Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 202-209 doi:10.4236/msa.2010.14032 Published Online October 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Célia Regina Tomachuk1, Alejandro Ramón Di Sarli2, Cecilia Inés Elsner2 1Energy and Nuclear Research Institute, IPEN/CNEN-SP, CCTM, Av. Prof. Lineu Prestes, São Paulo, Brazil; 2CIDEPINT: Research and Development Center in Paint Technology (CICPBA-CONICET), Av.52 s/n entre 121 y 122. CP. B1900AYB, La Plata-Argentina. Email: tomazuk@gmail.com, direccion@cidepint.gov.ar Received April 29th, 2010; revised July 22nd, 2010; accepted August 4th, 2010. ABSTRACT Hexavalent chromium-based passivation treatments have been successfully used as promoters of conversion coatings for many years. Their effectiveness is without question although there are many problems with regard to their environ- mental suitability. Hexavalent chromium compounds are carcinogenic and toxic. These problems have lead researchers to evaluate other potential systems, with lower toxicity, to ascertain if they can replace chromates as effective passiva- tors. Researchers have proposed several alternative passivation treatments; these are processes based on molybdates, permanganates, titanates, rare earth metal and Cr3+ (considered to be non-carcinogenic) compounds. In this work, zinc coatings obtained from free-cyanide alkaline bath and submitted to a Cr3+ based passivation treatment with different colors were studied. The corrosion behavior was studied by polarization measurements and mainly by electrochemical impedance spectroscopy in 0.6 N NaCl solution. Morphological observations on the coatings surface were also per- formed. The results indicate that the green-colored Cr3+ passivated coatings have a good corrosion resistance followed by yellow and blue-colored passivation respectively. They could be a less polluting alternative to the traditional chro- mated coatings. Keywords: Zinc, Conversion Treatment, Impedance Spectroscopy, Salt Spray, Corrosion 1. Introduction Electroplated zinc coating is employed as active galvanic protection for steel. However, as the zinc is an electro- chemically highly reactive metal, its corrosion rate may be also high in indoor but particularly under outdoor ex- posure conditions [1]. For this reason, it is necessary a post treatment in order to increase the lifetime of zinc coatings. In current industrial practice, this treatment consists of immersion in a chemical bath that forms a conversion layer on plated zinc. This latter layer is a di- electric passive layer with high corrosion resistance and is also a better surface for paint adherence. The main problem of traditionally used post treatments is the pres- ence of Cr6+ salts, considered carcinogenic substances which usage is forbidden by European norms [2]. Re- sponding to increasingly more rigorous environmental protection activities, recent years have shown progressive advances in order to reduce the use of environmen- tally-hazardous materials. In line with this purpose, the development of various kinds of chromate-free coated steel sheets, to be used in food, automotive, appliances, etc. industries, is being extensively explored all over the world. In this sense, the most common transitional alter- native to Cr6+ is Cr3+, which is used since the mid 1970’s [3-9]. According to Fonte et al. [10], the Cr3+ conversion layer formed in a bath containing transition metal ions such us Co2+, Ni2+ and Fe2+ showed higher corrosion re- sistance than those formed in a bath without transition metal ions. This finding was confirmed by Tomachuk et al. [11,12]. Molybdates, tungstates, permanganates and