,3,4]thiadiazol-7'- (4"-fluoro-phenyl)-3'-(4"-fluorophenyl)]thiourea (3)
A mixture of 1 (0.01 mol) and 4-fluorophenyl isothiocyanate (0.01 mol) in DMF (20 ml) was refluxed for 1h, cooled then poured onto ice. The yielded solid filtered off and crystallized from EtOH to give 3 as yellow crystals. Yield 72%, m.p. 164˚C - 165˚C. IR (γ) cm−1: 3272 (NH), 3016 (aromatic CH), 1695 (C=O), 1612 (C=N), 1600 (C=C), 1328 (cyclic NCSN), 1225 (C-F), 1208 (C=S), 1154 (C-S), 829,732, 719 (p-substituted phenyl), 639 (C-F).1H NMR (DMSO-d6) δ (ppm): 9.76, 8.73 (each s, 2H, NH), 7.97, 7.95, 7.47, 7.46, 7.45, 7.44, 7.38, 7.35, 7.18, 7.14, 7.11 (m, m, 11H, aromatic protons). 13C NMR (DMSO-d6) δ (ppm): 180.29 (C=S), 164.7 (C=O), 135.98, 135.62, 135.61 (C-N), 130.71, 126.24 (aromatic carbons), 119.97, 119.92 (C-N), 116.14, 115.32, 114.98 (C-F). CHNSF analysis for C23H13N6S2F3O (510). Calcd: C, 54.11; H, 2.54; N, 16.47; S, 12.54; F, 11.17%. Found: C, 53.98; H, 2.48; N, 16.24; S, 12.38; F, 10.27%.
4.4. 3-[(2'-Acetylamino)-5'-fluorophenyl]-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b] [1,3,4]thiadiazol-4-one (4)
A mixture of 1 (0.200 gm) and glacial acetic acid (5 ml) was warmed for 5 min, cooled then poured onto ice. The solid produced filtered off and crystallized from AcOH to give 4 as pall yellow crystals. Yield 55%, m.p. 182˚C - 183˚C. IR (γ) cm−1: 3123 (NH), 1632 (C=O), 1603 (C=N), 1414 (deformation CH3), 1321 (cyclic NCSN), 1225 (C-F), 1155 (C-S), 827, 795 (p-substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (m, 4H, aromatic protons), 7.38, 7.37, 7.3 (m, 3H, aromatic protons) 2.51 (s, 3H, CH3). 13C NMR (DMSO-d6) δ (ppm): 164.71 (C=O), 160.50 (C=O), 130.71, 130.65, 130.36, (aromatic carbons), 116.15, 116.0 (C-F), 39.03 (CH3). CHNSF analysis for C18H11N5SF2O (399). Calcd: C, 54.13; H, 2.75; N, 17.54; S, 8.0; F, 9.52%. Found: C, 53.88; H, 2.69; N, 17.32; S, 7.75; F, 9.40%.
4.5. 3-(2'-Trifluoroacetylamino-5'-fluorophenyl)-7-(4'-fluorophenyl)-1,2,4-triazino [3,4-b][1,3,4]thiadiazol-4-one (5)
Equimolar mixture of 1 and trifluoroethyl acetate in THF (50 ml) refluxed for 2 h, cooled. The solid thus obtained filtered off and crystallized from dioxan to give 5 as white crystals. Yield 82%, m.p. 179˚C - 180˚C. IR (γ) cm−1: 1632 (C=O), 1603 (C=N), 1321 (cyclic NCSN), 1225 (C-F), 1155 (C-S), 827, 795 (p-substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (4H, aromatic protons), 7.38, 7.37, 7.35 (3H, aromatic protons). 13C NMR (DMSO-d6) δ (ppm): 164.71 (C=O), 160.50 (C=O), 130.71, 130.65, 130.38, 130.36 (aromatic carbons), 116.14, 116.00 (C-F). M/S (Int.%): 455 (M+2, 0.11%), 190 (100), 153 (85.0), 138 (3.33), 95 (100), 66 (1.181, 60, 13.0). CHNSF analysis for C18H8N5SF2O2 (455, M+2). Calcd: C, 47.68; H, 1.76; N, 15.45; S, 7.06; F, 20.97%. Found: C, 47.38; H, 1.73; N, 15.19; S, 6.88; F, 20.67%.
