Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2010, 1, 191-198 doi:10.4236/msa.2010.14030 Published Online October 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA 191 The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Fathia S. Mohammed1, Alyaa G. Elramady2, Salheddin E. Abu Yahya2 1Department of Chemical Engineering, American University of Sharjah, Sharjah, United Arab Emirates; 2The Petroleum Institute, Abu Dhabi, United Arab Emirates. Email: fmohammed@aus.edu Received April 18th, 2010; revised August 6th, 2010; accepted August 16th, 2010. ABSTRACT This work investigates the rela tive agg ressiveness of n itrate so lu tions at different pH va lues on mild steel towa rds stress corrosion cracking (SCC) and general corrosion. Electrochemical behavior and stress corrosion cracking susceptibility measurements were carried out in 52 Wt% ammonium nitrate solutions at 368° K and various pH values ranging from 0.77 to 9.64. Constant load stress corrosion test at 90% yield stress was conducted. Tested specimens were prepared and examined using the scanning electron microscope (SEM). The potentiod ynamic polarizatio n curves for different pH values again emphasized the validity of the gravimetric measurements and hence the mechanism of cracking was at- tributed to the stress that assisted the dissolution process. Keywords: Stress Corrosion Cracking, Ammonium Nitrate Solution, Mild Steel, Constant Load Test, Effect of PH 1. Introduction Stress corrosion cracking (SCC) can lead to rapid and catastrophic failure in many different metals and alloys. This phenomenon occurs under conditions where a com- ponent is exposed to a mildly corrosive environment while under applied and/or residual tensile stress. Hence, metal parts with severe SCC can appear bright and shiny whilst being filled with microscopic cracks [1]. These factors along with the rapid progress of SCC make it common for SCC to go undetected prior to failure. Steel/ nitrate interaction is an issue in nitrogenous fertilizer plants, waste heat recovery boilers (WHRBs) in power generating plant and nuclear wastes [2]. The effect of pH and types of nitrate solution has been investigated [3], which concluded that the order of de- creasing aggressiveness of nitrate solutions corresponded to the order of increasing (initial) pH for a given chemi- cal strength, i.e., NH4+, Ca++, K+, Na+. The aggressiveness of ammonium nitrate when com- pared to other nitrates was attributed to its lower pH. Parkins [4] reported that the marked decrease in potency at initial pH values in the region number 4 compared to either slightly higher or lower values depends probably upon pH changes in the solution during the test. The above work was concerned with the changes in pH of the bulk of solution and not with the pH at the crack tip re- gion, which undoubtedly more acidic. Steel has been characterized as being very susceptible to SCC at near- neutral pH [5]. In this work a comprehensive study on th e effect of the pH of ammonium nitrate solution on the susceptibility of mild steel to stress corrosion cracking and general corro- sion was carried out. The results indicate that in some pH values the general and localized corrosion were the cause of failure. In other pH values the stress corrosion crack- ing were the cause of failures. The severity was con- firmed by the calculation of crack growth rate, morphol- ogy of the fracture surface by SEM and by polarization work. 2. Experimental Approach 2.1. Material The work was carried out on mild steel of the following 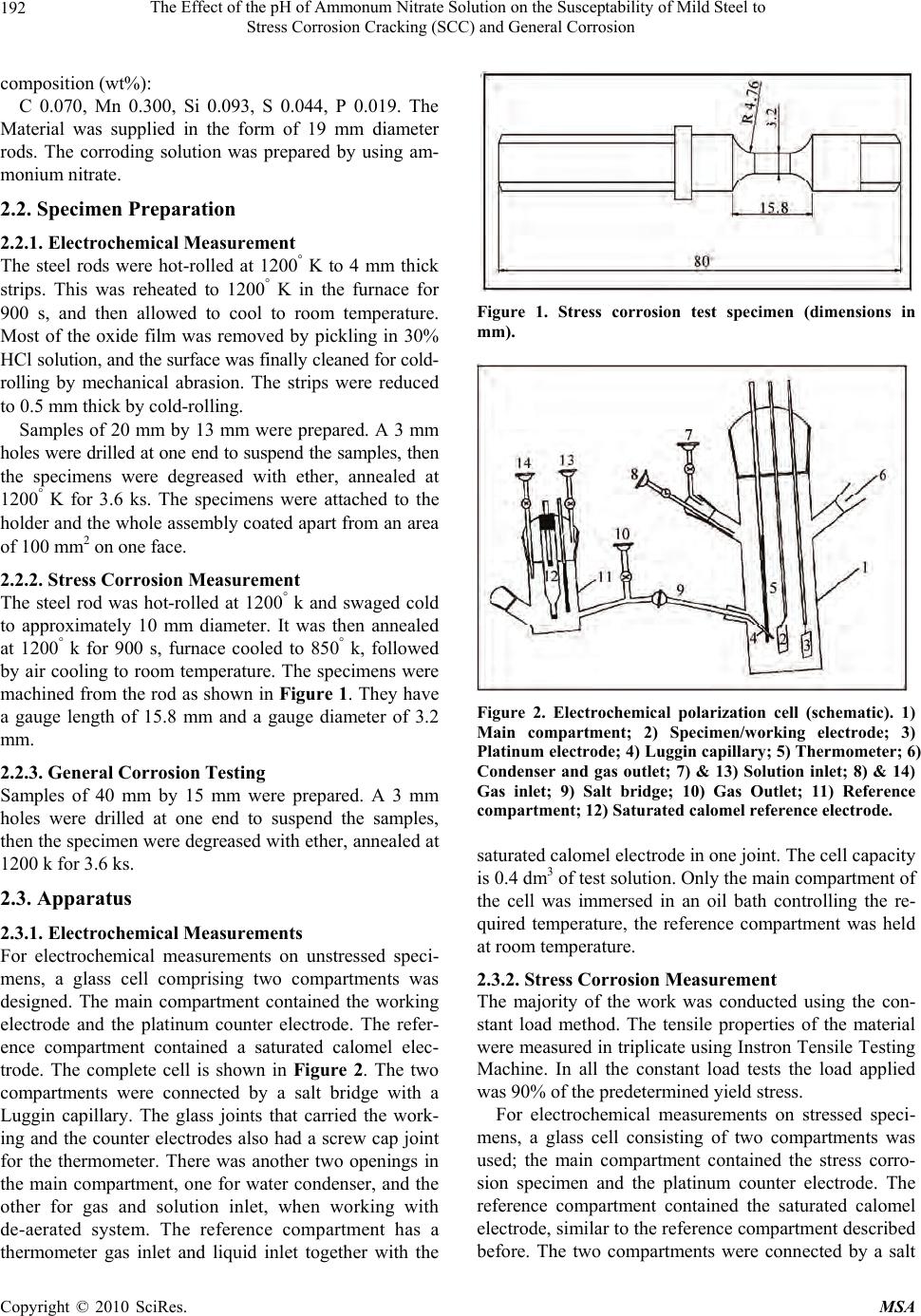 The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 192 composition (wt%) : C 0.070, Mn 0.300, Si 0.093, S 0.044, P 0.019. The Material was supplied in the form of 19 mm diameter rods. The corroding solution was prepared by using am- monium nitrate. 