vanadates, including chromium like elements, were the first chemi- cal elements tried as hexavalent chromium substitutes [13-17]. Recently many alternative coatings were devel- oped based on zirconium and titanium salts [18-20], co- balt salts [21,22], organic polymers [23,24] and rare earth salts [25]. However, preparation and corrosion behavior of these coatings is not clear and their practical usage is doubtful. In order to find an alternative treatment to Cr6+ con-  Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 203 version coating, several treatments that present a good anti-corrosive behavior, a high benefit/cost relation and, mainly, low environmental impact are still to be devel- oped. Usually, the corrosion behavior of coatings is eva- luated using traditional tests such as Salt Spray [26], Kesternich test [27], saturated humidity [28]. However the authors consider important the application of elec- trochemical methods to obtain fast information about the corrosion reactions kinetics. Among the electrochemical techniques that can be used, the electrochemical impedance spectroscopy (EIS) was selected based on the already obtained results for metal and metal-coated corrosion evaluation [29-32]. The main purpose of the present work was to find an environmentally friendly conversion treatment able to replace satisfactorily those passivating ones based on Cr6+. Electrogalvanised steel covered with Cr6+-free pas- sivating layers were investigated using AC and DC elec- trochemical techniques. Morphological studies of the coatings surface were also performed. 2. Experimental Details 2.1. Samples Preparation Electrogalvanised steel samples (7.5 × 10 × 0.1 cm) were industrially produced and covered with the following conversion treatments: 1) blue-colored Cr3+ based pas- sivation; 2) yellow-colored Cr3+ based passivation; 3) green-colored Cr3+ based passivation. For each conver- sion layer, an individual commercial conversion bath was formulated and the coating was produced according to the respective supplier recommendations. 2.2. Thickness Measurements The coatings thickness was measured using the Helmut Fischer equipment DUALSCOPE MP4. 2.3. Morphology The coatings morphology was determined from scanning electron microscopy (SEM) analyses using a LEICA S440 microscope. 2.4. Electrochemical Behavior The electrochemical cell consisted of a classic three-elec- trode arrangement, where the counter electrode was a platinum sheet, the reference one a saturated calomel electrode (SCE) and the working electrode each coated steel sample with a defined area of 7 cm2. All measure- ments were performed at a constant room temperature (22 3℃) in 0.6 N NaCl solution. Potentiodynamic polarization experiments were car- ried out using a Solartron 1280 electrochemical system at a swept rate of 1 mV.s-1, over the range ±0.300 V(SCE) from the open-circuit potential OCP). Before each swept, the electrode in contact with the electrolyte was stabi- lized for several minutes. The corrosion current density (j) and corrosion potential (Ecorr) were obtained from a Tafel slope by extrapolation of the linear portion of anodic and cathodic branches. Impedance spectra in the frequency range 2.10-2 Hz < f < 4.104 Hz were performed in the potentiostatic mode at the OCP, and as a function of the exposure time in the electrolyte solution, using a Solartron 1260 Frequency Response Analyzer (FRA) coupled to a Solartron 1286 electrochemical interface (EI). The amplitude of the ap- plied AC voltage was 3 mV peak to peak. Each sample’s surface evolution was analyzed until white corrosion products could be seen by the naked eye. The experi- mental spectra were interpreted on the basis of equivalent electrical circuits’ models using the ZView fitting soft- ware by Scribner Associates. All impedance measure- ments were carried out by triplicate in a Faraday cage in order to minimize external interference on the system studied. 