4.6. 10-(4'-Fluorophenyl)-4-fluoro-1,3,4-thiadiazolo[2,3-c][1,2,4]triazino[5,6-b]indole (6)
Compounds 1 (0.20 gm) in DMF (20 ml) refluxed for 3h, cooled then poured onto ice. The yielded solid filtered off and crystallized from EtOH to give 6 as yellowish crystals. Yield 60%, m.p. 184˚C - 185˚C. IR (γ) cm−1: 1603 (C=N), 1321 (cyclic NCSN), 1226 (C-F), 1155 (C-S), 827, 796 (p-substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 7.97, 7.96, 7.95, 7.94 (4H, aromatic protons), 7.38, 7.37, 7.35 (3H, aromatic protons).13C NMR (DMSO-d6) δ (ppm): 130.71, 130.65, 130.38, 130.36 (aromatic carbons), 116.14, 116.0 (C-F). CHNSF analysis for C16H7N5SF2 (339). Calcd: C, 56.63; H, 20.6; N, 20.6; S, 9.43; F, 11.20%. Found: C, 56.55; H, 2.03; N, 20.18; S, 9.14; F, 10.98%.
4.7. 3-(2'-Diphenylphosphatoamino-5'-fluorophenyl)-7-(4'-fluorophenyl)-1,2,4- triazino[3,4-b][1,3,4]thiadiazol-4-one (7)
An equimolar mixture of 1 (0.01 mol) and diphenyl phosphoryl chloride (0.01mol) in DMF (20 ml) refluxed for 30 min, then, cooled and poured onto ice. The produced solid filtered off and crystallized from THF to give 7 as yellowish crystals, yield 60%, m.p. 173˚C - 175˚C. IR (γ) cm−1: 3061(aromatic CH), 1632 (C=O), 1602 (C=N), 1320 (cyclic NCSN), 1225 (C-F), 1155 (C-S), 1097 (P=O), 1016 (Ph-O-P), 962, 936, 870, 827, 795 (substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (4H, aromatic protons), 7.38, 7.37, 7.35 (3H, aromatic protons), 7.2-6.8 (m,10H, phenyl protons). 13C NMR (DMSO-d6) δ (ppm): 160.50 (C=O), 130.71, 130.65, 130.38, 130.36 (aromatic carbons), 116.14, 116.0 (C-F). M/S (Int.%):590 (M+, 0.11%), 248 (100), 247 (11.8), 95 (95.0). CHNSF analysis for C28H18N5SF2PO4 (589). Calcd: C, 57.04; H, 3.05; N, 11.88; S, 5.43; F, 6.45%. Found: C, 57.01; H, 2.98; N, 11.60; S, 5.37; F, 6.14%.
4.8. 3-[5'-Fluoro-2'-(4"-fluorobenzoylamino)-phenyl]-7-(4'-fluorophenyl)-1,2,4- triazino[3,4-b][1,3,4]thiadiazol-4-one (8)
A mixture of 1 (0.01 mol) and 4-fluorobenzoyl chloride (0.01 mol) in DMF (20 ml) refluxed 1 h, cooled. The reaction mixture poured onto ice. The solid produced filtered off and crystallized from dioxan to give 8 as yellowish crystals, yield 65%, m.p. 149˚C - 150˚C. IR (γ) cm−1: 3200-3100 (b, OH, NH), 1677 (cyclic C=O), 1633 (NHCO), 1602 (C=N), 1315 (cyclic NCSN), 1225 (C-F), 1156 (C-S), 827, 796, 768 (p-substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 13.09 (s,1H, NH), 8.02, 8.05, 8.01, (m, 3H, aromatic protons), 7.97, 7.96, 7.95, 7.94 (m, 4H, aromatic protons), 7.38-7.32 (m, 4H, aromatic protons). 13C NMR (DMSO-d6) δ (ppm): 166.36, 164.04 (2 C=O), 132.11, 132.05, 130.65, 130.38, 130.36, 127.33 (aromatic carbons), 116.15, 116.0 (C-F), 115.69, 115.54 (C-N). CHNSF analysis for C22H12N5SF3O2 (467). Calcd: C, 56.53; H, 2.56; N, 14.98; S, 6.85; F, 12.20%. Found: C, 56.28; H, 2.50; N, 14.53; S, 6.67; F, 12.00%.
