International Journal of Organic Chemistry
Vol.3 No.3A(2013), Article ID:39034,13 pages DOI:10.4236/ijoc.2013.33A003
Efficient Synthesis of a New Class of N-Nucleosides of 4H-Thiochromeno[2,3-d]pyrimidine-10-Sulfone as Potential Anticancer and Antibacterial Agents
1Department of Chemistry, Al-Imam Mohammad Ibn Saud Islamic University (IMSIU), Faculty of Science, Riyadh, KSA
2Photochemistry Department, Heterocyclic & Nucleosides Unit, National Research Centre, Cairo, Egypt
Email: aalshamm@yahoo.com
Copyright © 2013 Abdulrahman G. Alshammari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 2, 2013; revised October 5, 2013; accepted October 15, 2013
Keywords: Thiochromene; Pyrimidine; Antibacterial; Anticancer Agents
ABSTRACT
A highly practical and efficient preparation of 6-methy-4H-thiochromene and 7-methyl-thiochromene[2,3-d]pyrimidine derivatives was developed via a multi-component reaction of 3-methyl-thiophenol (1), aldehydes (2), and malononitrile (3). A series of pyrimidine nucleoside, thiochromene[2,3-d]pyrimidine and thiochromene[2,3-d]pyrimidine-10-sulfone was efficiently obtained. These hybrid compounds were evaluated as potential antibacterial and anticancer agents and showed encouraging biological activities. Some of these derivatives showed broad-spectrum antitumour activity against the nine tumour subpanels tested, and demonstrated significant activity in the in vitro antitumour screening expressed by MG-MID log10GI50 value of −4.55, −4.67 and −4.73 of compounds 9a, 9b and 9c, respectively.
1. Introduction
Derivation of thiopyranopyrimidine and pyrimidine nucleosides has attracted much attention because of potent antitumor and antiviral activity [1]. Pyrimidine nucleosides substituted at the C-5 position constitute a class of biologically significant molecules [2]. The well-known cancer chemotherapeutic 5-fluorouracil and antiviral agents, such as 5-iodo-2’-deoxyuridine and 5-(trifluoro methyl)-2’-deoxyuridine, have been in clinical use for several years [3]. Meanwhile, in recent years, there have been significant interests in the potential usages of the C-5-substituted pyrimidine nucleosides in synthetic oligonucleotide probes as a tether site for linking reporter groups to nucleic acids [4]. When the modified nucleosides are incorporated into the duplex B-DNA, C-5-substituents are located in the major groove [5], and so do not disrupt Watson-Crick base pairing. As a result, the methodologies for constructing suitable linker arms and generating bonds to C-5 are important for the synthesis of potential therapeutic agents and synthetic oligonucleotide probes. Therefore, the 5-position of pyrimidine nucleosides has been the target of extensive studies on modifications [1].
Also, 1-benzothiopyran-4-ones are the thioanaloggues of flavones [6] which are a class of naturally occurring pharmacologically active compounds. The thioflavones also exhibit various pharmacological activities [7] such as antimalarial, antimicrobial, and antifungal activity and are useful as potent inhibitors of steroid sulfatase [8]. In general, 4H-1-benzothio pyran-4-ones are synthesized by the condensation of thiophenols with ethyl benzoylacetates in polyphosphoric acid, but the yields are low to moderate [9]. The cyclization of ethyl β-(aryl thio)-cinnamates, derived from Michael addition of thiophenols to ethyl phenylpropiolates, with stannic chloride or phosphorus pentoxide, methanesulfonic acid, gives 2-phenyl- 4H-1-benzo-thiopyran-4-ones [10]. However, this method could not be applied for the synthesis of methoxy substituted thioflavones because competitive cyclization into the cinnamyl aromatic ring, rather than the sulfur-bearing ring, occurs when cinnamyl ring is activated by methoxy substituent. The reaction of S-aroyl derivatives of thio salicylic acid with N-phenyl-(triphenylphosphoranylidene) ethemine [11] or (trimethylsilyl)-methylene-triphenylphosphorane [12] leads to the acylphosphoranes which undergo subsequent intramolecular Wittig cyclization to afford 2-phenyl-4H-1-benzothiopyran-4-ones, but the separation of phenyl isocyanate is tedious. Alternatively, the condensation of methyl thiosalicylate with trilithiated acetoacetanilides [13] or dilithiated N-benzoyl hydrazones [14] with excess lithium diisopropylamide gives the C-acylated intermediates which undergo subsequent cyclo-dehydration and hydrolysis with HCl to afford 2- phenyl-4H-1-benzothiopyran-4-ones in moderate to high yields. With the aim to develop and identify novel active compounds, we carried out a number of modifications on the structure of organic reagents.
I was devoid of any activity [15], first obtaining the planer isosteric derivative II (Figure 1). Also, as a part of our research program devoted to the preparation and evaluation of new anticancer agents, we extensively studied several polycyclic chromophores [16-19] among which we recently disclosed that the pyrido [2,3-d] pyrimidine, pyrimido-quinolines, thieno [2,3-d] pyrimidine and triazolo [4,3-a] pyrimidin-6-sulfonamide showed a detectable cytotoxic activity on human tumor cell lines which was ascribed to its ability to interfere with mitochondrial functions.
2. Chemistry
In this paper, we describe a new efficient synthesis of 2- amino-3-cyano-4H-thiochromene-4-aryl 4 via cyclocondensation of arylidenemalononitriles (aldehyde + malono nitrile) with 3-methyl-thio-phenol in high yields under the mild conditions, in which the thiopyran moiety gave us the potential to insert a pendant amino and cyano groups both at the 2- and in the 3-position of the chromophore. The reactivity of the new derivatives towards the organic reagents such as formic acid has been studied. The preparation of the thiochromene and the 4-aryl substituted derivatives were performed following the synthetic route described in Scheme 1. The starting 2- amino-4-(4-aryl)-6-methyl-4H-thiochromeno-3-carbonitrile (4a-f) were prepared by the following described procedure via the condensation of thiophenol derivatives 1 with aldehydes and malononitrile 3 in ethanol/piperidine [19].
The compounds were purified by recrystalization and Characterized by analytical and spectral data. In particular, the most discriminating features of the 1H NMR
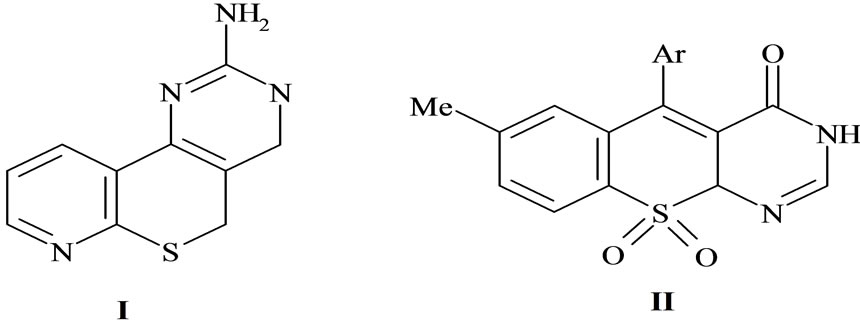
Figure 1. The planer isosteric derivative I, II.
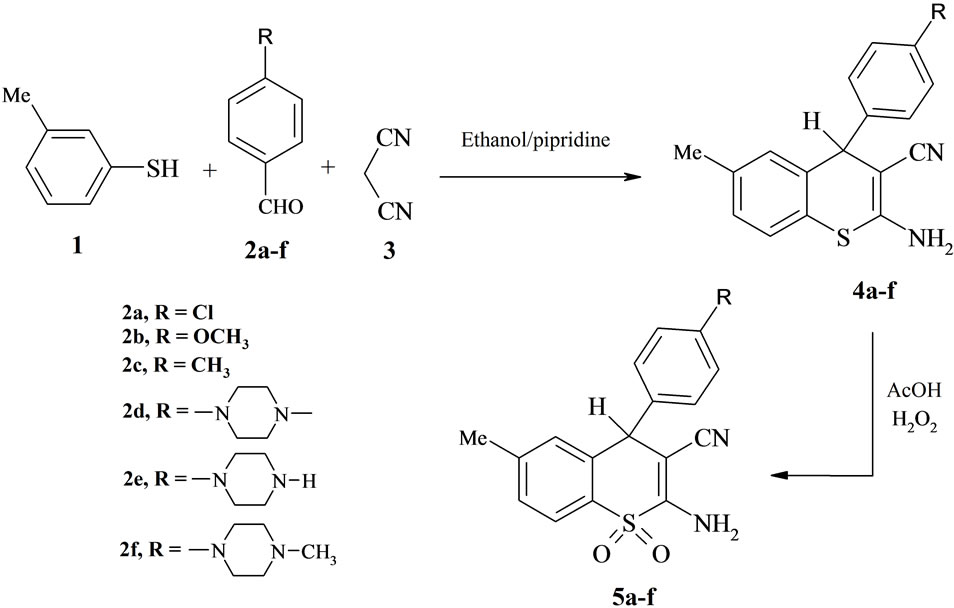
Scheme 1. Synthesis of the starting materials.
spectra of compounds 6a-f was a singlet at ≈4.50 ppm attributed to the proton in the 4-position of the thiopyran, also at ≈8.80 ppm the resonated proton of the pyrimidine at 2-position.The synthetic sequence leading to the Nglycosides substituted thiochormen [2,3-d] pyrimidine (Scheme 2) involved the conversion of the pyrimidine moiety of (6a-f) into the intermediate potassium salt, in which the reactive NH functionality was protected as previously reported by us [16]. The subsequent addition of potassium salt of 6 with the appropriate bromo furanosyl/pyranosyl, in anhydrous acetone at room temperature, involves the removing of KBr as a first step Scheme 2.
The glycosylation of 6d-f with 2,3,5-tri-O-acetyl-β-Darabinopyranosyl bromide 11 or 2,3,4,6-tetra-O-acetyl -α-D-glycosyl bromide 12a,b in acetone and in the presence of aqueous potassium hydroxide afforded the corresponding acetylated nucleosides 7a-c, 8a-f respectively in good yields ≈65% - 70% Scheme 2. Thin layer chromatography (chloroform: methanol, 8:2) indicated the formation of the pure compounds. The structures of 7a-c, 8a-f were confirmed by elemental analysis and spectral data (IR, 1H NMR, 13C.NMR) (cf. Exp.). The 1H NMR spectrum of compound 8a as an example, showed the anomeric proton of the glucose moiety as a doublet at d 5.95 ppm with a coupling constant J1’-2’ = 10.67 Hz indicating β-configuration of the anomeric center. The other protons of the glucopyranose ring resonated around d 3.98 - 5.19 ppm, while the four acetoxy groups appeared as four singlets at d 1.94, 2.02, 2.11 and 2.14 ppm. The 13C.NMR revealed the signals at d 166.9 for CO-imide and at d 169.3, 170.2, 171.3, 171.9 ppm for four acetoxy groups, signals at d 60.21, 65.23, 67.70, 69.35, 75.34 and 87.19 ppm are assigned to C-6’, C-3’, C-2’, C-4’, C-5’ and C-1’ respectively.