3. Results and Discussion The overall coating thickness and description of the sam- ples investigated in this work are reported in Table 1. In it can be seen that these showed similar and uniform thickness; besides, they also exhibited a bright appear- ance throughout their extension. Unfortunately, informa- tion related with the passive layer thickness was not pos- sible to be obtained. 3.1. Morphology The consideration of the coating morphology after the coating/drying process is very important since the pres- ence of flaws such as pores and/or other defects could be areas were localized corrosion of the treated zinc surface starts from its exposure to a given environment [33]. Therefore, after applying the conversion treatment, the coatings surface morphology was observed up to 1,000X Table 1. Characteristics of the samples. IdentificationDescription Thickness (Zn + conversion treatment) (µm) A blue-colored Cr3+ passivation UniFix Zn-3-50 (LABRITS®) 10.8 B yellow-colored Cr3+ passivation UniYellow 3 (LABRITS®) 11.2 C green-colored Cr3+ passivation SurTec S680® 10.4 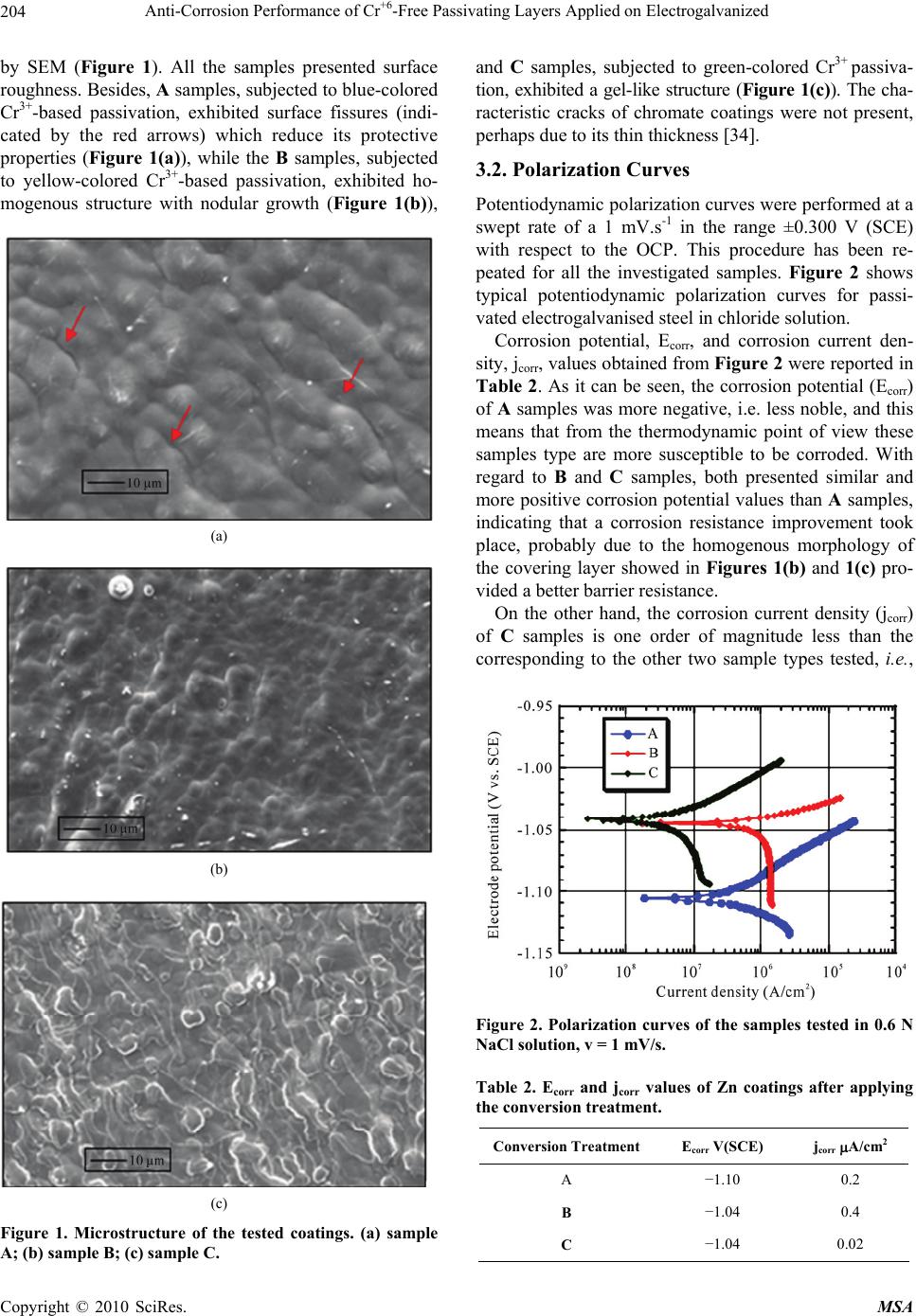 Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 204 by SEM (Figure 1). All the samples presented surface roughness. Besides, A samples, subjected to blue-colored Cr3+-based passivation, exhibited surface fissures (indi- cated by the red arrows) which reduce its protective properties (Figure 1(a)), while the B samples, subjected to yellow-colored Cr3+-based passivation, exhibited ho- mogenous structure with nodular growth (Figure 1(b)), (a) (b) (c) Figure 1. Microstructure of the tested coatings. (a) sample A; (b) sample B; (c) sample C. and C samples, subjected to green-colored Cr3+ passiva- tion, exhibited a gel-like structure (Figure 1(c)). The cha- racteristic cracks of chromate coatings were not present, perhaps due to its thin thickness [34]. 3.2. Polarization Curves Potentiodynamic polarization curves were performed at a swept rate of a 1 mV.s-1 in the range ±0.300 V (SCE) with respect to the OCP. This procedure has been re- peated for all the investigated samples. Figure 2 shows typical potentiodynamic polarization curves for passi- vated electrogalvanised steel in chloride solution. Corrosion potential, Ecorr, and corrosion current den- sity, jcorr, values obtained from Figure 2 were reported in Table 2. As it can be seen, the corrosion potential (Ecorr) of A samples was more negative, i.e. less noble, and this means that from the thermodynamic point of view these samples type are more susceptible to be corroded. With regard to B and C samples, both presented similar and more positive corrosion potential values than A samples, indicating that a corrosion resistance improvement took place, probably due to the homogenous morphology of the covering layer showed in Figures 1(b) and 1(c) pro- vided a better barrier resistance. On the other hand, the corrosion current density (jcorr) of C samples is one order of magnitude less than the corresponding to the other two sample types tested, i.e., Figure 2. Polarization curves of the samples tested in 0.6 N NaCl solution, v = 1 mV/s. Table 2. Ecorr and jcorr values of Zn coatings after applying the conversion treatment. Conversion TreatmentEcorr V(SCE) jcorr A/cm2 A −1.10 0.2 B −1.04 0.4 C −1.04 0.02  Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 205 its corrosion rate is lower. 