4.9. 3-(5'-Fluoro-2'-carboxymethylanilino)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b] [1,3,4]thiadiazol-4-one (9)
A mixture of 1(0.01 mol) and monochloroacetic acid (0.01 mol) in DMF (20 ml) refluxed for 1h, cooled then poured onto ice. The solid produced filtered off and crystallized from THF to give 9 as yellowish crystals, yield 72%, m.p. 180˚C - 182˚C. IR (γ) cm−1: 3500 - 3100 (b, OH, NH), 1720, 1633 (2 C=O), 1603 (C=N), 1490, 1413 (deformation CH2), 1321 (cyclic NCSN), 1226 (C-F), 1156 (C-S), 826, 796 (substituted phenyl), 636 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (m, 4H, aromatic), 7.38, 7.37, 7.35 (m, 3H, aromatic), 4.55 (s, 1H, OH), 2.51 (s, 2H, J,8.7, CH2). 13C NMR (DMSO-d6) δ (ppm): 164.71 (C=O), 163.06 (C=O), 130.70, 130.65, 130.38, 130.36(aromatic carbons), 116.14, 116.0 (C-F), 39.03 (CH2). CHNSF analysis for C18H11N5SFO3 (415). Calcd: C, 52.53; H, 2.65; N, 16.86; S, 7.71; F, 9.15%. Found: C, 52.33; H, 2.62; N, 16.59; S, 7.62; F, 9.04%.
4.10. 3-(5'-Fluoro-2'-carboxymethinicanilino)-7-(4'-fluorophenyl)-1,2,4-triazino [3,4-b][1,3,4]thiadiazol-4-one (10)
A mixture of 1 (0.01 mol) and 1,1'-dichloroacetic acid (0.01 mol) in DMF (20 ml) refluxed for 30 min, cooled then poured onto ice. The solid thus obtained filtered off and crystallized from EtOH to give 10 as yellowish crystals, yield 60%, m.p. 154-155˚C. IR (γ) cm−1: 3500 - 3300 (OH), 1696, 1632 (C=O), 1602 (C=N), 1321 (cyclic NCSN), 1226 (C-F), 1155 (C-S), 826, 796 (substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.8 (s,1H, N=CH), 7.97, 7.96, 7.95, 7.94 (m, 4H, aromatic), 7.38, 7.37, 7.35 (m, 3H, aromatic), 4.41 (s, 1H, OH). 13C NMR (DMSO-d6) δ (ppm): 164.71 (C=O), 163.06 (C=O), 130.71, 130.65, 130.38, 130.36, 128.72, 127.25 (aromatic carbons), 116.14, 116.0 (C-F). CHNSF analysis for C18H9N5SFO3 (413). Calcd: C, 52.30; H, 2.17; N, 16.94; S, 7.74; F, 9.20%. Found: C, 52.08; H, 2.11; N, 16.55; S, 7.54; F, 8.89%.