Also, the four signals around d 22.12 - 22.55 ppm are assigned to the acetate methyl carbon atoms. Deacetylation of acetylated nucleosides 7a-c, 8a-f using saturated solution of ammonia in methanol at room temperature afforded the corresponding deacetylated nucleosides 9a-c,
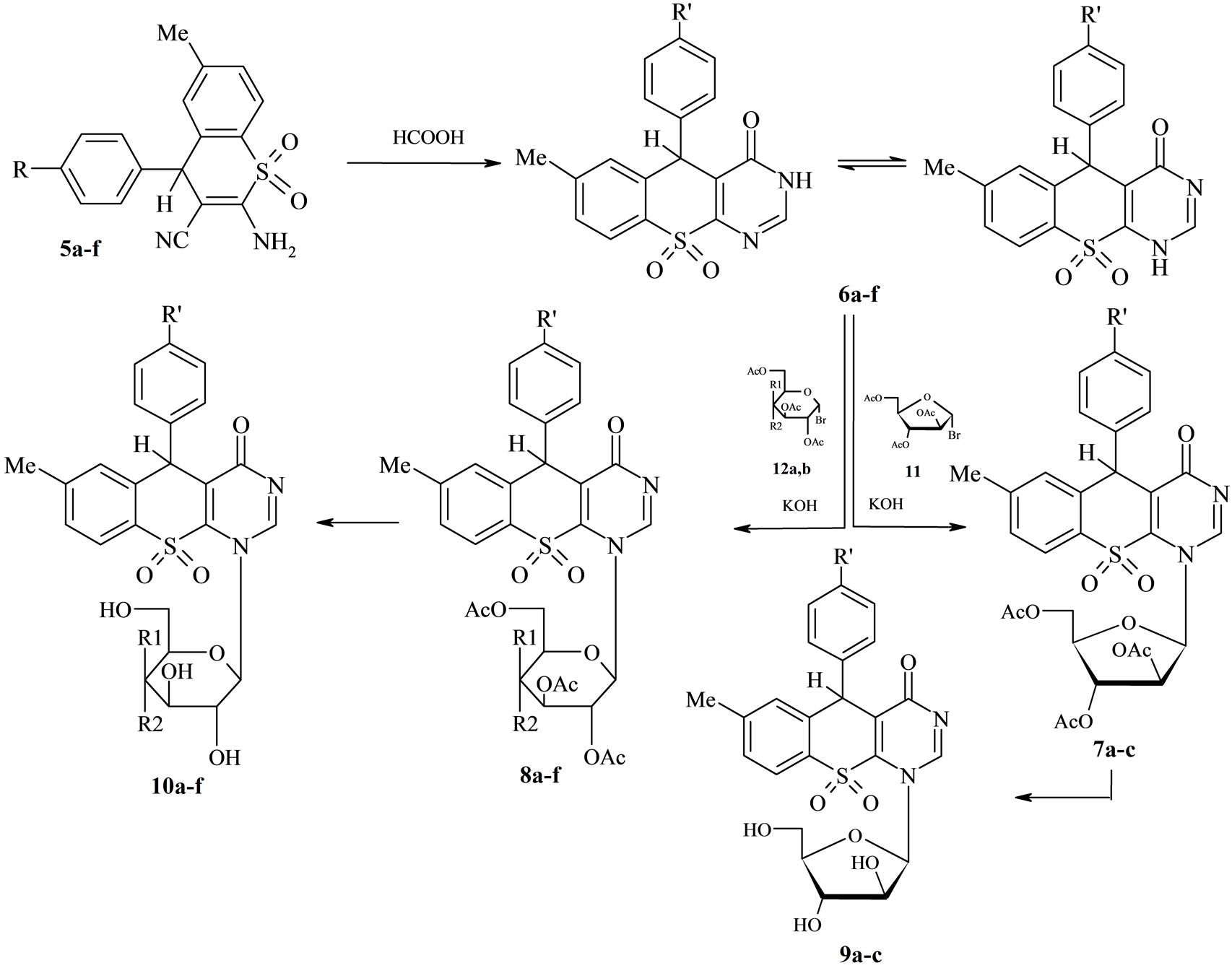
Scheme 2. Synthesis of the nucleosides of thiochromen derivatives.
10a-f respectively. The structures of free nucleosides 9 and 10 have been established on the basis of their spectral data and elemental analyses. Thus, the 1H NMR spectrum of 10a showed the anomeric proton as a doublet at d 6.04 ppm, J1’-2’ = 10.61 Hz indicating a β-Dconfiguration. The signals of the other six glucose protons appeared around d 3.87 - 5.13 ppm, while the signals that disappear on rapid exchange with D2O are observed at d 4.60, 5.04, 5.18 and 5.57 ppm, were assigned as the four hydroxyl groups.
3. Results and Discussions
3.1. Antibacterial Activity
The compounds 9a-c, 10a-f was evaluated for their efficacy as antibacterial in vitro by disc diffusion method against various bacterial strains. The antibacterial activity has been compared to some standard antibacterial agents like sulfanilamide and sulfadiazine that contain a pamino benzene sulfonamide moiety. From the results in Table 1 Compound (9a-c) exhibited excellent activity toward Gram(+ve) and Gram(−ve) bacteria E. coli, P.aeruginosa, S. epidermidis, B. subtilis and Vibrio species, as compared with the reference drug (sulfa-diazine, This may be due to the presence of 4-methyl-piprazine on p-positions of 4-thiochromene ring and arabinofuranos ring at N-1of the pyrimidine, while the other compounds exhibited mild to moderate activity compared to sulfadiazine against B. subtilis. All of compounds exhibited excellent activity toward Vibrio species. Compounds 10a, 10d exhibited high activity toward Pseudomonos aeruginosa while the other compounds exhibited mild to moderate activity. Also all of compounds exhibited excellent activity toward E. coli as compared with the reference drug (sulfadiazine) (Table 2).
3.2. Antitumour Activity
Amongst the substituted 4-piperazino/morpholine-Nnucleoside thiochromene derivatives synthesized, compounds 9a-c and 10a-f were chosen by National Cancer Institute (NCI) as prototypes for preliminary test and were evaluated in three cell lines one dose prescreen [20-22] comprising of MCF-7 (breast), NCI-H460 (lung) and SF-268 (CNS) cell lines. These have been in use by DTP (Development Therapeutic Program) for several years to evaluate combinatorial libraries and have proven to be an effective test of agents, which exhibited some capability level to inhibit the growth of human tumour cells in culture. The compounds were added at a single
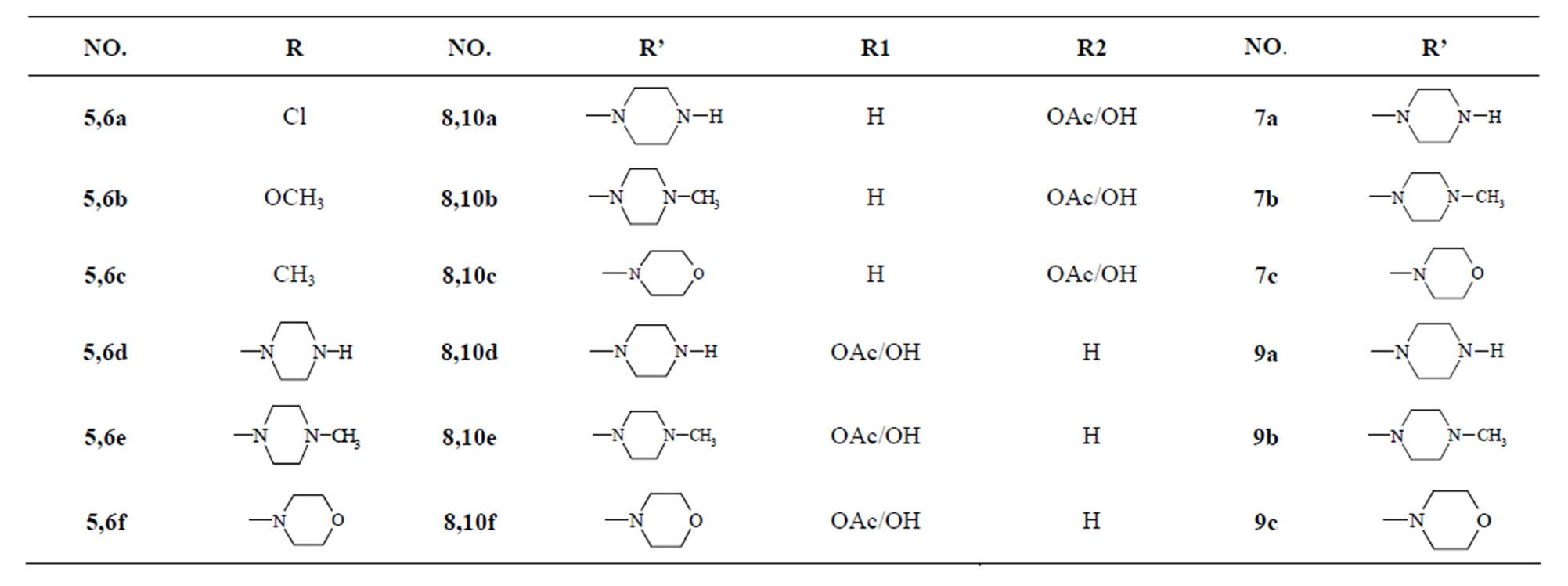
Table 1. Substituation of the thiochromen nucleosides.
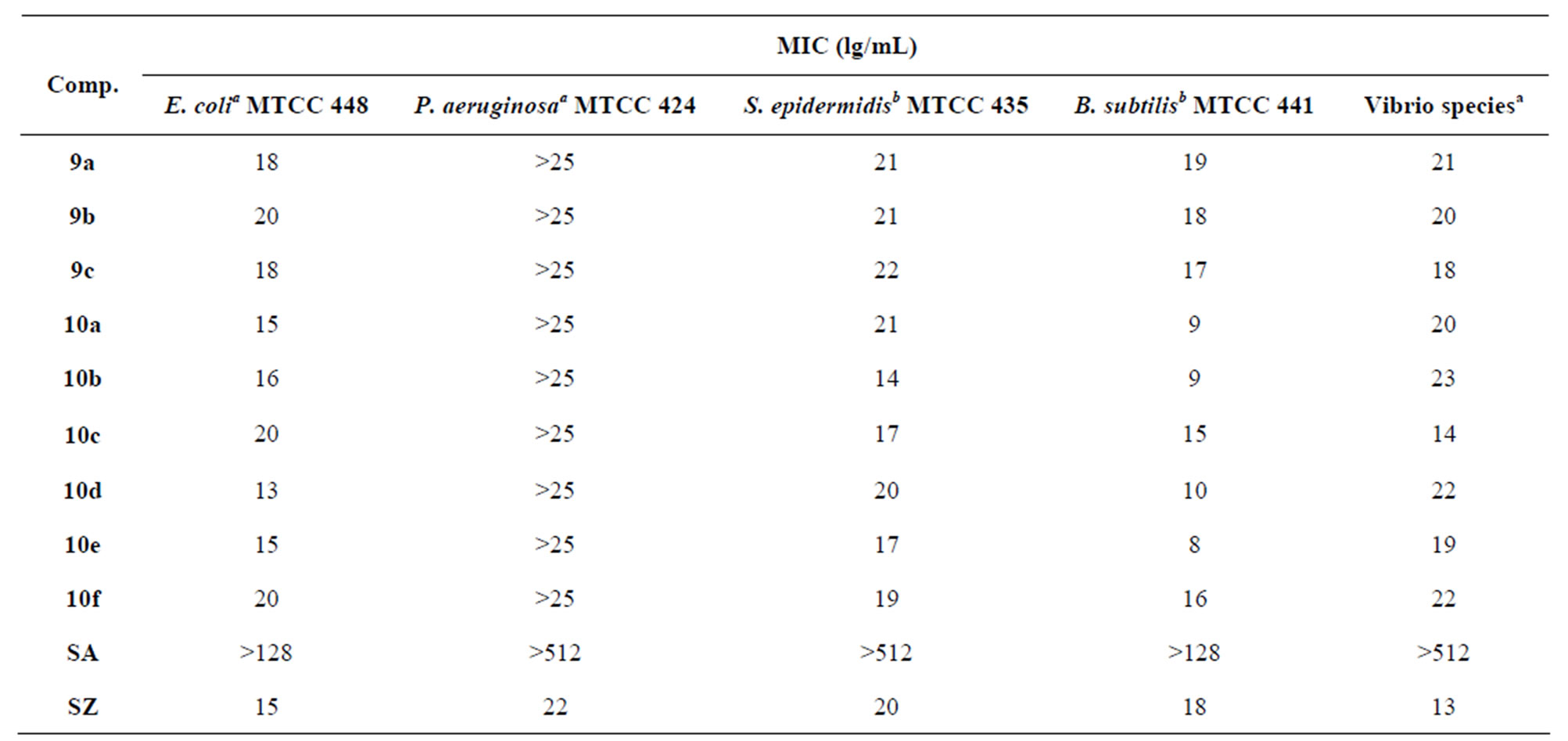
Table 2. In vitro antibacterial activity of 4H-thiochromen[2,3-d]pyrimidine nucleosides (9a-c), (10a-f).
SA, sulfanilamide, SZ, sulfadiazine, aGram-negative, bGram-positive.
concentration (10−4 M) and the culture was incubated for 48 h. End point determination were Compounds 9a, 9b and 9c were further evaluated at 10-fold dilutions of five concentrations ranging from 10−4 to 10−8 M against 60 different human tumour cell lines organized in subpanels representing melanoma, leukaemia and cancers of breast, prostate, lung, colon, ovary, CNS and kidney. The details of the cell lines used are shown in Table 3 and the ex- perimental procedures have been described in the literature in detail [23-25]. Three dose response parameters were calculated for each experimental agent: the compound concentration required to carry 50% of net cell growth (GI50) which signifies the growth inhibitory power of the test agents, the compound concentrations resulting in total growth inhibition (TGI) which signifies the cytostatic effect of the test agent, and the concentration of the compound leading to the 50% of net cell death (LC50) which signifies the cytotoxic effect of the test agent. The log10GI50, log10TGI and log10LC50 were then determined defined as the means of the log10’s of the individual GI50, TGI and LC50 value as shown in Table 3, respectively. Compounds having log10GI50 values −4 and <−4 were declared to be active. The mean graph points (MG-MID) represent average values for each of the mentioned parameters and indicate the average sensitivity of all cell lines to each tested compound.