3.3. Electrochemical Impedance Spectroscopy EIS measurements carried out in the 0.6 N NaCl solution were discontinued upon the white corrosion products on the surface could be seen by naked eye. Figure 3 shows a Nyquist representation of the time dependent electrochemical impedance, while Figure 4 illustrates the electrical equivalent circuits able of simu- lating the physicochemical processes taking place at the coated steel surface. It is important to emphasize that experimental impedance data obtained for A and B sam- ples were analyzed on the basis of the electric equivalent circuit depicted in Figure 4(a)., while for the C samples was used the shown in Figure 4(b) [35]. In these figures, Rsol represents the electrolyte resistance between the ref- erence and working (coated steel) electrodes; the first time constant (R1Q1) - where R1 and (Q1 C1) are re- spectively the resistance to the ionic flux and the dielec- tric capacitance of the conversion layer - appears at the higher frequencies. Once the permeating and corro- sion-inducing chemicals (water, oxygen and ionic species) reach electrochemically active areas of the substrate, particularly at the bottom of the coating defects, the me- tallic corrosion becomes measurable so that its associated parameters, the charge transfer resistance, R2, and the electrochemical double layer capacitance, (Q2 C2), can be estimated [3]. Sometimes, the Q2 parameter can be associated to a diffusional process, which not only could be the rate-determining step (rds) of the corrosion reac- tion but also mask part of - or completely its time con- stant. It is important to remark that R2 and C2 values vary directly (R2) and inversely (C2) with the size of the electrochemically active metallic surface. Distortions observed in these resistive-capacitive con- tributions indicate a deviation from the theoretical mod- els in terms of a time constants distribution due to either lateral penetration of the electrolyte at the metal/coating interface (usually started at the base of intrinsic or artifi- cial coating defects), underlying metallic surface hetero- geneity (topological, chemical composition, surface en- ergy) and/or diffusional processes that could take place along the test. Since all these factors cause the imped- ance/frequency relationship to be non-linear, they are taken into consideration by replacing one or more ca- pacitive components (Ci) of the equivalent circuit trans- fer function by the corresponding constant phase element Qi (CPE), for which the impedance may be expressed as [36,37]: n 0 jω Y Z where: (a) (b) Figure 3. Evolution of the A and C samples impedance (Nyquist representation). (a) sample A; (b) sample C. (a) (b) Figure 4. Equivalent circuit models used for fitting the im- pedance data. Z() impedance of the CPE (Z = Z´ + jZ´´)() j imaginary number (j2 = −1) angular frequency (rad) 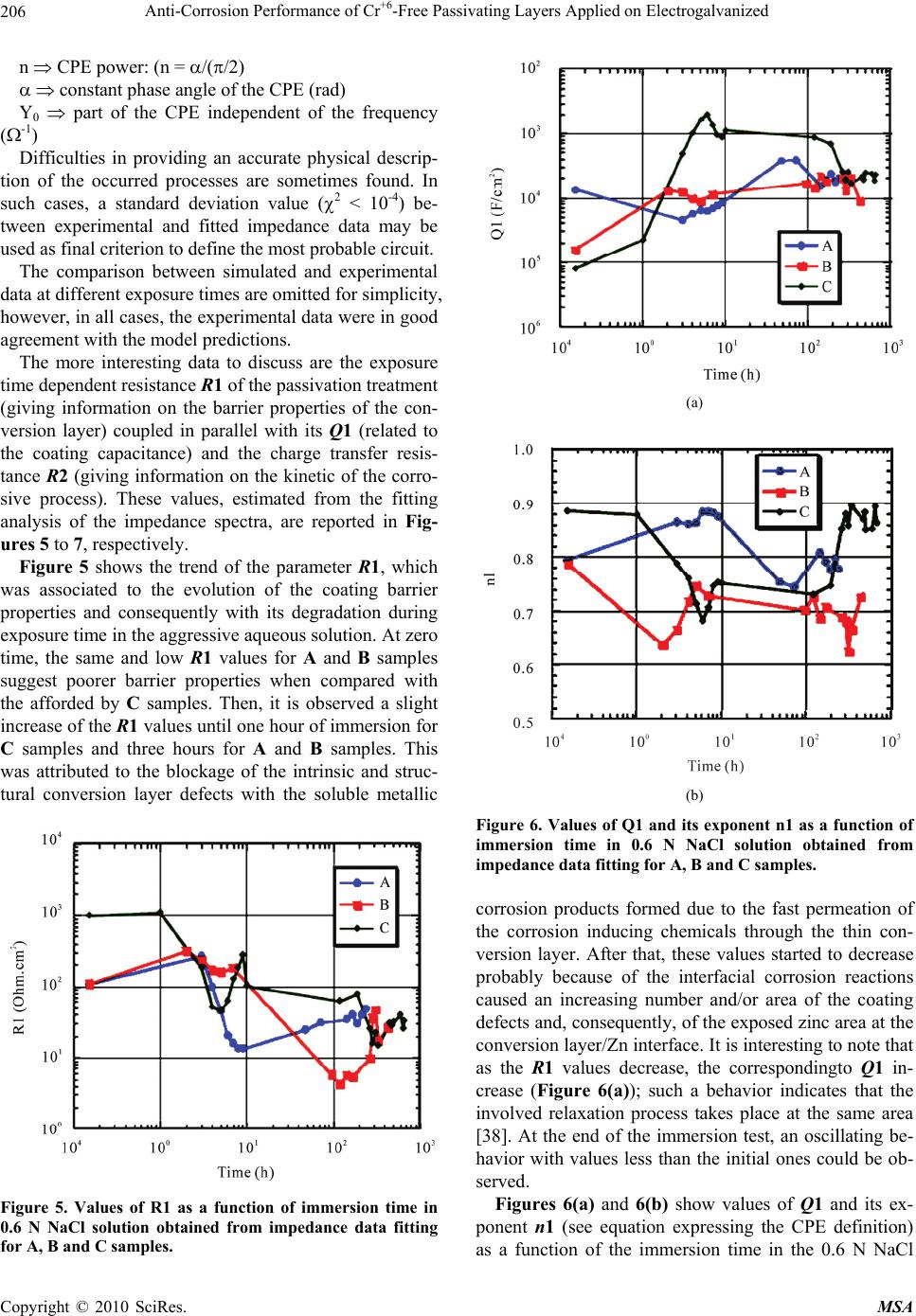 Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 206 n CPE power: (n = / constant phase angle of the CPE (rad) Y0 part of the CPE independent of the frequency (-1) Difficulties in providing an accurate physical descrip- tion of the occurred processes are sometimes found. In such cases, a standard deviation value (2 < 10-4) be- tween experimental and fitted impedance data may be used as final criterion to define the most probable circuit. The comparison between simulated and experimental data at different exposure times are omitted for simplicity, however, in all cases, the experimental data were in good agreement with the model predictions. The more interesting data to discuss are the exposure time dependent resistance R1 of the