4.11. 3-(5"-Fluoro-2'-cyanomethylanilino)-7-(4'-fluorophenyl)-1,2,4-triazino[3,4-b] [1,3,4]thiadiazol-4-one (11)
Equimolar amounts of 1 and chloroacetonitrile in DMF (20 ml) refluxed for 1h, cooled then poured onto ice. The solid produced filtered off and crystallized from THF to give 11 as brown crystals, yield 65%, m.p. 177˚C - 178˚C. IR (γ) cm−1: 3180 (NH), 2220 (C N), 1635 (C=O), 1507, 1413 (deformation CH2), 1320 (Cyclic NCSN), 1225 (C-F), 1155 (C-S), 226, 796 (Substituted phenyl), 635 (C-F).1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (4H, aromatic), 7.38, 7.37, 7.35 (m, 3H, aromatic), 3.36 (2H, J, 6.6 p.c.s, CH2).13C NMR (DMSO-d6) δ (ppm): 160.50 (C=O), 130.71, 130.65, 130.38, 130.36, 128.91, 127.88 (aromatic carbons), 116.14, 116.0 (C-F), 39.03 (CH2CN). CHNSF analysis for C18H10N6SF2O(396). Calcd: C, 54.54; H, 2.52; N, 21.21; S, 8.08; F, 9.59%. Found: C, 54.31; H, 2.50; N, 20.89; S, 7.98; F, 9.41%.
4.12. 3-(5'-fluoro-2'-Methylanilino)-7-(4'-Fluorophenyl)-1,2,4-Triazino[3,4-b][1,3,4] Thiadi-Azol-4-One (12)
A mixture of 9 (0.20 gm) and K2CO3solution (5%, 50 ml) refluxed for 1h, cooled then acidification use 5% HCl. The solid obtained filtered off and crystallized from EtOH to give 12 as brownish crystals, yield 55%, m.p. 178˚C - 180˚C. IR (γ) cm−1: 3062 (aromatic CH), 1700, 1632 (C=O), 1600 (C=N), 1507, 1413 (deformation CH3), 1321 (cyclic NCSN), 1225 (C-F), 1155 (C-S), 820, 795 (Substituted phenyl), 635 (C-F). 1H NMR (DMSO-d6) δ (ppm): 8.73 (s,1H, NH), 7.97, 7.96, 7.95, 7.94 (m, 4H, aromatic), 7.38, 7.37, 7.35 (m, 3H, aromatic), 2.22 (s, 3H, CH3N). 13C NMR (DMSO-d6) δ (ppm): 160.11 (C=O), 130.71, 130.65, 130.38, 130.36, 128.72 (aromatic carbons), 116.14, 116.0 (C-F), 22.65(CH3). CHNSF analysis for C17H11N5SF2O(371). Calcd: C, 54.98; H, 2.96; N, 18.86; S, 8.62; F, 10.24%. Found: C, 54.69; H, 2.22; N, 18.43; S, 8.49; F, 9.98%.
4.13. Formation of 9
A mixture of 11 (0.20 gm) and diluted HCl (10%, 50 ml) refluxed for 1h, coled. The solid produced filtered off and crystallized from THF to give 9 as yellowish crystals, yield 60%, m.p. 178˚C - 179˚C. Mixed melting point no depression.
4.14. N'[2'-(4"-Fluorophenyl)-5-oxo-6-(5'-fluorophenyl-2"-yl)-N3-(4'-fluorophenyl)- thiobarbituric acid (13)
Equimolar mixture of 3 and malonic acid in glacial acetic acid (20 ml) refluxed for 4 h, cooled and poured onto ice. Extracted the organic layer by diethyl ether and leaf at room temperature. The solid obtained crystallized from dioxan to give 13 faint yellow crystals, yield 65%, m.p. 150˚C - 151˚C. IR (γ) cm−1: 3530 (OH), 1660 (C=O), 1488 (deformation CH2), 1385 (NCSN), 1255 (C-F), 1205 (C=S), 670 (C-F). 1H NMR (DMSO-d6) δ (ppm): 10.04 (s,1H,OH), 8.2 - 8.0 (m, 4H, aromatic protons), 7.60-7.44 (m, 4H, aromatic protons),7.0 - 6.98 (m, 3H, aromatic protons), 3.55, 2.59 - 2.58 (s, 2H, CH2). 13C NMR (DMSO-d6) δ (ppm): 181.10, 165.71, 160.00, 159.38, 138.2, 133.40, 134.20, 126.91, 126.80, 125.1, 121.69, 115.45, 115.21, 77.79, 77.57, 77.36, 44.36, 40.46-39.8). CHNSF analysis for C36H13N6S2F3O3 (578). Calcd: C, 53.97; H, 2.24; N, 14.53; S, 11.67; F, 9.86%. Found: C, 53.79; H, 2.11; N, 14.33; S, 11.55; F, 9.59%.