Made with a protein binding dye, sulforhodamine B (SRB). Results for each compound were reported as per centage test cell growth compared with untreated control cells (PTC) as illustrated in Table 2. Compounds, whi-
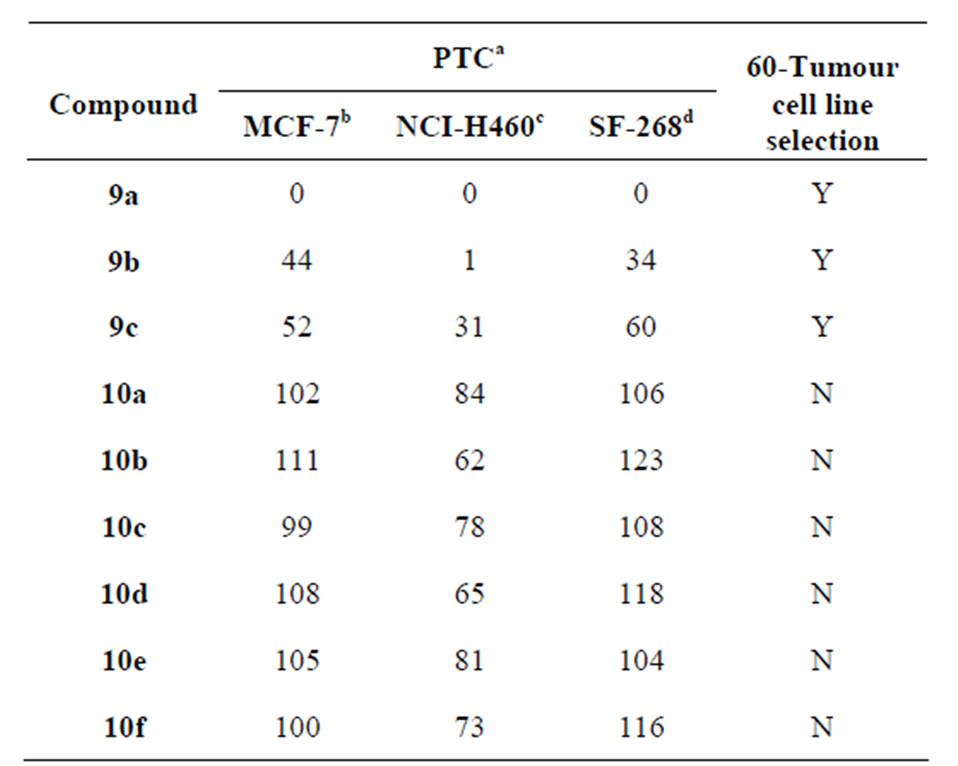
Table 3. Primary in vitro growth inhibition assay results at 10−4 M concentration.
Y, yes selected, N, not selected. aPTC, percent test cell growth compared with untreated control cells. bBreast cell line. cLung cell line. dCNS cell line.
chreduce the growth of any one of the cell lines to 32% or less, were selected for further evaluation in the full panel of 60 human tumour cell lines. Compound 9a has shown 0% of growth inhibition against all the three cell lines. Similarly, 9b and 9c presented the 1% and 31% of growth inhibition for the NCI-H460 cell line, respectively. However, compounds 10a-f have not reduced the growth of any cell lines by 32% or less. Therefore, only three compounds 9a, 9b and 9c have been selected for 60-cell line panel assay (Table 3).
From Table 4, we can conclude that, all the active compounds in this test showed broad-spectrum antitumour activity against the nine tumour subpanels tested, and demonstrated significant activity in the in vitro antitumour screening expressed by MG-MID log10GI50 value of −4.55, −4.67 and −4.73 of compounds 9a, 9b and 9c, respectively, whereas compounds 10a-f were inactive (log10GI50 > −4). Substitution of N-glucopyranosyl (10a-c) and N-galactopyranosyl (10e-f) on thiochromen [2,3-d] pyrimidine ring has reduced the activity. Log10GI50 value of compound 9a is −4.50 and −4.54 in the breast cancer cell lines (MCF-7 and MDA-MB-231, respectively), whereas this value in case of E7010 is −6.14 and −5.07 for the same cell lines [25]. Substitution of arabinofuranosyl group on the N-1 position of pyrimidine, and piperazine, 4-methylpiperazine and morpholine at 4 positions of thiochromene, respectively, (9a-c) also exhibits similar log10GI50 value for these cell lines, that is, −4.45 and −4.72, however the activity is enhanced particularly in case of leukaemia CCRF-CEM cell line (log10GI50 −5.18, GI50 is 6.62 µM). In case of compound 9c log10GI50 value is −5.36 and −4.38 in the breast cancer cell lines (MCF-7 and MDA-MB-231 cell lines, respectively) and also demonstrated significant activity in other cell lines.
4. Conclusion
The synthesis and screening of anticancer and antibacterial activities of a novel series of Nnucleoside-thiochromene-5-piperazino/5-methyl-piperazino/and or 5- morpholino have been investigated. Compounds 9a-c were screened against 60 human cancer cell lines and exhibited a broad spectrum of activity against almost all the cancer cell lines and in the case of certain cancer cell lines, the activity was comparable to E7010. Furthermore, most of the compounds showed better activity than the controls in antibacterial screening except against P. aeruginosa.
5. Experimental
All starting materials, solvents, and reagents were very pure grade. Chromatography solvents were HPLC grade. Reactions were monitored by thin layer chromatography (TLC), (Silica gel 60 F254). Melting points were determined on the Electrothermal 9100 melting point apparatus and are uncorrected. The IR spectra (KBr) were recorded on a FT-IR NEXCES spectrophotometer (Shimadzu, Japan). The NMR spectra were measured with a Jeol ECA 500 MHz. Mass spectra (EI) were run at 70 eV with a Finnigan SSQ 7000 spectrometer. Compounds were properly characterized by elemental analyses. The Pharmacological evaluations of the products were carried out in Pharmacological Unit Pharmacology department, (NCI, Cairo University, Egypt).
Preparation of the 2-amino-4-(4-aryl)-6-methyl-4Hthiochromeno-3-carbonitrile (4a-f), General procedure: The solution of each of 3-methylthiopenol (0.13 mol), aldehyde (2a-f) (0.01 mol) and malononitrile (0.01 mol) in ethanol absolute (50 ml) and piperidine (1 ml) was heated under reflux for 3 - 5 hours (under TLC control). The reaction mixture was allowed to cool to room temperature, poured into water (100 mL), acidify with acetic acid. The formed solid was collected by filtration, washed with water (50 mL), dried and crystallized from ethanol (100 ml), in 56% - 68% yields.
2-amino-4-(4-chlorophenyl)-6-methyl-4H-thiochromeno-3-carbonitrile (4a), It was obtained from 2a, in 65% yield, as yellow crystals, m. p. 238˚C - 240˚C, IR (cm−1, ν), 3440 (br, NH), 2212 (CN), 1H.NMR (DMSO-d6, d, ppm): 2.29 (s, CH3), 4.39 (s, thiopyran-H), 7.00 (m, Ar-H), 7.06 (m, Ar-H), 7.08 (s, Ar-H), 7.11 (m, Ar-H), 7.18 (d, 2H, Ar-H), 7.82 (d, 2H, Ar-H) 9.85 (br, NH, D2O exchangeable), 13C.NMR: 22.34 (CH3), 39.28 (Cthiopyran-4), 117.9 (CN), 125.5-140.8 (12C-Ar), 141.2, 143.9 (2C-thiopyran), Its MS (m/z), 312 (M+, 72%), 313 (M+ + 1, 19%), C17H13ClN2S (312.8).
2-amino-4-(4-methoxyphenyl)-6-methyl-4H-thiochromeno-3-carbonitrile (4b), It was obtained from 2b, in
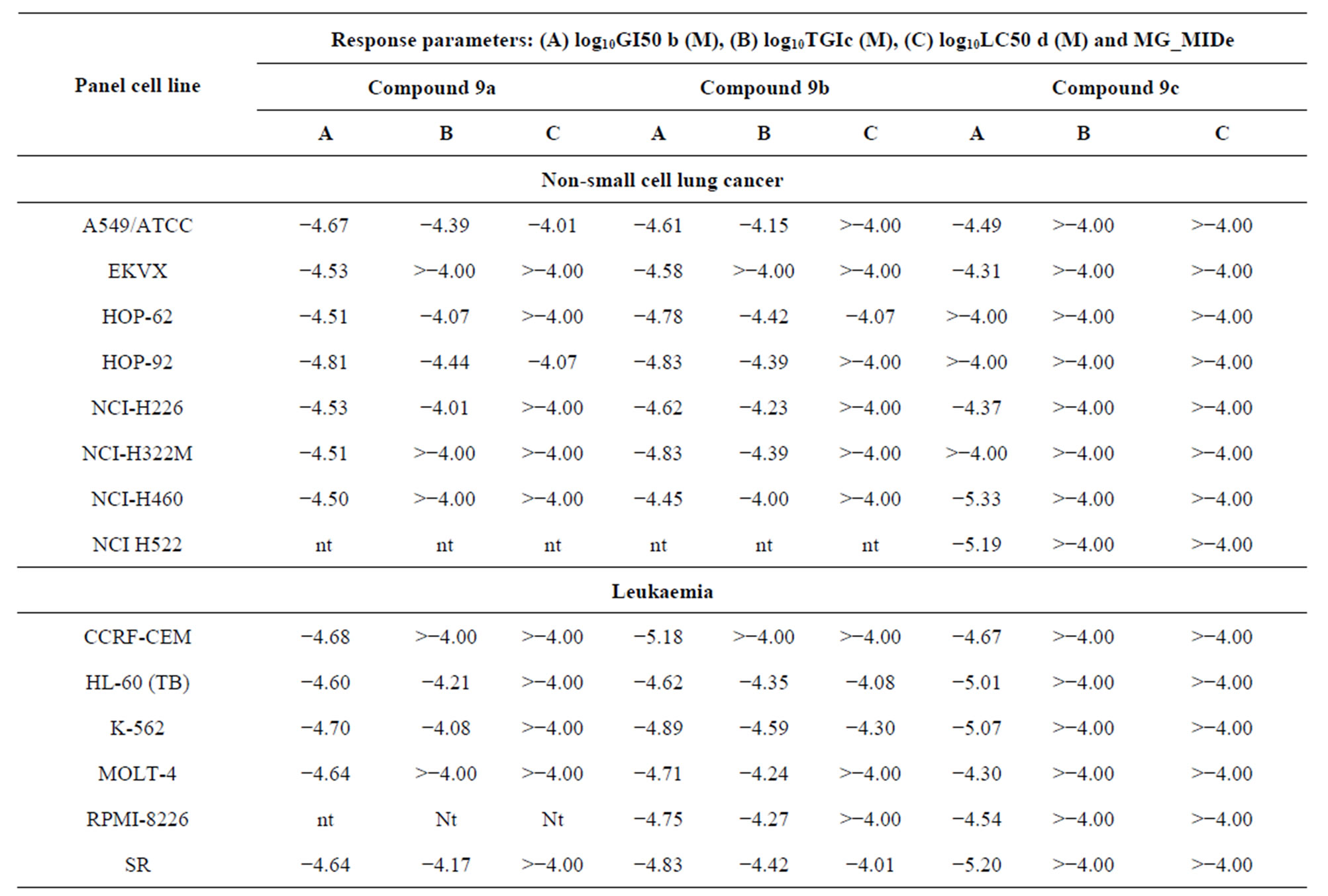
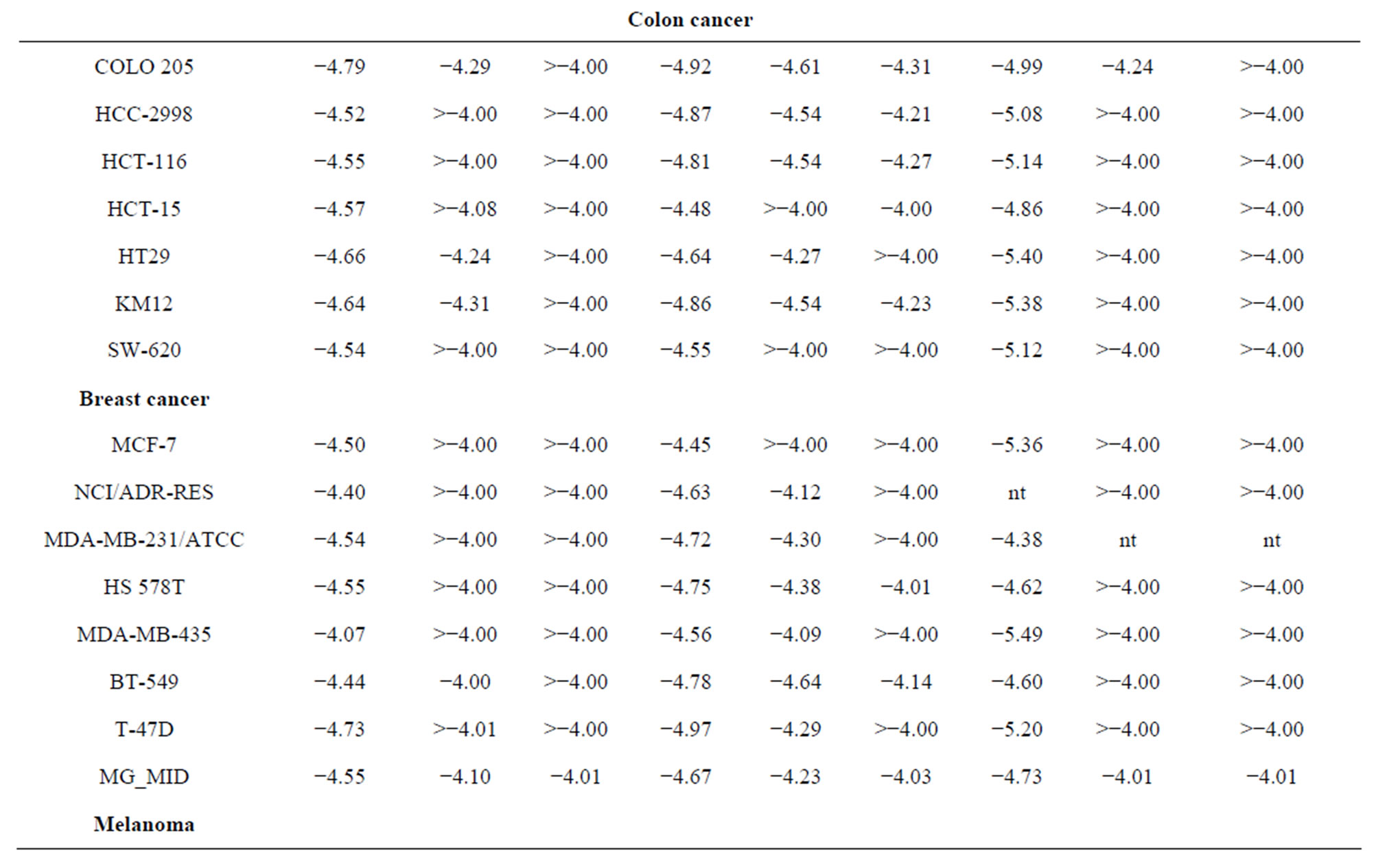
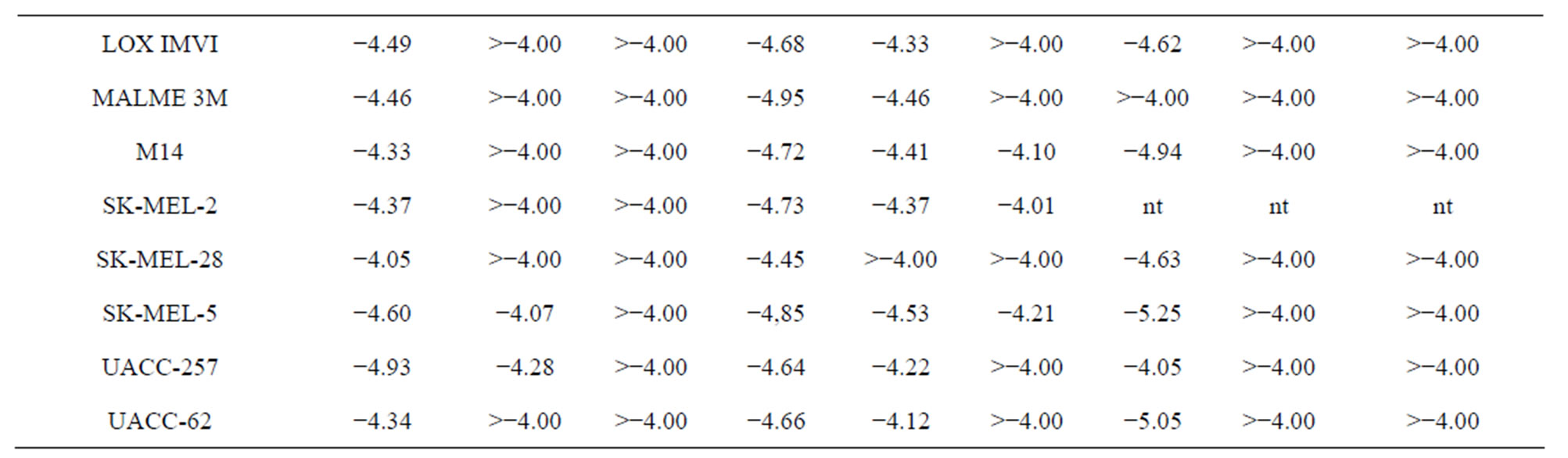
Table 4. Inhibition of in vitro cancer cell lines by selected 4H-thiochromen[2,3-d]-pyrimidine nucleosides 9a-ca.