passivation treatment (giving information on the barrier properties of the con- version layer) coupled in parallel with its Q1 (related to the coating capacitance) and the charge transfer resis- tance R2 (giving information on the kinetic of the corro- sive process). These values, estimated from the fitting analysis of the impedance spectra, are reported in Fig- ures 5 to 7, respectively. Figure 5 shows the trend of the parameter R1, which was associated to the evolution of the coating barrier properties and consequently with its degradation during exposure time in the aggressive aqueous solution. At zero time, the same and low R1 values for A and B samples suggest poorer barrier properties when compared with the afforded by C samples. Then, it is observed a slight increase of the R1 values until one hour of immersion for C samples and three hours for A and B samples. This was attributed to the blockage of the intrinsic and struc- tural conversion layer defects with the soluble metallic Figure 5. Values of R1 as a function of immersion time in 0.6 N NaCl solution obtained from impedance data fitting for A, B and C samples. (a) (b) Figure 6. Values of Q1 and its exponent n1 as a function of immersion time in 0.6 N NaCl solution obtained from impedance data fitting for A, B and C samples. corrosion products formed due to the fast permeation of the corrosion inducing chemicals through the thin con- version layer. After that, these values started to decrease probably because of the interfacial corrosion reactions caused an increasing number and/or area of the coating defects and, consequently, of the exposed zinc area at the conversion layer/Zn interface. It is interesting to note that as the R1 values decrease, the correspondingto Q1 in- crease (Figure 6(a)); such a behavior indicates that the involved relaxation process takes place at the same area [38]. At the end of the immersion test, an oscillating be- havior with values less than the initial ones could be ob- served. Figures 6(a) and 6(b) show values of Q1 and its ex- ponent n1 (see equation expressing the CPE definition) as a function of the immersion time in the 0.6 N NaCl 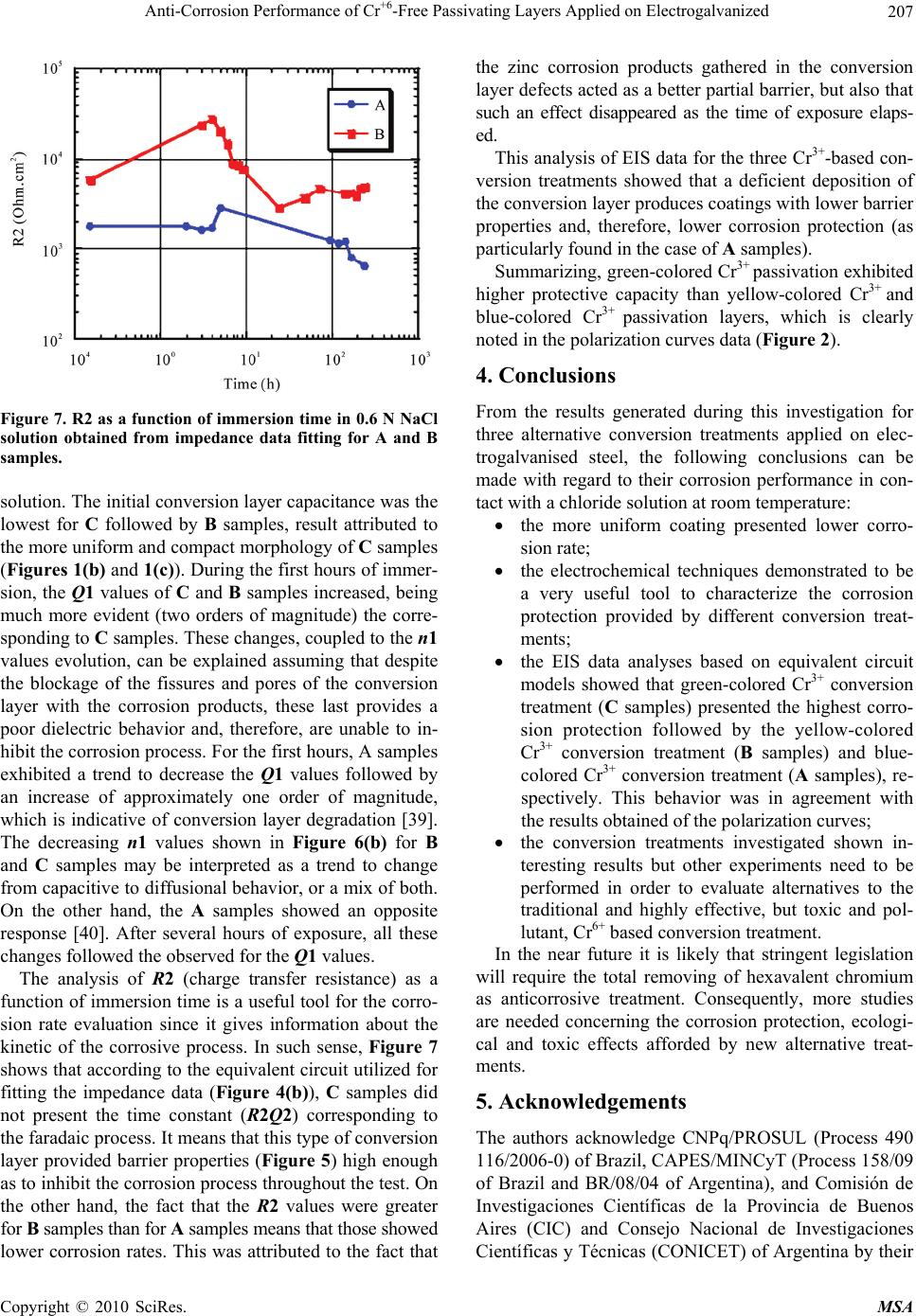 Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 207 Figure 7. R2 as a function of immersion time in 0.6 N NaCl solution obtained from impedance data fitting for A and B samples. solution. The initial conversion layer capacitance was the lowest for C followed by B samples, result attributed to the more uniform and compact morphology of C samples (Figures 1(b) and 1(c)). During the first hours of immer- sion, the Q1 values of C and B samples increased, being much more evident (two orders of magnitude) the corre- sponding to C samples. These changes, coupled to the n1 values evolution, can be explained assuming that despite the blockage of the fissures and pores of the conversion layer with the corrosion products, these last provides a poor dielectric behavior and, therefore, are unable to in- hibit the corrosion process. For the first hours, A samples exhibited a trend to decrease the Q1 values followed by an increase of approximately one order of magnitude, which is indicative of conversion layer degradation [39]. The decreasing n1 values shown in Figure 6(b) for B and C samples may be interpreted as a trend to change from capacitive to diffusional behavior, or a mix of both. On the other hand, the A samples showed an opposite response [40]. After several hours of exposure, all these changes followed the observed for the Q1 values. The analysis of R2 (charge transfer resistance) as a function of immersion time is a useful tool for the corro- sion rate