5. Pharmacological Evaluation
1,2,4-Triazine derivatives showed a wide biocidal spectrum [13] -[15] . Also, 1,3,4-thiadiazoles exhibited a large biocidal agents [11] [12] . In addition, introduction of both fluorine atoms and/or amino groups to heterocyclic systems often improve their medicinal properties [5] -[9] . Thus, in search for new drugs as potential anthelmintic to control on the smoke diseases, the present work, aim to obtain new drugs. All the new synthesized compounds were screened for their anthelmintic activity against H. nana infection in mice, by using the standard method of steward. The oral dose was 200 mg/Kg given for 2 days. Only the compounds 1, 3, 5, 6, 7, 9 and 13 recorded a weak activity (10 <%). On the other hand, evaluation of these compounds against N-brasiliensis infection in rats ta the same oral dose, by using other standard method [26] [27] . The obtained results showed that the activity in range of 25% - 60% (Table 1).
From the obtained results (Table 1) we can be conclude that compounds containing COCF3 are highly effect than aromatic C-F. also, presence of phosphate group bonded to NH enhance that activity. Full fluorinated
Table 1. Anthelmintic activity of the new synthesized compounds.
N,N'-diarylthiourea showed a rise activity towards N-brasifiensis. As well as N-alkyl systems exhibited a moderate activity. Thus, atype of both compounds 5 and 7 would present a fruitful matrix for the future development of a new class of potential anthelmintic agents, that deserves further investigation and derivation. A simple of nucleophilic attack of amino group of fluorinated 1,2,4-triazino[2,3-c]thiadiazolone to various electrophilic agents was deduced to give N-substituted analogues. The anthelmintic activity of these systems was evaluated. Among these tested analogs, compounds 5 and 7 showed 50% - 60% activity, while all the tested compounds exhibited below 10% activity towards H. nana.
Cite this paper
Mohammed Saleh TawfekMakki,Reda Mohammady AbdelRahman,Ola Ahmad AbuAli, (2015) Synthesis of New Fluorinated 1,2,4-Triazino [3,4-b][1,3,4]thiadiazolones as Antiviral Probes-Part II-Reactivities of Fluorinated 3-Aminophenyl-1,2,4-triazinothiadiazolone. International Journal of Organic Chemistry,05,153-165. doi: 10.4236/ijoc.2015.53017
References
- 1. Al-Harbi, A.S., Abdel-Rahman, R.M. and Asiri, A.M. (2014) Synthesis of Some New Fluorine Substituted Thiobarbituric Acid Derivatives as Anti-HIV1 and Cyclin-Dependent Kinase 2(CDK2) for Cell Tumor Division—Part II. International Journal of Organic Chemistry, 4, 142-153.
http://dx.doi.org/10.4236/ijoc.2014.42016 - 2. Makki, M.S.T., Ab-del-Rahman, R.M. and Khan K.A. (2014) Fluorine Substituted 1,2,4-Triazinones as Potential Anti-HIV-1 and CDK2 Inhicitors. Journal of Chemistry, 2014, Article ID: 430573.
- 3. Makki, M.S.T., Abdel-Rahman, R.M., Faidallah, H.M. and Khan K.A. (2013) Synthesis of New Fluorine Substituted Heterocyclic Nitrogen Systems Derived from p-Aminosalicyclic Acid as Anti-Mycobacterial Agents. Journal of Chemistry, 2013, Article ID: 819462.