aData obtained from the NCI’s in vitro disease-oriented human tumour cells screen. bLog10GI50 = log of molar concentration that inhibits 50% net cell growth. cLog10TGI = log of molar concentration that produces a total growth inhibition. dLog10LC50 = log of molar concentration that leads to 50% net cell death. eMG-MID = mean graph midpoint = arithmetical mean value for all tested cell lines 67% yield, as white powder, m. p. 219˚C - 221˚C, IR (cm−1, ν), 3315 (br, NH), 2215 (CN), 1H.NMR (DMSOd6, d, ppm): 2.29 (s, CH3), 3.78 (s, OCH3), 4.42 (s, thiopyran-H), 6.95 (m, Ar-H), 7.02 (m, Ar-H), 7.07 (s, Ar-H), 7.12 (m, Ar-H), 7.28 (d, 2H, Ar-H), 7.96 (d, 2H, Ar-H), 10.05 (br, D2O exchangeable-NH), 13C.NMR: 21.89 (CH3), 41.32 (C-thiopyran-4), 57.42 (OCH3), 118.2 (CN), 121.9-141.3 (12C-Ar), 141.9, 144.2 (2C-thiopyran), Its MS (m/z), 308 (M+, 100%), C18H16N2OS (308.4).
.1.3. 2-amino-4-(4-methylphenyl)-6-methyl-4H-thio- chromeno-3-carbonitrile (4c), It was obtained from 2c, in 64% yield, as white crystals, m. p. 202˚C - 204 ˚C, IR (cm−1, ν), 3280 (br, NH), 2217 (CN), 1H.NMR (DMSOd6, d, ppm): 2.29 (s, CH3), 2.38 (s, CH3), 4.48 (s, thiopyran-H), 6.98 (m, Ar-H), 7.03 (m, Ar-H), 7.09 (s, Ar-H), 7.13 (m, Ar-H), 7.32 (d, 2H, Ar-H), 7.95 (d, 2H, Ar-H), 9.75 (br, D2O exchangeable-NH), 13C.NMR: 21.9 (CH3), 24.54 (CH3), 41.37 (C-thiopyran-4), 118.7 (CN), 120.7-141.1 (12C-Ar), 142.9, 144.7 (2C-thiopyran), Its MS (m/z), 292 (M+, 68%), C18H16N2S (292.4).
2-amino-6-methyl-4-(4-piperazin-1-ylphenyl)-4H-thio chromeno-3-carbonitrile (4d), It was obtained from 2d, in 59% yield, as brown crystals, m.p. 179˚C - 181˚C, IR (cm−1, ν), 3370 (br, NH), 2220 (CN), 1H.NMR (DMSOd6, d, ppm): 2.26 (s, CH3), 2.56 (m, 4H, N(CH2)2), 3.03 (m, 4H, HN(CH2)2), 4.56 (s, thiopyran-H), 7.01 (m, Ar-H), 7.04 (m, Ar-H), 7.09 (s, Ar-H), 7.14 (m, Ar-H), 7.26 (d, 2H, Ar-H), 8.05 (d, 2H,Ar-H), 9.65, 10.15 (2brs, D2O exchangeable-NH), 13C.NMR: 25.12(CH3), 38.76 (C-thiopyran-4), 43.5 (2C, HN(CH2)2), 47.6 (2C, N(CH2)2), 116.8(CN), 120.9 - 139.7(12C-Ar), 141.3, 145.1(2C-thio pyran), Its MS (m/z), 362 (M+, 43%), C21H22N4S (362.5).
2-amino-6-methyl-4-[4-(4-methylpiperazin-1-yl)phenyl]-4H-thiochromeno-3-carbonitrile (4e), It was obtained from 2e, in 61% yield, as yellow crystals, m. p. 193˚C - 195˚C, IR (cm−1, ν), 3260 (br, NH), 2215 (CN), 1H.NMR (DMSO-d6, d, ppm): 2.21 (s, CH3), 2.29 (s, 3H, CH3), 2.56 (m, 4H, N(CH2)2), 2.68 (m, 4H, N(CH2)2), 4.51 (s, thiopyran-H), 6.98 (m, Ar-H), 7.03 (m, Ar-H), 7.09 (s, Ar-H), 7.15 (m, Ar-H), 7.21 (d, 2H, Ar-H), 8.01 (d, 2H, Ar-H), 9.80 (br, D2O exchangeable-NH), 13C.NMR: 22.36 (CH3), 39.83 (C-thiopyran-4), 46.01 (N-CH3), 47.8 (2C, N(CH2)2), 52.7 (2C, N(CH2)2), 114.7 (CN), 118.7 - 139.3 (12C-Ar), 140.5, 144.3 (2C-thiopyran), Its MS (m/z), 376 (M+, 51%), C22H24N4S (376.5).
2-amino-6-methyl-4-(4-morpholin-4-ylphenyl)-4H-thio chromeno-3-carbonitrile (4f), It was obtained from 2f, in 58% yield, as brown powder, m. p. 211˚C - 213˚C, IR (cm−1, ν), 3290 (br, NH), 2213 (CN), 1H.NMR (DMSOd6, d, ppm): 2.28 (s, CH3), 2.55 (m, 4H, N(CH2)2), 3.58 (m, 4H, O(CH2)2), 4.52 (s, thiopyran-H), 7.04 (m, Ar-H), 7.07 (m, Ar-H), 7.13 (s, Ar-H), 7.16 (m, Ar-H),7.23 (d, 2H, Ar-H), 8.12 (d, 2H, Ar-H), 9.60 (br, D2O exchangeable-NH), 13C.NMR: 23.49 (CH3), 40.13 (C-thiopyran-4), 46.5 (2C, N(CH2)2), 65.8 (2C, O(CH2)2), 117.6 (CN), 119.9 - 140.3 (12C-Ar), 142.1, 145.3 (2C-thiopyran), Its MS (m/z), 363 (M+, 65%), C21H21N3OS (363.5).
Preparation of the 2-amino-4-(4-aryl)-6-methyl-4Hthiochromeno-3-carbonitrile-1,1-dioxide (5a-f), General procedure: The solution of 4a-f (0.01 mol) in hydrogen peroxide solution (20 ml) (AcOH, H2O2, 2:1) was stirred at room temperature for 18 - 24 hs (TLC control). The solvent was evaporated under reduced pressure at 40˚C, and the crude product was filtered off. The product was dried, and crystallized from the proper solvent.
2-amino-4-(4-chlorophenyl)-6-methyl-4H-thiochrome no-3-carbonitrile-1,1-dioxide (5a), It was obtained from 4a, in 62% yield, as yellow crystals, m. p. 251˚C - 253˚C, IR (cm−1, ν), 3280 (br, NH), 2215 (CN),1340 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.30 (s, CH3), 4.42 (s, thiopyran-H), 7.02 (m, Ar-H), 7.07 (m, Ar-H), 7.11 (s, Ar-H), 7.13 (m, Ar-H), 7.23 (d, 2H, Ar-H), 7.90 (d, 2H, Ar-H), 10.05 (br, D2O exchangeable-NH), 13C.NMR: 24.20 (CH3), 40.08 (C-thiopyran-4), 116.9 (CN), 123.5 - 140.6 (12C-Ar), 141.6, 143.4 (2C-thiopyran), Its MS (m/z), 344 (M+, 65%), 313 (M+ + 1, 20%), C17H13ClN2O2S (344.8).
2-amino-4-(4-methoxyphenyl)-6-methyl-4H-thiochrome no-3-carbonitrile-1,1-dioxide (5b), It was obtained from 4b, in 56% yield, as white powder, m. p. 243˚C - 245˚C, IR (cm−1, ν), 3305 (br, NH), 2212 (CN), 1324 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.32 (s, CH3), 3.86 (s, OCH3), 4.40 (s, thiopyran), 6.98 (m, Ar-H), 7.04 (m, Ar-H), 7.09 (s, Ar-H), 7.15 (m, Ar-H), 7.27 (d, 2H, Ar-H), 7.95 (d, 2H, Ar-H), 9.45 (br, D2O exchangeableNH), 13C.NMR: 23.61 (CH3), 40.45 (C-thiopyran-4), 58.26 (OCH3), 117.6 (CN), 120.6 - 141.5 (12C-Ar), 141.3, 144.5 (2C-thiopyran), Its MS (m/z), 340 (M+, 83%), C18H16N2O3S (340.4).
2-amino-4-(4-methylphenyl)-6-methyl-4H-thiochrome no-3-carbonitrile-1,1-dioxide (5c), It was obtained from 4c, in 60% yield, as white crystals, m. p. 253˚C -255˚C, IR (cm−1, ν), 3315 (br, NH), 2221(CN), 1352 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.28 (s, CH3), 2.35 (s, CH3), 4.44 (s, thiopyran), 6.99 (m, Ar-H), 7.02 (m, Ar-H), 7.08 (s, Ar-H), 7.12 (m, Ar-H), 7.30 (d, 2H, Ar-H), 7.91 (d, 2H, Ar-H), 9.90 (br, D2O exchangeable-NH), 13C.NMR: 21.92 (CH3), 23.22 (CH3), 40.23 (C-thiopyran- 4), 118.4 (CN), 121.4 - 141.3 (12C-Ar), 142.5, 144.2 (2C-thiopyran), Its MS (m/z), 324 (M+, 63%), C18H16N2O2S (324.4).