evaluation since it gives information about the kinetic of the corrosive process. In such sense, Figure 7 shows that according to the equivalent circuit utilized for fitting the impedance data (Figure 4(b)), C samples did not present the time constant (R2Q2) corresponding to the faradaic process. It means that this type of conversion layer provided barrier properties (Figure 5) high enough as to inhibit the corrosion process throughout the test. On the other hand, the fact that the R2 values were greater for B samples than for A samples means that those showed lower corrosion rates. This was attributed to the fact that the zinc corrosion products gathered in the conversion layer defects acted as a better partial barrier, but also that such an effect disappeared as the time of exposure elaps- ed. This analysis of EIS data for the three Cr3+-based con- version treatments showed that a deficient deposition of the conversion layer produces coatings with lower barrier properties and, therefore, lower corrosion protection (as particularly found in the case of A samples). Summarizing, green-colored Cr3+ passivation exhibited higher protective capacity than yellow-colored Cr3+ and blue-colored Cr3+ passivation layers, which is clearly noted in the polarization curves data (Figure 2). 4. Conclusions From the results generated during this investigation for three alternative conversion treatments applied on elec- trogalvanised steel, the following conclusions can be made with regard to their corrosion performance in con- tact with a chloride solution at room temperature: the more uniform coating presented lower corro- sion rate; the electrochemical techniques demonstrated to be a very useful tool to characterize the corrosion protection provided by different conversion treat- ments; the EIS data analyses based on equivalent circuit models showed that green-colored Cr3+ conversion treatment (C samples) presented the highest corro- sion protection followed by the yellow-colored Cr3+ conversion treatment (B samples) and blue- colored Cr3+ conversion treatment (A samples), re- spectively. This behavior was in agreement with the results obtained of the polarization curves; the conversion treatments investigated shown in- teresting results but other experiments need to be performed in order to evaluate alternatives to the traditional and highly effective, but toxic and pol- lutant, Cr6+ based conversion treatment. In the near future it is likely that stringent legislation will require the total removing of hexavalent chromium as anticorrosive treatment. Consequently, more studies are needed concerning the corrosion protection, ecologi- cal and toxic effects afforded by new alternative treat- ments. 5. Acknowledgements The authors acknowledge CNPq/PROSUL (Process 490 116/2006-0) of Brazil, CAPES/MINCyT (Process 158/09 of Brazil and BR/08/04 of Argentina), and Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina by their  Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 208 financial support to this research. REFERENCES [1] N. Zaki, “Chromate Conversion Coating for Zinc,” Metal Finishing, Vol. 86, No. 2, February 1988, pp. 75-78. [2] P. L. Hagans and C. M. Haas, “ASM Handbook,” Surface Engineering, ASM International, Vol. 5, 1994. [3] F. Deflorian, S. Rossi, L. Fedrizzi and P. L. Bonora, “EIS Study of Organic Coating on Zinc Surface Pretreated with Environmentally Friendly Products,” Progress in Organic Coatings, Vol. 52, No. 4, 2005, pp. 271-279. [4] R. Berger, U. Bexell, T. M. Grehk and S. E. Hörnström, “A Comparative Study of the Corrosion Protective Prop- erties of Chromium and Chromium Free Passivation Me- thods,” Surface and Coatings Technology, Vol. 202, No. 2, 2007, pp. 391-397. [5] K. W. Cho, V. S. Rao and H. Kwon, “Microstructure and Electrochemical Characterization of Trivalent Chromium Based Conversion Coating on Zinc,” Electrochimica Acta, Vol. 52, No. 13, 2007, pp. 4449-4456. [6] Y.