- 4. Makki, M.S.T., Abdel-Rahman, R.M., Faidallah, H.M. and Khan, K.A. (2013) Synthesis of Substituted Thioureas and Their Sulfur Heterocyclic Systems of p-Aminosalicylic Acid as Anti-Mycobacterial Agents. Journal of Chemistry, 2013, Article ID: 862463.
- 5. Abdel-Rahman, R.M. and Ali, T.S. (2013) Synthesis and Biological Evaluation of Some New Polyfluorinated 4-Thiazolidinone and α-Aminophosphonic Acid Derivatives. Monatshefte für Chemie, 144, 1243-1252.
http://dx.doi.org/10.1007/s00706-013-0934-6 - 6. Makki, M.S.T., Bakhotmah, D.A., Abdel-Rahman, R.M. and Elshahawy, M.S. (2012) Designing and Synthesis of New Fluorine Substituted Pyrimidine-Thion-5-Carbonitriels and the Related Derivatives as Photochemical Probe Agents for Inhibition of Vitiligo Diseases. International Journal of Organic Chemistry, 2, 311-320.
- 7. Makki, M.S.T., Bakhotmah, D.A. and Abdel-Rahman, R.M. (2012) Highly Efficient Synthesis of Novel Fluorine Bearing Quinolone-4-carboxylic Acid the Related Compounds as Amylolytic Agents. International Journal of Organic Chemistry, 2, 49-55.
http://dx.doi.org/10.4236/ijoc.2012.21009 - 8. Abdel-Rahman, R.M., Makki, M.S.T. and Bawazir, W.A. (2011) Synthesis of Some More Fluorine Heterocyclic Nitrogen Systems Derived from Sulfa Drugs as Photochemical Probe Agents for Inhibition of Vitiligo Disease—Part I. E-Journal of Chemistry, 8, 405-414.
http://dx.doi.org/10.1155/2011/586063 - 9. Abdel-Rahman, R.M., Makki, M.S.T. and Bawazir, W.A. (2010) Synthesis of Some More Fluorine Heterocyclic Nitrogen Systems Derived from Sulfa Drugs as Photochemical Probe Agents for Inhibition of Vitiligo Disease—Part II. E-Journal of Chemistry, 7, S93-S102.
- 10. Abdel-Rahman, R.M. (2001) Chemistry of Uncondensed 1,2,4-Triazines, Part IV—Synthesis and Chemistry of Bioactive 3-Amino-1,2,4-triazines and Related Compounds. Pharmacies, 56, 275-286.
- 11. Shawali, A.S, (2014) 1,3,4-Thiadiazoles of Pharmalogical Interest: Resent Trends in Their Synthesis via Tandem 1,3- Dipolar-Cycloaddition: Review. Journal of Advanced Research, 5, 1-17.
http://dx.doi.org/10.1016/j.jare.2013.01.004 - 12. Kushwaha, N., Kushwaha, S.K.S. and Rai, A.K. (2011) Biological Activities of Thiadiazole Derivatives. International Journal of ChemTech Research, 4, 517-531.
- 13. Abdel-Rahman, R.M., Makki, M.S.T., Ali, T.S. and Ibrahim, M.A. (2012) 1,2,4-Triazine Chemistry Part III-Synthesis Strategies to Functionalized Brideghead Nitrogen Hetero Annulated 1,2,4-Triazine Systems and Their Region Specific and Pharmacological Properties. Current Organic Synthesis, 9, 1-25.
- 14. Abdel-Rahman, R.M., Makki, M.S.T., Ali, T.S. and Ibrahim, M.A. (2010) 1,2,4-Triazine Chemistry Part I: Orientation of Cyclization Reaction of Functionalized 1,2,4-Triazine Derivatives. European Journal of Chemistr, 1, 236-245.
http://dx.doi.org/10.5155/eurjchem.1.3.236-245.54 - 15. Abdel-Rahman, R.M., Makki, M.S.T., Ali, T.S. and Ibrahim, M.A. (2014) 1,2,4-Triazine Chemistry Part IV: Synthesis and Chemical Behavior of 3-Functionalized-5,6-Diphenyl-1,2,4-Triazines towards Some Nucleophilic and Electrophilic Reagents. Journal of Hetero-cyclic Chemistry, ID JHET, 12-0734.