2-amino-6-methyl-4-(4-piperazin-1-ylphenyl)-4H-thio chromeno-3-carbonitrile-1,1-dioxide (5d), It was obtained from 4d, in 59% yield, as colorless crystals, m.p. 219˚C - 221˚C, IR (cm−1, ν), 3267 (br, NH’s), 2214 (CN), 1353 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.28 (s, CH3), 2.58 (m, 4H, N(CH2)2), 3.12 (m, 4H, HN(CH2)2), 4.52 (s, thiopyran-H), 7.00 (m, Ar-H), 7.05 (m, Ar-H), 7.12 (s, Ar-H), 7.16 (m, Ar-H), 7.24 (d, 2H, Ar-H), 8.12 (d, 2H, Ar-H), 10.23, 10.50 (2brs, D2O exchangeable-NH), 13C.NMR: 22.47 (CH3), 40.16 (C-thiopyran-4), 42.9 (2C, HN(CH2)2), 47.8 (2C, N(CH2)2), 118.3(CN), 121.4 - 140.6 (12C-Ar), 141.6, 145.7 (2C-thiopyran), Its MS (m/z), 394 (M+, 51%), 395 (M+ + 1, 13%), C21H22N4O2S (394.5).
2-amino-6-methyl-4-[4-(4-methylpiperazin-1-yl)phenyl]-4H-thiochromeno-3-carbonitrile-1,1-dioxide (5e), It was obtained from 4e, in 63% yield, as yellow crystals, m. p. 231˚C - 233˚C, IR (cm−1, ν), 3256 (br, NH), 2214 (CN), 1320 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.25 (s, CH3), 2.43 (s, CH3), 2.56 (m, N(CH2)2), 2.68 (m, N(CH2)2), 4.51 (s, thiopyran-H), 6.98 (m, Ar-H), 7.03 (m, Ar-H), 7.09 (s, Ar-H), 7.15 (m, Ar-H), 7.21 (d, 2H, ArH), 8.01 (d, 2H, Ar-H), 9.80 (br, D2O exchangeable-NH), 13C.NMR: 23.25 (CH3), 39.83 (C-thiopyran-4), 46.01 (N-CH3), 47.8 (2C, N(CH2)2), 52.7(2C, N(CH2)2), 114.7 (CN), 118.7 - 139.3(12C-Ar), 140.5, 144.3 (2Cthiopyran), Its MS (m/z), 376 (M+, 51%), C22H24N4O2S (376.5).
2-amino-6-methyl-4-(4-morpholin-4-ylphenyl)-4H-thio chromeno-3-carbonitrile-1,1-dioxide (5f), It was obtained from 4f, in 53% yield, as brown powder, m. p. 253˚C -255˚C, IR (cm−1, ν), 3290 (br, NH), 2213 (CN), 1353 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.25 (s, CH3), 2.58 (m, 4H, N(CH2)2), 3.61 (m, 4H, O(CH2)2), 4.50 (s, thiopyran-H), 7.02 (m, Ar-H), 7.06 (m, Ar-H), 7.11 (s, Ar-H), 7.15 (m, Ar-H), 7.21 (d, 2H, Ar-H), 8.10 (d, 2H, Ar-H), 9.65 (br, D2O exchangeable-NH), 13C.NMR: 23.49 (CH3), 41.17(C-thiopyran-4), 46.51 (2C, N(CH2)2), 65.80 (2C, O(CH2)2), 119.3 (CN), 120.3 - 140.8 (12C-Ar), 142.3, 144.9 (2C-thiopyran), Its MS (m/z), 395 (M+, 53%), C21H21N3O3S (395.5).
Preparation of 7-methyl-5-(4-substituted-phenyl)-3, 5-dihydro-4H-thiochromeno-[2,3-d]pyrimidine-4-one- 10,10-dioxide (6a-f). General procedure: A mixture of compounds 5a-f (0.01mol), formic acid (10 mL) and catalytic amount of concentrated hydrochloric acid was heated under reflux for 10 - 15 hours (TLC control). The reaction mixture was allowed to cool to room temperature, poured into water (100 mL). The formed solid was collected by filtration, washed with absolute ethanol (50 mL), dried and crystallized from dimethylformamide.
5-(4-Chlorophenyl)-7-methyl-3,5-dihydro-4H-thiochro meno[2,3-d]pyrimidine-4-one-10,10-dioxide (6a). It was obtained from 5a, in 76% yield, as yellow powder, m.p. 288˚C - 290˚C, IR (cm−1, ν), 3240 (br, NH), 1678 (CO), 1330 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.29 (s, CH3), 4.47 (s, thiopyran-H), 6.98 (m, Ar-H), 7.05 (m, Ar-H), 7.13 (s, Ar-H), 7.16 (m, Ar-H), 7.20 (d, 2H, Ar-H), 8.04 (d, 2H, Ar-H), 8.84 (s, pyrimidine-H), 9.15 (br, D2O exchangeable-NH), 13C.NMR: 23.19 (CH3), 40.11 (Cthiopyran-4), 123.5 - 143.7 (15C-Ar), 167.4 (CO), Its MS (m/z), 372 (M+, 87%), 373 (M+ + 1, 23%), C18H13ClN2O3S (372.8).
5-(4-Methoxyphenyl)-7-methyl-3,5-dihydro-4H-thio chromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (6b). It was obtained from 5b, in 76% yield, as white powder, m. p. 278˚C - 280˚C, IR (cm−1, ν), 3270 (br, NH), 1685 (CO), 1345 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.30 (s, CH3), 3.84 (s, OCH3), 4.39 (s, thiopyran-H), 7.00 (m, Ar-H), 7.03 (m, Ar-H), 7.11 (s, Ar-H), 7.17 (m, Ar-H), 7.29 (d, 2H, Ar-H), 7.87 (d, 2H, Ar-H), 8.78 (s, pyrimidine-H) 10.05 (br,D2O exchangeable-NH), 13C.NMR: 23.61 (CH3), 59.18 (OCH3), 119.8 - 146.5 (15C-Ar), 166.9 (CO), Its MS (m/z), 368 (M+, 61%), C19H16N2O4S (368.4).
5-(4-Methylphenyl)-7-methyl-3,5-dihydro-4H-thiochro meno[2,3-d]pyrimidine-4-one-10,10-dioxide (6c). It was obtained from 5c, in 75% yield, as white powder, m.p. 301˚C - 303˚C, IR (cm−1, ν), 3276 (br, NH), 1678 (CO), 1343 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.24 (s, CH3), 2.31 (s, CH3), 4.38 (s, thiopyran-H), 6.96 (m, Ar-H), 7.01 (m, Ar-H), 7.06 (s, Ar-H), 7.11 (m, Ar-H), 7.28 (d, 2H, Ar-H), 7.96 (d, 2H, Ar-H), 8.79 (s, pyrimidine-H), 10.35 (br, D2O exchangeable-NH), 13C.NMR: 21.49 (CH3), 24.21 (CH3), 43.13(C-thiopyran-4), 121.6 - 146.8 (15C-Ar), 168.5 (CO), Its MS(m/z), 352 (M+, 71%), C19H16N2O3S (352.4).
7-methyl-5-(4-piperazin-1-ylphenyl)-3,5-dihydro-4H- thiochromeno-[2,3-d]-pyrimidine-4-one-10,10-dioxide (6d). It was obtained from 5d, in 65% yield, as white crystals, m. p. 263˚C - 265˚C, IR (cm−1, ν), 3335 (br, NH’s), 1687 (CO), 1339 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.30 (s, CH3), 2.54 (m, 4H, N(CH2)2), 3.09 (m, 4H, HN(CH2)2), 4.45 (s, thiopyran-H), 7.02 (m, Ar-H), 7.07 (m, Ar-H), 7.13 (s, Ar-H), 7.19 (m, Ar-H), 7.26 (d, 2H, Ar-H), 8.00 (d, 2H, Ar-H), 8.93 (s, pyrimidine-H), 10.20, 10.65 (2brs, D2O exchangeable-NH), 13C.NMR: 21.97 (CH3), 40.07 (C-thiopyran-4), 43.5 (2C, HN(CH2)2), 46.9 (2C, N(CH2)2), 120.5 - 145.9 (15C-Ar), 167.8 (CO), Its MS (m/z), 422 (M+, 43%) , C22H22N4O3S (422.4).
7-Methyl-5-[4-(4-methylpiperazin-1-yl)phenyl]-3,5- dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10, 10-dioxide (6e). It was obtained from 5e, in 71% yield, as yellow crystals, m. p. 271˚C -273˚C, IR (cm−1, ν), 3258 (br, NH), 1685 (CO), 1337(SO), 1H.NMR (DMSOd6, d, ppm): 2.23 (s, CH3), 2.41 (s,CH3), 2.54 (m, N(CH2)2), 2.66 (m, N(CH2)2), 4.47 (s, thiopyran-H), 6.99(m, Ar-H), 7.05 (m, Ar-H), 7.12(s, Ar-H),7.17(m, Ar-H), 7.24 (d, Ar-H), 7.96 (d, Ar-H), 8.85 (s, pyrimidine-H), 10.45 (br, D2O exchangeable-NH), 13C. NMR: 21.35 (CH3), 39.92 (C-thiopyran-4), 45.89 (N-CH3), 48.21 (2C, N(CH2)2, 53.67 (2C, N(CH2)2), 119.8 - 147.6 (15C-Ar), 167.5(CO), Its MS (m/z), 376 (M+, 51%), C23H24N4O3S (436.4).
7-Methyl-5-(4-morpholin-1-ylphenyl)-3,5-dihydro-4H-thiochromeno[2,3-d]-pyrimidine-4-one-10,10-dioxide (6f). It was obtained from 5f, in 65% yield, as yellow powder, m. p. 303˚C - 305˚C, IR (cm−1, ν), 3268 (br, NH), 1678 (CO), 1348 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.22 (s, CH3), 2.55 (m, 4H, N(CH2)2), 3.64 (m, 4H, O(CH2)2), 4.53 (s, thiopyran-H), 7.00 (m, Ar-H), 7.04 (m, Ar-H), 7.09 (s, Ar-H), 7.13 (m, Ar-H), 7.20 (d, 2H, Ar-H), 8.02 (d, 2H, Ar-H), 8.93 (s, pyrimidine-H), 9.95 (br, D2O exchangeable-NH), 13C.NMR: 22.89 (CH3), 43.26 (Cthiopyran-4), 45.91 (2C, N(CH2)2), 64.92 (2C, O(CH2)2), 120.6 - 147.8 (15C-Ar), 166.8 (CO), Its MS (m/z), 423 (M+, 65%), C22H21N3O4S (423.4).
Preparation of the acetylated N-nucleosides of 3, 5-dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (7a-c) and (8a-f), General procedure: To a solution of 6d-f (0.01 mol) in aqueous potassium hydroxide (0.01 mol) in distilled water (5 ml) was added a solution of 1-bromo-2,3,5-tri-O-acetyl-α-D-arabino furanose (11) or 2,3,4,6-tetra-O-acetyl-α-D-gluco-/or galactopyranosyl bromide (12a,b) (0.015 mol) in acetone (40 ml). The reaction mixture was stirred at room temperature for 24 h (under TLC control). The solvent was evaporated under reduced pressure at 40˚C, and the crude product was filtered off and washed with distilled water to remove KBr formed. The product was dried, and crystallized from the proper solvent.
N-(2’,3’,5’-tri-O-acetyl-β-D-arabinofuranosyl)-7- methyl-5-(4-piperazin-1-yl-phenyl)-3,5-dihydro-4H- thiochrom eno[2,3-d]pyrimidine-4-one-10,10-dioxide (7a). It was obtained from 6d and 2,3,5-tri-O-acetyl-α-D-arabino furanosyl)-bromide (11), as white powder, m. p. 209˚C - 211˚C, IR (cm−1, ν), 3260 (br, NH), 1741 (3CO), 1686 (CO), 1330 (SO), 1H.NMR (DMSO-d6, d, ppm): 1.95, 1.99, 2.01 (3s, 3CH3CO), 2.31 (s, CH3), 2.45 (m, 4H, N(CH2)2), 3.03 (m, 4H, HN(CH2)2), 4.08 (m, H-4’), 4.15 (m, H-5’, H-5’’), 4.48 (s, thiopyran-H), 5.31 (m, H-3’), 5.38 (m, H-2’), 6.73 (d, J = 3.67 Hz, H-1’), 7.04 (m, Ar-H), 7.11 (m, H, Ar-H), 7.15 (s, Ar-H), 7.21 (m, Ar-H), 7.30 (d, 2H, Ar-H), 8.00 (d, 2H, Ar-H), 8.86 (s, pyrimidine-H), 10.25 (br, NH), 13C. NMR: 21.43 (CH3), 22.19, 22.24, 22.61 (3CH3), 39.83 (C-thiopyran-4), 43.71 (2C, HN(CH2)2), 45.93 (2C, N(CH2)2), 61.52 (C-5’), 66.23 (C-3’), 66.78 (C-2’), 67.37 (C-4’), 85.73 (C-1’), 121.3 - 147.5 (15C-Ar), 167.8 (CO), 169.2, 170.5, 172.6 (3CO), Its MS (m/z), 680 (M+, 28%), C33H36N4O10S (680.7).