-T. Chang, N.-T. Wen, W.-K. Chen, M.-D. Ger, G.-T. Pan and T. C.-K. Yang, “The Effects of Immersion Time on Morphology and Electrochemical Properties of the Cr(III)-Based Conversion Coatings on Zinc Coated Steel Surface,” Corrosion Science, Vol. 50, No. 12, 2008, pp. 3494-3499. [7] X. Zhang, C. van den Bos, W. G. Sloof, A. Hovestad, H. Terryn and J. H. W. de Wit, “Comparison of the Mor- phology and Corrosion Performance of Cr(VI)- and Cr(III)-Based Conversion Coatings on Zinc,” Surface and Coatings Technology, Vol. 199, No. 1, 2005, pp. 92-104. [8] N. T. Wen, F. J. Chen, M. D. Ger, Y. N. Pan and C. S. Lin, “Microstructure of Trivalent Chromium Conversion Coating on Electrogalvanized Steel Plate,” Electro- chemical Solid State Letter, Vol. 11, No. 8, 2008, pp. C47-C50. [9] N.-T. Wen, C.-S. Lin, C.-Y. Bai and M.-D. Ger, “Struc- tures and Characteristics of Cr(III)-Based Conversion Coatings on Electrogalvanized Steels,” Surface and Coat- ings Technology, Vol. 203, No. 3-4, 2008, pp. 317-323. [10] B. da Fonte, Jr. and M. C. Mich, US Patent 4.359.345, 1982. [11] C. R. Tomachuk, C. I. Elsner, A. R. Di Sarli and O. B. Ferraz, “Morphology and Corrosion Resistance of Cr(III)- Based Conversion Treatments Applied on Electrogalva- nised Steel,” Journal of Coatings Technology and Research, Vol. 7, No. 4, 2010, pp. 493-502. [12] C. R. Tomachuk, C. I. Elsner, A. R. di Sarli and O. B. Ferraz, “Corrosion Resistance of Cr(III) Conversion Treatments Applied on Electrogalvanised Steel and Sub- jected to Chloride Containing Media,” Materials Chemis- try and Physics, Vol. 119, No. 1-2, 2009, pp. 19-29. [13] E. Almeida, T. C. Diamantino, M. O. Figueiredo and C. Sá, “Oxidizing Alternative Species to Chromium VI in Zinc Galvanized Steel Surface Treatment. Part 1. A Morphological and Chemical Study,” Surface and Coat- ings Technology, Vol. 106, No. 1, July 1998, pp. 8-17. [14] E. Almeida, L. Fedrizzi and T. C. Diamantino, “Oxidizing Alternative Species to Chromium VI in Zinc Galvanized Steel Surface Treatment. Part 2. An Electrochemical Study,” Surface and Coatings Technology, Vol. 105, No. 1-2, June 1998, pp. 97-101. [15] G. D. Wilcox and D. R. Gabe, “Passivation Studies Using Group VIA Anions. V. Cathodic Treatment of Zinc,” British Corrosion Journal, Vol. 22, No. 4, 1987, pp. 254- 258. [16] G. D. Wilcox, D. R. Gabe and M. E. Warwick, “The De- velopment of Passivation Coatings by Cathodic Reduc- tion in Sodium Molybdate Solutions,” Corrosion Science, Vol. 28, No. 6, 1988, pp. 577-587. [17] V. I. Korobov, Y. M. Loshkarev and O. V. Kozhura, “Cathodic Treatment of Galvanic Zinc Coatings in Solu- tions of Molybdates,” Russian Journal of Electrochemis- try, Vol. 34, No. 11, 1998, pp. 1154-1157. [18] P. D. Deck and D. M. Reichgott, “Characterization of Chromium-Free No-Rinse Prepaint Coatings on Alumin- ium and Galvanized Steel,” Metal Finishing, Vol. 90, No 9, 1992, pp. 29-35. [19] B. R. W. Hinton, “Corrosion Prevention and Chromates, the End of an Era?” Metal Finishing, Vol. 89, No. 9, 1991, pp. 55-61. [20] B. R. W. Hinton, “Corrosion Prevention and Chromates, the End of an Era?” Metal Finishing, Vol. 89, No. 4, 1991, pp. 15-20. [21] A. Barbucci, M. Delucchi and G. Cerisola, “Study of Chromate-Free Pretreatments and Primers for the Protec- tion of Galvanized Steel Sheets,” Progress in Organic Coatings, Vol. 33, No. 2, 1998, pp. 131-138. [22] G. D. Wilcox and J. A. Wharton, “A