http://dx.doi.org/10.1002/jhet.2014 - 16. El-Gendy, Z., Morsy, J.M., Allimony, H.A., Abdel-Monem, W.R. and Abdel-Rahman, R.M. (2001) Synthesis of Heterobicyclic Nitrogen Systems Bearing the 1,2,4-Triazine Moiety as Anti-HIV and Anticancer Drugs, Part III. Pharmazie, 56, 376-383.
- 17. Makki, M.S.T., Ab-del-Rahman, R.M. and Abu-Ali, O.A.(2015) Synthesis of Some More New Fluorinated 1,2,4-Triazino[3,4-b][1,3,4] Thiadiazolones and Their Molluscicidal Against Selective Snails—Part I. Journal of Chemistry and Chemical Engineering, 9, 162-175.
- 18. Abdel-Rahman, R.M. (1991) Synthesis and Anti-Human Immune Virus Activity of Some New Fluorine Con-taining Substituted 3-Thioxo-1,2,4-Triazin-5-Ones. Farmaco, 46, 379-389.
- 19. Abdel-Rahman, R.M. (1992) Synthesis of New Fluorine Bearing Trisubstituted 3-Thioxo-1,2,4-Triazin-5-Ons as Potential Anticancer Agents. Farmaco, 47, 319-326.
- 20. Al-Romazian, A.N., Makki, M.S.T. and Abdel-Rahman, R.M. (2014) Synthesis of New Fluorine/Phosphorus Substituted 6-(2’-Aminophenyl)-3-Thioxo-1,2,4-5(2H,4H)One and Their Related Alkylated Systems as Molluscicidal Agent as against the Snails Responsible for Bilharziiasis Diseases. International Journal of Organic Chemistry, 4, 154-168.
http://dx.doi.org/10.4236/ijoc.2014.42017 - 21. Breuer, E. (1996) The Chemistry of Organophosphorus Compounds. John Wiley and Sons, NewYork.
- 22. Blakley, B., Broussea, U., Fournier, M. and Voccia, A.I. (1999) Immunotoxicity of Pesticides: A Review. Toxicology and Industral Health, 15, 119-132.
http://dx.doi.org/10.1177/074823379901500110 - 23. Ibrahim, M.A., Abdel-Rahman, R.M., Abdel-Halim, A.M., Ibracim, S.S. and Allimony, H.A., (2008) Synthesis and Antifungal Activity of Novel Polyheterocyclic Compounds Containing 1,2,4-Triazine Moiety. Archive for Organic Chemistry, 2008, 202-215.
http://dx.doi.org/10.3998/ark.5550190.0009.g19 - 24. Abdel-Rahman, R.M. and Islam, E.I. (1993) Synthesis and Reactions of Acetonitrile Dervatives Bearing a 5,6-Diphenyl-1,2,4-Tryazin-3-yl Moiety. Indian Journal of Chemistry, 32, 526-529.
- 25. Al-Harbi, A.S., Abdel-rahman, R.M. and Asiri, A.M. (2015) Synthesis of Some New Fluorine Substituted Thiobarbituric Acid Derivatives as Anti HIV-1 and Cyclin-Dependent Kinase 2(CDK2) for Cell Tumor Division-Part I. European Journal of Chemistry, 6, 63-70.
http://dx.doi.org/10.5155/eurjchem.6.1.63-70.1147 - 26. Steward, J.S. (1955) Anthelmintic Studies: II. A Double Entero-Nemacidal Anthelmintic Test Covering a Wide Range of Activities. Parasitology, 45, 242-254.
http://Dx.Doi.Org/10.1017/S003118200002761x - 27. Stadden, O.P. (1963) Experimental Chemotherapy. Academic Press, New York.
NOTES
*Corresponding author.