N-(2’,3’,5’-tri-O-acetyl-β-D-arabinofuranosyl)-7- methyl -5-[4-(4-methylpiperazin-1-yl)phenyl]-3,5-dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (7b). It was obtained from compound 6e and 2,3, 5-tri-O-acetyl-α-D-arabinofuranosyl)-bromide (11), as pale yellow powder, m. p. 219˚C - 221˚C, IR (cm−1, ν), 1737 (3CO), 1679 (CO), 1341 (SO), 1H.NMR(DMSOd6, d, ppm): 1.92, 1.97, 2.03 (3s, 3CH3CO), 2.24 (s, CH3), 2.28 (s, CH3), 2.53 (m, 4H, N(CH2)2), 3.11 (m, 4H, HN(CH2)2), 4.05 (m, H-4’), 4.12 (m, H-5’, H-5’’), 4.46 (s, thiopyran-H), 5.28 (m, H-3’), 5.31 (m, H-2’), 6.72 (d, J = 3.71 Hz, H-1’), 7.03 (m, Ar-H), 7.09 (m, Ar-H), 7.14 (s, Ar-H), 7.19 (m, Ar-H), 7.25 (d, Ar-H), 8.01 (d, Ar-H), 8.89 (s, pyrimidine-H), 13C. NMR: 21.29 (CH3), 22.23, 22.32, 22.70 (3CH3),40.13(C-thiopyran-4), 43.56 (2C, HN(CH2)2), 45.11 (2C, N(CH2)2), 46.04 (NCH3), 61.39 (C-5’), 66.19 (C-3’), 66.82 (C-2’), 67.35 (C-4’), 85.61 (C-1’), 121.1 - 147.6 (15C-Ar), 167.5 (CO), 169.5, 170.3, 173.1 (3CO), Its MS (m/z), 694 (M+, 19%), C34H38N4O10S (694.7).
N-(2’,3’,5’-tri-O-acetyl-β-D-arabinofuranosyl)-7- methyl-5-(4-morpholin-1-yl-phenyl)-3,5-dihydro-4H- thiochro meno[2,3-d]pyrimidine-4-one-10,10-dioxide (7c). It was obtained from compound 6f and 2,3,5-tri-O-acetyl-α-D-arabinofuranosyl)-bromide (11), as yellow powder, m. p. 261˚C - 263˚C, IR (cm−1, ν), 1728 (3CO), 1672 (CO), 1335 (SO), 1H.NMR (DMSO-d6, d, ppm): 1.93, 1.99, 2.11 (3s, 3CH3CO), 2.23 (s, CH3), 2.54 (m, 4H, N(CH2)2), 3.61 (m, 4H, O(CH2)2), 4.13 (m, H-4’), 4.18 (m, H-5’, H-5’’), 4.51 (s, thiopyran-H), 5.31 (m, H-3’), 5.39 (m, H-2’), 6.81 (d, J = 3.72 Hz, H-1’), 7.03 (m, Ar-H), 7.09 (m, Ar-H), 7.15 (s, Ar-H), 7.19 (m, Ar-H), 7.27 (d, 2H, Ar-H), 8.00 (d, 2H, Ar-H), 8.80 (s, pyrimidine-H), 13C.NMR: 21.90 (CH3), 22.21, 22.30, 22.57 (3CH3), 41.32 (C-thiopyran-4), 45.76 (2C, N(CH2)2), 60.98 (C-5’), 63.28 (2C, O(CH2)2), 66.30 (C-3’), 66.87 (C-2’), 67.41 (C-4’), 86.03 (C-1’), 119.8 - 148.3 (15CAr), 166.8 (CO), 170.1, 171.3, 172.9 (3CO), Its MS (m/z), 681 (M+, 21%), C33H35N3O11S (681.7).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-glucopyranosyl)-7- methyl-5-(4-piperazin-1-yl-phenyl)-3,5-dihydro-4H-thio chromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (8a). It was obtained from compound 6d and 2,3,4,6-tetra-Oacetyl-α-D-glucopyranosyl)-bromide (12a), as a pale yellow powder, m. p. 192˚C - 194˚C, IR (cm−1, ν), 3285 (br, NH), 1725(4CO), 1680(CO), 1345(CS), 1H.NMR (DMSO-d6, d, ppm): 1.94, 2.02, 2.11, 2.14 (4s, 12H, 4CH3CO), 2.28 (s, CH3), 2.53 (m, 4H, N(CH2)2), 3.05 (m, 4H, HN(CH2)2), 3.98 (m, H-5’), 4.25 (m, H-6’, H-6’’), 4.36 (m, H-4’), 4.39 (s, thiopyran-H), 5.01 (t, H-2’), 5.19 (t, 1H, J = 9.60 Hz, H-3’), 5.95 (d, J =10.67 Hz, H-1’), 7.01 (m, Ar-H), 7.08 (m, Ar-H), 7.17 (s, Ar-H), 7.21 (m, Ar-H), 7.27 (d, Ar-H), 8.02 (d, Ar-H), 8.87 (s, pyrimidine-H), 10.17 (br, NH), 13C. NMR: 21.67 (CH3), 22.12, 22.23, 22.36, 22.55(4CH3), 39.87(C-thiopyran-4), 43.7 (2C, HN(CH2)2), 46.3 (2C, N(CH2)2), 60.21 (C-6’), 65.23 (C-3’), 67.70 (C-2’), 69.35 (C-4’), 75.34 (C-5’), 87.19 (C-1’), 120.7-147.9 (15C-Ar), 166.9 (CO), 169.3, 170.2, 171.3, 171.9 (4CO), Its MS (m/z), 752 (M+, 32%), C36H40N4O12S (752.7).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-glucopyranosyl)-7- methyl-5-[4-(4-methyl-piperazin-1-yl)phenyl]-3,5-dihy- dro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10,10-di-oxide (8b). It was obtained from compound 6e and 2,3,4, 6-tetra-O-acetyl-α-D-glucopyranosyl)-bromide (12a), as a pale yellow powder, m. p. 207˚C - 209˚C, IR (cm−1, ν), 1728 (CO),1667(CO),1342(SO), 1H.NMR (DMSO-d6, d, ppm): 1.92, 1.99, 2.02, 2.11 (4s, 12H, 4CH3CO), 2.26 (s, CH3), 2.46 (s, CH3), 2.58 (m, N(CH2)2), 2.69 (m, N(CH2)2), 3.83 (m, H-5’), 4.11 (m, H-6’, H-6’’), 4.28 (m, H-4’), 4.46 (s, thiopyran-H), 4.89 (t, H-2’), 5.45 (t, J = 9.60 Hz, H-3’), 5.96 (d, J = 10.54 Hz, H-1’), 7.01 (m, Ar-H), 7.08 (m, Ar-H), 7.13 (s, Ar-H), 7.19 (m, Ar-H), 7.26 (d, 2H, Ar-H), 7.95 (d, 2H, Ar-H), 8.84 (s, pyrimidine-H), Its MS (m/z), 766 (M+, 31%), C37H42N4O12S (766.8).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-glucopyranosyl)-7- methyl-5-(4-morpholin-1-yl-phenyl)-3,5-dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (8c). It was obtained from compound 6f and 2,3,4,6-tetra-Oacetyl-α-D-glucopyranosyl)-bromide (12a), as yellow powder, m. p. 258˚C - 260˚C, IR (cm−1, ν), 1728 (CO), 1678 (CO), 1341 (SO), 1H.NMR (DMSO-d6, d, ppm): 1.98, 2.04, 2.15, 2.19 (4s, 12H, 4CH3CO), 2.24 (s, CH3), 2.56 (m, 4H, N(CH2)2), 3.63 (m, 4H, O(CH2)2), 4.01 (m, H-5’), 4.22 (m, H-6’, H-6’’), 4.36 (m, H-4’), 4.51 (s, thiopyran-H), 4.87 (t, H-2’), 5.21 (t, J = 9.61 Hz, H-3’), 6.03 (d, J = 10.70 Hz, H-1’),7.01 (m, Ar-H),7.07 (m, Ar-H), 7.11 (s, Ar-H), 7.18 (m, Ar-H), 7.23 (d, Ar-H), 7.98 (d, Ar-H), 8.90 (s, pyrimidine-H), 13C.NMR: 21.89 (CH3), 22.17, 22.25, 22.38, 22.63(4CH3), 43.26 (C-thiopyran-4), 45.91(2C,N(CH2)2), 64.92(2C,O(CH2)2), 121.9 - 147.6(15C-Ar), 165.9(CO), 169.3, 170.2, 171.3, 171.9 (4CO), Its MS(m/z), 753(M+, 25%), C36H39N3O13S (753.7).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-galactopyranosyl)-7-methyl-5-(4-piperazin-1-yl-phenyl)-3,5-dihydro-4H-thio chromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (8d). It was obtained from compound 6d and 2,3,4,6-tetra-Oacetyl-α-D-galactopyranosyl)-bromide (12b), as a pale yellow powder, m. p. 178˚C - 180˚C, IR (cm−1, ν), 3274 (br, NH), 1730(4CO), 1676 (CO)1345 (CS), 1H.NMR (DMSO-d6, d, ppm): 1.97, 2.05, 2.13, 2.16(4s, 12H, 4CH3CO), 2.25 (s, CH3), 2.55 (m, 4H, N(CH2)2), 3.02 (m, 4H, HN(CH2)2), 3.96 (m, H-5’), 4.23 (m, H-6’, H-6’’), 4.38 (m, H-4’), 4.41 (s, thiopyran-H), 5.13 (t, 1H, H-2’), 5.20 (t, J = 9.78 Hz, H-3’), 6.01 (d, J = 10.64 Hz, H-1’), 7.03 (m, Ar-H), 7.11 (m, Ar-H), 7.19 (s, Ar-H), 7.23 (m, Ar-H), 7.31 (d, Ar-H), 8.00 (d, Ar-H), 8.90 (s, pyrimidine-H), 10.25 (br, NH), 13C.NMR:21.88(CH3), 22.17, 22.26, 22.38, 22.59 (4CH3), 40.56(C-thiopyran-4), 43.8 (2C, HN(CH2)2), 46.7 (2C, N(CH2)2), 60.56 (C-6’), 65.78 (C-3’), 67.78 (C-2’), 70.13 (C-4’), 74.64 (C-5’), 87.21 (C-1’), 121.4 - 147.6 (15C-Ar), 167.2 (CO), 170.1, 170.7, 171.8, 172.4 (4CO), Its MS (m/z), 752(M+, 41%), C36H40N4O12S (752.7).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-galactopyranosyl)-7-methyl-5-[4-(4-methyl-piperazin-1-yl)phenyl]-3,5- dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10, 10-dioxide (8e). It was obtained from compound 6e and 2,3, 4,6-tetra-O-acetyl-α-D-galacto-pyranosyl)-bromide (12b), as yellow powder, m. p. 219˚C - 221˚C, IR (cm−1, ν), 1723 (CO),1673(CO), 1340(SO), 1H.NMR (DMSO-d6, d, ppm): 1.94, 2.02, 2.12, 2.19 (4s, 12H,4CH3CO), 2.29 (s, CH3), 2.45 (s, CH3), 2.53(m, N(CH2)2), 2.70 (m, N(CH2)2), 3.92 (m, 1H, H-5’), 4.07 (m, 2H, H-6’, H-6’’), 4.19(m, H-4’), 4.52 (s, thiopyran-H), 4.87 (t, H-2’), 5.29 (t, 1H, J = 9.78 Hz, H-3’), 5.99 (d, J = 10.64 Hz, H-1’), 7.00(m, Ar-H), 7.07(m, H, Ar-H), 7.12 (s, Ar-H), 7.21 (m, Ar-H), 7.28 (d, Ar-H), 7.98 (d, Ar-H), 8.87 (s, pyrimidine-H), Its MS (m/z), 766 (M+, 31%), C37H42N4O12S (766.8).