Review of Chromate Free-Passivation Treatments for Zinc and Zinc Alloys,” Transactions of the Institute of Metal Finishing, Vol. 75, No. 4, 1997, pp. B140-B146. [23] T. F. Child and W. J. van Ooij, “Application of Silane Technology to Prevent Corrosion of Metal and Improve Paint Adhesion,” Transactions of the Institute of Metal Finishing, Vol. 77, No. 2, 1999, pp. 64-70. [24] S. González, M. A. Gil, J. O. Hernández, V. Fox and R. M. Souto, “Resistance to Corrosion of Galvanized Steel Covered with an Epoxy – Polyamide Primer Coating,” Progress in Organic Coatings, Vol. 41, No. 1-3, March 2001, pp. 167-170. [25] M. F. Montemor, A. M. Simões, M. G. S. Ferreira and C. B. Breslin, “Composition and Corrosion Behavior of Galvanized Steel Treated with Rare-Earth Salts: The Ef- fect of the Cation,” Progress in Organic Coatings, Vol. 44, No. 2, 2002, pp. 111-120. [26] “Standard Practice for Operating Salt Spray (Fog) Appa- ratus,” ASTM B 117 Review A07. [27] “Testing in a Saturated Atmosphere in the Presence of Sulphur Dioxide,” DIN 50018, 1997. [28] “Atmospheres and their Technical Application Condensa- tion Water Test Atmospheres,” DIN 50017, 1982. [29] J. R. Mac Donald, “Impedance Spectroscopy Emphasiz-  Anti-Corrosion Performance of Cr+6-Free Passivating Layers Applied on Electrogalvanized Copyright © 2010 SciRes. MSA 209 ing Solid State Materials,” John Wiley & Sons, New York, 1987. [30] F. Mansfeld, “Models for the Impedance Behavior of Protective Coatings and Cases of Localized Corrosion,” Electrochimica Acta, Vol. 38, No. 14, 1993, pp. 1891- 1897. [31] P. Carbonini, T. Monetta, L. Nicodemo, P. Mastronardi, B. Scatteia and F. Bellucci, “Electrochemical Characteri- zation of Multilayer Organic Coatings,” Progress in Or- ganic Coatings, Vol. 29, No. 1-4, 1996, pp. 13-20. [32] L. de Rosa, T. Monetta, D. B. Mitton and F. Bellucci, “Monitoring Degradation of Single and Multilayer Or- ganic Coatings. I. Absorption and Transport of Water: Theoretical Analysis and Methods,” Journal of the Elec- trochemical Society, Vol. 145, No. 11, 1998, pp. 3830- 3838. [33] A. Boukamp, “Equivalent Circuit,” Report CT88/265/128, CT89/214/128, University of Twente, Twente, 1989. [34] N. M. Martyah and J. E. McCaskie, “Corrosion Behavior of Zinc Chromate Coatings,” Metal Finishing, Vol. 94, No. 2, 1996, pp. 65-67. [35] C. Delouis, M. Duprat and C. Tournillon, “The Kinetics of Zinc Dissolution in Aerated Sodium Sulphate Solu- tions. A Measurement of the Corrosion Rate by Imped- ance Techniques,” Corrosion Science, Vol. 29, No. 1, 1989, pp. 13-20. [36] B. del Amo, L. Véleva, A. R. Di Sarli and C. I. Elsner, “Performance of Coated Steel Systems Exposed to Dif- ferent Media. Part 1: Painted Galvanized Steel,” Progress in Organic Coatings, Vol. 50, No. 3, 2004, pp. 179-192. [37] E. P. M van Westing, G. M. Ferrari, F. M. Geenen and J. H. W. de Wit, “In Situ Determination of the Loss of Ad- hesion of Barrier Epoxy Coatings Using Electrochemical Impedance Spectroscopy,” Progress in Organic Coatings, Vol. 23, No. 1, 1993, pp. 89-103. [38] G. G. Nascimento, O. R. Mattos, J. L. C. Santos and I. C. P. Margarit, “Impedance Measurements on Lacquered Tinplate: Fitting with Equivalent Circuits,” Journal of Applied Electrochemistry., Vol. 29, No. 3, March 1999, pp. 383-392. [39] F. Mansfeld, “Use of Electrochemical Impedance Spec- troscopy for the Study of Corrosion Protection by Poly- mer Coatings,” Journal of Applied Electrochemistry, Vol. 25, No. 3, March 1995, pp. 187-202. [40] R. L. Zeller and R. F. Savinell, “Interpretation of AC Impedance Response of Chromated Electrogalvanized Steel,” Corrosion Science, Vol. 26, No. 5, 1986, pp. 389- 399. |