N-(2’,3’,4’,6’-tetra-O-acetyl-β-D-galactopyranosyl)-7-methyl-5-(4-morpholin-1-yl-phenyl)-3,5-dihydro-4H- thiochromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (8f). It was obtained from compound 6f and 2,3,4,6-tetra- O-acetyl-α-D-galactopyranosyl)-bromide (12b), as yellow powder, m. p. 239˚C - 241˚C, IR (cm−1, ν), 1727 (CO), 1672 (CO), 1341(SO), 1H.NMR (DMSO-d6, d, ppm): 1.96, 2.03, 2.07, 2.13 (4s, 12H, 4CH3CO), 2.25 (s, CH3), 2.56 (m, 4H, N(CH2)2), 3.63 (m, 4H, O(CH2)2), 3.92 (m, H-5’), 4.07 (m, 2H, H-6’, H-6’’), 4.19 (m, H-4’), 4.53 (s, thiopyran-H), 4.87 (t, H-2’), 5.29 (t, J = 9.64 Hz, H-3’), 5.99 (d, J = 10.60 Hz, H-1’), 6.98 (m, Ar-H), 7.06 (m, Ar-H), 7.11 (s, Ar-H), 7.15 (m, Ar-H), 7.21 (d, 2H, Ar-H), 8.00 (d, 2H, Ar-H), 8.89 (s, pyrimidine-H), 13C. NMR: 22.67 (CH3), 43.31(C-thiopyran-4), 45.87 (2C, N(CH2)2), 60.39 (C-6’), 63.76 (2C, O(CH2)2), 66.73 (C-3’), 67.84 (C-2’), 70.16 (C-4’), 74.71 (C-5’), 87.23 (C-1’), 120.8-148.9 (15C-Ar), 166.6 (CO), 169.8, 170.2, 171.8, 172.7 (4CO), Its MS (m/z), 753 (M+, 23%), C36H39N3O13S (753.7).
Synthesis of diacetylated N-(β-D-glycosidylthio)-3, 5-dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one- 10,10-dioxide (9a-c) and (10a-f). General procedure: Acetylated compound 7a-c or 8a-f (1.0 mmol) was dissolved in methanolic ammonia (saturated with NH3 at 0˚C, 100 ml). The reaction mixture was stirred overnight and then heated the reaction mixture for 1 h at 120˚C - 130˚C. The mixture was then cooled and the solvent was evaporated to provide the crude nucleoside. Purification by heating the crude in n-hexane (100 ml, three times) provided 9a-c or 10a-f as yellow solid. Crystallization from methanol gave a pale yellow powder.
N-(β-D-arabinofuranosyl)-7-methyl-5-(4-piperazin-1-ylphenyl)-3,5-dihydro-4H-thiochromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (9a). It was obtained from 7a, as white powder, m. p. 239 C - 241˚C, IR (cm−1, ν), 3500 (brs, OH), 3280 (br, NH),1676(CO),1330 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.24 (s, CH3), 2.51 (m, 4H, N(CH2)2), 3.00 (m, 4H, HN(CH2)2), 3.80 (m, H-5’, H-5’’), 4.12 (m, H-4’), 4.50 (s, thiopyran-H), 4.80 (t, H-2’), 5.14 (t, J = 5.41 Hz, J = 4.97 Hz, OH-C(5’), 5.22 (d, J = 4.46 Hz, OH-C(3’), 5.41 (d, J = 5.95 Hz, OH-C(2’), 5.66 (t, J = 9.80 Hz, H-3’), 6.88 (d, J = 5.63 Hz, H-1’), 7.03 (m, Ar-H), 7.10 (m, Ar-H), 7.16 (s, Ar-H), 7.23 (m, Ar-H), 7.28 (d, 2H, Ar-H), 8.07 (d, 2H, Ar-H), 8.78 (s, pyrimidineH), 10.55 (br, NH), 13C.NMR: 22.03 (CH3), 40.13 (Cthiopyran-4), 43.53 (2C, HN(CH2)2), 46.02 (2C, N(CH2)2), 60.86 (C-5’), 65.33 (C-3’), 67.58 (C-2’), 69.26 (C-4’), 87.71 (C-1’), 120.6 - 147.9 (15CAr), 166.7 (CO), Its MS (m/z), 554 (M+, 31%), C27H30N4O7S (554.6).
N-(β-D-arabinofuranosyl)-7-methyl-5-[4-(4-methylpip erazin-1-yl)phenyl]-3,5-dihydro-4H-thiochromeno [2,3-d]pyrimidine-4-one-10,10-dioxide (9b). It was obtained from compound 7b, as pale yellow powder, m. p. 271˚C - 273˚C, IR (cm−1, ν), 3480 (brs, OH), 1682 (CO), 1354 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.21 (s, CH3), 2.27 (s, CH3), 2.56 (m, 4H,N(CH2)2),3.09 (m, 4H, HN(CH2)2), 3.78 (m, H-5’, H-5’’), 4.13 (m, H-4’), 4.50 (s, thiopyran-H), 4.69 (t, H-2’), 5.12 (t, J = 5.41 Hz, J = 4.94 Hz, OH-C(5’), 5.22 (d, J = 4.45 Hz, OH-C(3’), 5.35 (d, J = 5.95 Hz, OH-C(2’), 5.70 (t, J = 9.58 Hz, H-3’), 6.91 (d, J = 5.69 Hz, H-1’), 7.01 (m, Ar-H), 7.09 (m, Ar-H), 7.13 (s, Ar-H), 7.20 (m, Ar-H), 7.26 (d, Ar-H), 8.02 (d, Ar-H), 8.69 (s, pyrimidine-H), 13C.NMR: 21.35(CH3), 40.17(C-thio-pyran-4), 43.56(2C, HN(CH2)2), 45.11(2C, N(CH2)2, 46.04 (NCH3), 61.41 (C-5’), 66.21 (C-3’), 66.84 (C-2’), 67.39 (C-4’), 85.70 (C-1’), 120.7 - 147.9 (15C-Ar), 166.8 (CO), Its MS (m/z), 568(M+, 28%), C28H32N4O7S (568.6).
N-(β-D-arabinofuranosyl)-7-methyl-5-(4-morpholin-1-yl)phenyl-3,5-dihydro-4H-thiochromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (9c). It obtained from 7c, as yellow powder, m. p. 289˚C - 291˚C, IR (cm−1, ν), 3460 (brs, OH), 1677 (CO), 1334 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.25 (s, CH3), 2.48 (m, 4H, N(CH2)2), 3.59 (m, 4H, O(CH2)2), 3.83 (m, H-5’, H-5’’), 4.09 (m, H-4’), 4.49 (s, thiopyran-H), 4.63 (t, H-2’), 5.07 (t, J = 5.40 Hz, J = 4.94 Hz, OH-C(5’), 5.21 (d, J = 4.41 Hz, OH-C(3’), 5.34 (d, J = 5.95 Hz, OH-C(2’), 5.67(t, J = 9.56 Hz, H-3’), 6.88 (d, J = 5.67 Hz, H-1’), 7.05 (m, Ar-H), 7.11 (m, Ar-H), 7.17 (s, Ar-H), 7.21 (m, Ar-H), 7.28 (d, Ar-H), 8.02 (d, Ar-H), 8.92 (s, pyrimidine-H), 13C.NMR: 22.07 (CH3), 41.26 (C-thiopyran-4), 46.06 (2C, N(CH2)2), 61.08 (C-5’), 63.45 (2C, O(CH2)2), 66.41 (C-3’), 66.65 (C-2’), 67.70 (C-4’), 85.73 (C-1’), 121.8 - 148.5 (15C-Ar), 166.9 (CO), Its MS (m/z), 555 (M+, 29%), C27H29N3O8S (555.6).
N-(β-D-glucopyranosyl)-7-methyl-5-(4-piperazin-1-yl-phenyl)-3,5-dihydro-4H-thio-chromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (10a). It obtained from 8a, as a white powder, m. p. 234˚C - 236˚C, IR (cm−1, ν), 3490 (brs, OH), 3250 (br, NH),1668 (CO), 1345 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.23 (s, CH3), 2.50 (m, 4H, N(CH2)2), 3.01 (m, 4H, HN(CH2)2), 4.35 (s, thiopyran-H), 3.87 (m, H-5’), 4.11 (m, H-6’, H-6’’), 4.27 (m, H-4’), 4.60 (br, D2O-exchangeable OH), 4.85 (t, H-2’), 5.04 (br, D2O-exchangeable OH), 5.13 (t, J = 9.61 Hz, H-3’), 5.18 (d, J = 4.83 Hz, D2O-exchangeable OH), 5.57 (br, D2Oexchangeable OH), 6.04 (d, J = 10.61 Hz, H-1’), 7.00 (m, Ar-H), 7.05 (m, Ar-H), 7.15 (s, Ar-H), 7.22 (m, Ar-H), 7.29 (d, Ar-H), 8.02 (d, Ar-H), 8.90 (s, pyrimidine-H), 10.28 (br, NH), 13C.NMR: 21.59 (CH3), 39.79 (Cthiopyran-4), 43.82 (2C, HN(CH2)2), 46.32 (2C, N(CH2)2), 61.56 (C-6’), 66.37 (C-3’), 68.40 (C-2’), 68.97 (C-4’), 77.79 (C-5’), 89.63 (C-1’), 120.5 - 148.2 (15C-Ar), 166.9 (CO), Its MS (m/z), 584 (M+, 26%), C28H32N4O8S (584.6).
N-(β-D-glucopyranosyl)-7-methyl-5-[4-(4-methyl piperazin-1-yl)phenyl]-3,5-dihydro-4H-thiochromeno[2,3-d]pyrimidine-4-one-10,10-dioxide (10b). It obtained from 8b, as a pale yellow powder, m. p. 243˚C - 245˚C, IR (cm−1, ν), 3480 (brs, OH), 1677(CO), 1339 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.28 (s, CH3), 2.44 (s, CH3), 2.56 (m, N(CH2)2), 2.70 (m, N(CH2)2), 3.81 (m, H-5’), 4.01 (m, H-6’, H-6’’), 4.31 (m, H-4’), 4.41 (s, thiopyran-H), 4.54 (br, D2O-exchangeable OH), 4.79 (t, H-2’), 5.03 (br, D2O-exchangeable-OH), 5.12 (t, J = 9.64 Hz, H-3’), 5.19 (d, J = 5.0 Hz, D2O-exchangeable OH), 5.60 (br,D2O-exchange-able OH), 6.07 (d, J = 10.54 Hz, H-1’), 7.00 (m, Ar-H), 7.06 (m, Ar-H), 7.11(s, Ar-H), 7.16 (m, Ar-H), 7.21 (d, Ar-H), 7.92 (d, Ar-H), 8.93 (s, pyrimidine-H), Its MS (m/z), 598 (M+, 27%), C29H34N4O8S (598.6).
N-(β-D-glucopyranosyl)-7-methyl-5-(4-morpholin-1-yl) phenyl-3,5-dihydro-4H-thiochromeno[2,3-d]pyrimi dine- 4-one-10,10-dioxide (10c). It obtained from 8c, as yellow powder, m. p. 289˚C - 291˚C, IR (cm−1, ν), 3470 (brs, OH), 1675 (CO), 1345 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.29 (s, CH3), 2.53 (m, 4H, N(CH2)2), 3.67 (m, 4H, O(CH2)2), 3.92 (m, H-5’), 4.06 (m, H-6’, H-6’’), 4.35 (m, H-4’), 4.56 (s, thiopyran-H), 4.97 (t, H-2’), 4.64 (br, D2O-exchangeable OH), 5.06 (br, D2O-exchangeable OH), 5.11 (d, J = 4.82 Hz, D2O-exchang-eable OH), 5.16 (t, J = 9.55 Hz, H-3’), 5.61 (br, D2O-exchangeable OH), 6.13 (d, J = 10.61 Hz, H-1’), 7.03 (m, Ar-H), 7.09 (m, Ar-H), 7.15 (s, Ar-H), 7.19 (m, Ar-H), 7.24 (d, 2H, ArH), 8.01 (d, 2H, Ar-H), 8.83 (s, pyrimidine-H), 13C.NMR: 21.89 (CH3), 43.26 (C-thiopyran-4), 45.91 (2C, N(CH2)2), 61.45 (C-6’), 64.81 (2C, O(CH2)2), 66.40 (C-3’), 67.89 (C-2’), 68.95 (C-4’), 77.82 (C-5’), 89.71 (C-1’), 120.8 - 148.4 (15C-Ar), 165.9 (CO), Its MS (m/z), 585 (M+, 30%), C28H31N3O9S (585.6)
N-(β-D-galactopyranosyl)-7-methyl-5-(4-piperazin-1-ylphenyl)-3,5-dihydro-4H-thiochromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (10d). It obtained from 8d, as a white powder, m. p. 229˚C - 231˚C, IR (cm−1, ν), 3530 (br, OH), 3315 (br, NH), 1675 (CO), 1339 (SO), 1H.NMR (DMS-O-d6, d, ppm): 2.31 (s, CH3), 2.52 (m, 4H, N(CH2)2), 3.07 (m, 4H, HN(CH2)2), 3.85 (m, H-5’), 4.13 (m, H-6’, H-6’’), 4.29 (m, H-4’), 4.37 (s, thiopyran-H), 4.57 (br, D2O-ex-changeable OH), 4.87 (t, H-2’), 5.08 (br, D2Oexchangeable OH), 5.15 (t, J = 9.70 Hz, H-3’), 5.20 (d, J = 4.84 Hz D2O-exchangeable-OH), 5.59 (br, D2O-exchange able OH), 6.07 (d, J = 10.65 Hz, H-1’), 7.02 (m, Ar-H), 7.09 (m, Ar-H), 7.17 (s, Ar-H), 7.23 (m, Ar-H), 7.31 (d, Ar-H), 8.05 (d, Ar-H), 8.86 (s, pyrimidine-H), 10.45 (br, NH), 13C. NMR: 21.45 (CH3), 40.19 (Cthiopyran-4), 43.74 (2C, HN(CH2)2), 46.29 (2C, N(CH2)2), 61.70 (C-6’), 66.41 (C-3’), 68.39 (C-2’), 69.07 (C-4’), 78.29 (C-5’), 89.87 (C-1’), 120.7 - 148.9 (15CAr), 167.5 (CO), Its MS (m/z), 584 (M+, 34%), C28H32N4O8S (584.6).
N-(β-D-galactopyranosyl)-7-methyl-5-[4-(4-methyl piperazin-1-yl)phenyl]-3,5-dihydro-4H-thiochromeno [2,3-d]pyrimidine-4-one-10,10-dioxide (10e). It obtained from 8e, m. p. 271˚C - 273˚C, IR (cm−1, ν), 3485 (brs, OH), 1668 (CO), 1341 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.23 (s, CH3), 2.47 (s, CH3), 2.53 (m, N(CH2)2), 2.69 (m, N(CH2)2), 3.78 (m, 1H, H-5’), 4.03 (m, H-6’, H-6’’), 4.33 (m, H-4’), 4.51 (s, thiopyran-H), 4.56 (br, D2O-exchangeable OH), 4.81 (t, H-2’), 5.11 (br, D2Oexchangeable OH), 5.15 (t, J = 9.70 Hz, H-3’), 5.21 (d, J = 5.0 Hz, D2O-exchangeable OH), 5.63 (br, D2O-exchangeable OH), 6.08 (d, J = 10.59 Hz, H-1’), 7.01 (m, Ar-H), 7.09 (m, Ar-H), 7.13 (s, Ar-H), 7.19 (m, Ar-H), 7.23 (d, Ar-H), 7.98 (d, Ar-H), 8.83 (s, pyrimidine-H), Its MS(m/z),598(M+, 27%),C29H34N4O8S (598.6).
N-(β-D-galactopyranosyl)-7-methyl-5-(4-morpholin-1-ylphenyl)-3,5-dihydro-4H-thiochromeno[2,3-d] pyrimidine-4-one-10,10-dioxide (10f). It obtained from 8f, as pale brown powder, m. p. 267˚C - 269˚C, IR (cm−1, ν), 3495 (brs, OH), 1668 (CO), 1350 (SO), 1H.NMR (DMSO-d6, d, ppm): 2.25 (s, CH3), 2.50 (m, 4H, N(CH2)2), 3.65 (m, 4H, O(CH2)2), 3.96 (m, H-5’), 4.05 (m, H-6’, H-6’’), 4.41 (m, H-4’), 4.60 (s, thiopyran-H), 4.98 (t, H-2’), 4.70 (br, D2O-exchangeable OH), 5.10 (br, D2O-exchangeable OH), 5.16 (d, J = 4.81 Hz, D2O-exchangeable OH), 5.19 (t, J = 9.64 Hz, H-3’), 5.63 (br, D2O-exchangeable OH), 6.15 (d, J = 10.65 Hz, H-1’), 6.98 (m, Ar-H), 7.04 (m, Ar-H), 7.12 (s, Ar-H), 7.20 (m, 1H, Ar-H), 7.25 (d, 2H, Ar-H), 8.03 (d, 2H, Ar-H), 8.87 (s, pyrimidine-H), 13C. NMR: 21.97 (CH3), 42.69 (Cthiopyran-4), 45.65 (2C, N(CH2)2), 61.38 (C-6’), 64.69 (2C, O(CH2)2), 66.35 (C-3’), 67.91 (C-2’), 68.93 (C-4’), 77.85 (C-5’), 89.76 (C-1’), 121.5-148.7 (15C-Ar), 166.4 (CO), Its MS (m/z), 585 (M+, 30%), C28H31N3O9S (585.6).
5. Acknowledgements
The authors are thankful to the Al-Imam Mohammad Ibn Saud Islamic University (IMSIU), Faculty of Science, for providing laboratory facilities, Micro-analytical Centre, and the Pharmacological Unit National Research Centre, for microanalyses and pharmacological screening of the compounds.
REFERENCES
- E. De Clercq, D. Desgranges, P. Herdewijin, I. S. Shim, A. S. Jones, M. J. Mclean and R. T. Walker, “Synthesis and Antiviral Activity of (E)-5-(2-Bromovinyl)uracil and (E)-5-(2-Bromovinyl)uridine,” Journal of Medicinal Chemistry, Vol. 29, No. 2, 1986, pp. 213-217. http://dx.doi.org/10.1021/jm00152a008
- T. S. Lin, J. T. Guo, R. F. Schinazi, C. K. Chu, J. N. Xiang and W. H. Prusoff, “Synthesis and Antiviral Activity of Various 3’-Azido Analogues of Pyrimidine Deoxyribonucleosides against Human Immunodeficiency Virus (HIV-1, HTLV-III/LAV),” Journal of Medicinal Chemistry, Vol. 31, No. 2, 1988, pp. 336-340. http://dx.doi.org/10.1021/jm00397a011
- C. Heidelberger, D. D. King and D. Shugar, “Antiviral Agents in Pharmacology and Therapeutics,” Pergamon, Oxford, 1979, p. 472.
- F. W. Hobbs, “Palladium-Catalyzed Synthesis of Alkynylamino Nucleosides: A Universal Linker for Nucleic Acids,” The Journal of Organic Chemistry, Vol. 54, No. 14, 1989, pp. 3420-3422. http://dx.doi.org/10.1021/jo00275a030
- B. C. Froehler, S. Wadwani, J. J. Terhorst and S. K. Gerrad, “Oligodeoxynucleotides Containing C-5 Propyne Analogs of 2′-Deoxyuridine and 2′-Deoxycytidine,” Tetrahedron Letters, Vol. 33, No. 37, 1992, pp. 5307-5307. http://dx.doi.org/10.1016/S0040-4039(00)79079-4
- A. W. Taylor and D. K. Dean, “A New Synthesis of Thioflavones,” Tetrahedron Letters, Vol. 29, No. 15, 1988, pp. 1845-1848. http://dx.doi.org/10.1016/S0040-4039(00)82060-2
- S. W. Schneller, “Thiochromanones and Related Compounds,” Advances in Heterocyclic Chemistry, Vol. 18, 1975, pp. 59-97. http://dx.doi.org/10.1016/S0065-2725(08)60128-2
- P. Kumar, A. T. Rao and B. Pandey, “An Efficient Approach to the Synthesis of 4H-1-Benzo-Thiopyran-4-Ones via Intramolecular Wittig Reaction,” Journal of the Chemical Society, Chemical Communications, No. 21, 1992, pp. 1580-1584. http://dx.doi.org/10.1039/c39920001580
- P. Kumar and M. S. Bodas, “A New Synthesis of 4H- 1-Benzothiopyran-4-Ones Using (Trimethylsilyl)-Methylenetriphenylphosphorane,” Tetrahedron, Vol. 57, 2001, pp. 9755-9758. http://dx.doi.org/10.1016/S0040-4020(01)00977-2
- A. J. Angel, A. E. Finefrock, K. L. French, D. R. Hurst, A. R. Williams, M. E. Rampey, S. L. Studer-Martinez and C. F. Beam, “Preparation of N-Aryl-4-oxo-4H-1-benzothiopyran-2-acetamides from Trilithiated Acetoacetanilides and Lithiated Methyl Thiosalicylate,” Canadian Journal of Chemistry, Vol. 77, No. 1, 1999, pp. 94-97. http://dx.doi.org/10.1139/v98-216
- K. L. French, A. J. Angel, A. R. Williams, D. R. Hurst and C. F. Beam, “A New Preparation of Substituted 4H- 1-Benzothiopyran-4-Ones from C(a), N-benzoyl Hydrazones or C(a), n-Carboalkoxy Hydrazones and Methyl Thiosalicylate,” Journal of Heterocyclic Chemistry, Vol. 35, No. 1, 1998, pp. 45-48. http://dx.doi.org/10.1002/jhet.5570350109
- C. E. Foster and P. R. Mackie, “Comprehensive Organic Functional Group Transformations II,” Elsevier Ltd, Oxford, 2005, p. 244.
- A. M. Marini, F. Da Settimo, S. Salerno, C. La Motta, F. Simorini, S. Taliani, D. Bertini, O. Gia and L. Dalla Via, “Synthesis and in Vitro Antiproliferative Activity of New Substituted Benzo[3′,2′:5,6]thiopyrano[4,3-d]pyrimidines,” Journal of Heterocyclic Chemistry, Vol. 45, No. 3, 2008, pp. 745-749. http://dx.doi.org/10.1002/jhet.5570450318
- A. S. Abbas, H. N. Hafez and A. B. A. El-Gazzar, “Synthesis, in Vitro Antimicrobial and in Vivo Antitumor Evaluation of Novel Pyrimidoquinolines and Its Nucleoside Derivatives,” European Journal of Medicinal Chemistry, Vol. 46, No. 1, 2011, pp. 21-30. http://dx.doi.org/10.1016/j.ejmech.2010.09.071
- H. N. Hafez, H. A. Hussein and A. B. A. El-Gazzar, “Synthesis of Substituted Thieno[2,3-d]pyramidine -2,4-dithiones and Their S-Glycoside Analogues as Potential Antiviral and Anti-Bacterial Agents,” European Journal of Medicinal Chemistry, Vol. 45, No. 9, 2010, pp. 4026- 4034. http://dx.doi.org/10.1016/j.ejmech.2010.05.060
- H. N. Hafez and A. B. A. El-Gazzar, “Synthesis and Antitumor Activity of Substituted Triazolo-[4,3-a]pyrimidin- 6-Sulfonamide with an Incorporated Thiazolidinone Moiety,” Bioorganic & Medicinal Chemistry Letters, Vol. 19, No. 15, 2009, pp. 4143-4147. http://dx.doi.org/10.1016/j.bmcl.2009.05.126
- A. B. A. El-Gazzar, H. N. Hafez and H. A. S. Abbas, “Sand C-Nucleosidoquinazoline as New Nucleoside Analogs with Potential Analgesic and Anti-Inflammatory Activity,” European Journal of Medicinal Chemistry, Vol. 44, No. 10, 2009, pp. 4249-4258. http://dx.doi.org/10.1016/j.ejmech.2009.05.025
- J. A. Halperin, A. Natarajan, Y. Guo, F. Harbinski, Y. H. Fan, H. Chen, L. Luus, J. Diercks, H. Aktas and M. Chorev, “Novel Arylsulfonamide-Oxindole Hybrid as an Anticancer Agent That Inhibits Translation Initiation,” Journal of Medicinal Chemistry, Vol. 47, No. 21, 2004, pp. 4979-4982. http://dx.doi.org/10.1021/jm0496234
- M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbot, J. G. Mayo, R. H. Shoemaker and M. R. Boyd, “Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay,” Cancer Research, Vol. 48, No. 3, 1988, pp. 589-601.
- M. R. Grever, S. A. Schepartz and B. A. Chabner, “The National Cancer Institute: Cancer Drug Discovery and Development Program,” Oncology, Vol. 19, No. 6, 1992, pp. 622-638.
- M. R. Boyd and K. D. Paull, “Some Particles Consideration and Applications of the NCI in Vitro Anticancer Drug Discovery Screen,” Drug Development Research, Vol. 34, No. 2, 1995, pp. 91-109. http://dx.doi.org/10.1002/ddr.430340203
- A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, M. Gray-Goodrich, H. Campbell, J. Mayo and M. Boyd, “Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines,” Journal of the National Cancer Institute, Vol. 83, No. 11, 1991, pp. 757-766. http://dx.doi.org/10.1093/jnci/83.11.757
- J. N. Weinstein, T. G. Myers, P. M. Connor, S. H. Friend A. J. Fornace Jr., K. W. Kohn, T. Fojo, S. E. Bates, L. V. Rubinstein, N. L. Anderson, J. K. Buolamwini, W. W. van Osdol, A. P. Monks, D. A. Scudiero, E. A. Sausville, D. W. Zaharevitz, B. Bunow, V. N. Viswanadhan, G. S. Johnson, R. E. Wittes and K. D. Paull, “An InformationIntensive Approach to the Molecular Pharmacology of Cancer,” Science, Vol. 275, No. 5298, 1997, pp. 343-349. http://dx.doi.org/10.1126/science.275.5298.343
- K. Yoshimatsu, A. Yamaguchi, H. Yoshino, N. Koyanagi and K. Kitoh, “Mechanism of Action of E7010, an Orally Active Sulfonamide Antitumor Agent: Inhibition of Mitosis by Binding to the Colchicine Site of Tubulin,” Cancer Research, Vol. 57, No. 1, 1997, pp. 3208-3213.