2.2. Specimen Preparation 2.2.1. Electrochemical Measurement The steel rods were hot-rolled at 1200° K to 4 mm thick strips. This was reheated to 1200° K in the furnace for 900 s, and then allowed to cool to room temperature. Most of the oxide film was removed by pickling in 30% HCl s olution, and the surface was finally c leaned for cold- rolling by mechanical abrasion. The strips were reduced to 0.5 mm thick by cold-rolling. Samples of 20 mm by 13 mm were prepared. A 3 mm holes were drilled at one end to suspend the samples, then the specimens were degreased with ether, annealed at 1200° K for 3.6 ks. The specimens were attached to the hold er and the who le assemb ly coated ap art from an ar ea of 100 mm2 on one face. 2.2.2. Stress Corrosion Measurement The steel rod was hot-rolled at 1200° k and swaged cold to approximately 10 mm diameter. It was then annealed at 1200° k for 900 s, furnace cooled to 850° k, followed by air cooling to room temperature. The specimens were machined from the rod as shown in Figure 1. They have a gauge length of 15.8 mm and a gauge diameter of 3.2 mm. 2.2.3. General Corrosion Testing Samples of 40 mm by 15 mm were prepared. A 3 mm holes were drilled at one end to suspend the samples, then the specimen were degreased with ether, annealed at 1200 k for 3. 6 ks. 2.3. Apparatus 2.3.1. Electrochemical Measurements For electrochemical measurements on unstressed speci- mens, a glass cell comprising two compartments was designed. The main compartment contained the working electrode and the platinum counter electrode. The refer- ence compartment contained a saturated calomel elec- trode. The complete cell is shown in Figure 2. The two compartments were connected by a salt bridge with a Luggin capillary. The glass joints that carried the work- ing and the counter electrodes also had a screw cap joint for the thermometer. There was another two openings in the main compartment, one for water condenser, and the other for gas and solution inlet, when working with de-aerated system. The reference compartment has a thermometer gas inlet and liquid inlet together with the Figure 1. Stress corrosion test specimen (dimensions in mm). Figure 2. Electrochemical polarization cell (schematic). 1) Main compartment; 2) Specimen/working electrode; 3) Platinum electrode; 4) Luggin capillary; 5) Thermometer; 6) Condenser and gas outlet; 7) & 13) Solution inlet; 8) & 14) Gas inlet; 9) Salt bridge; 10) Gas Outlet; 11) Reference compartment; 12) Saturated calomel reference el ectrode. saturated calomel electrode in one joint. The cell capacity is 0.4 dm3 of test solution. Only the main compartment of the cell was immersed in an oil bath controlling the re- quired temperature, the reference compartment was held at room temperature. 2.3.2. Stress Corrosion Measurement The majority of the work was conducted using the con- stant load method. The tensile properties of the material were measured in triplicate using Instron Tensile Testing Machine. In all the constant load tests the load applied was 90% of the predetermined yield stress. For electrochemical measurements on stressed speci- mens, a glass cell consisting of two compartments was used; the main compartment contained the stress corro- sion specimen and the platinum counter electrode. The reference compartment contained the saturated calomel electrode, similar to the reference compartment described before. The two compartments were connected by a salt 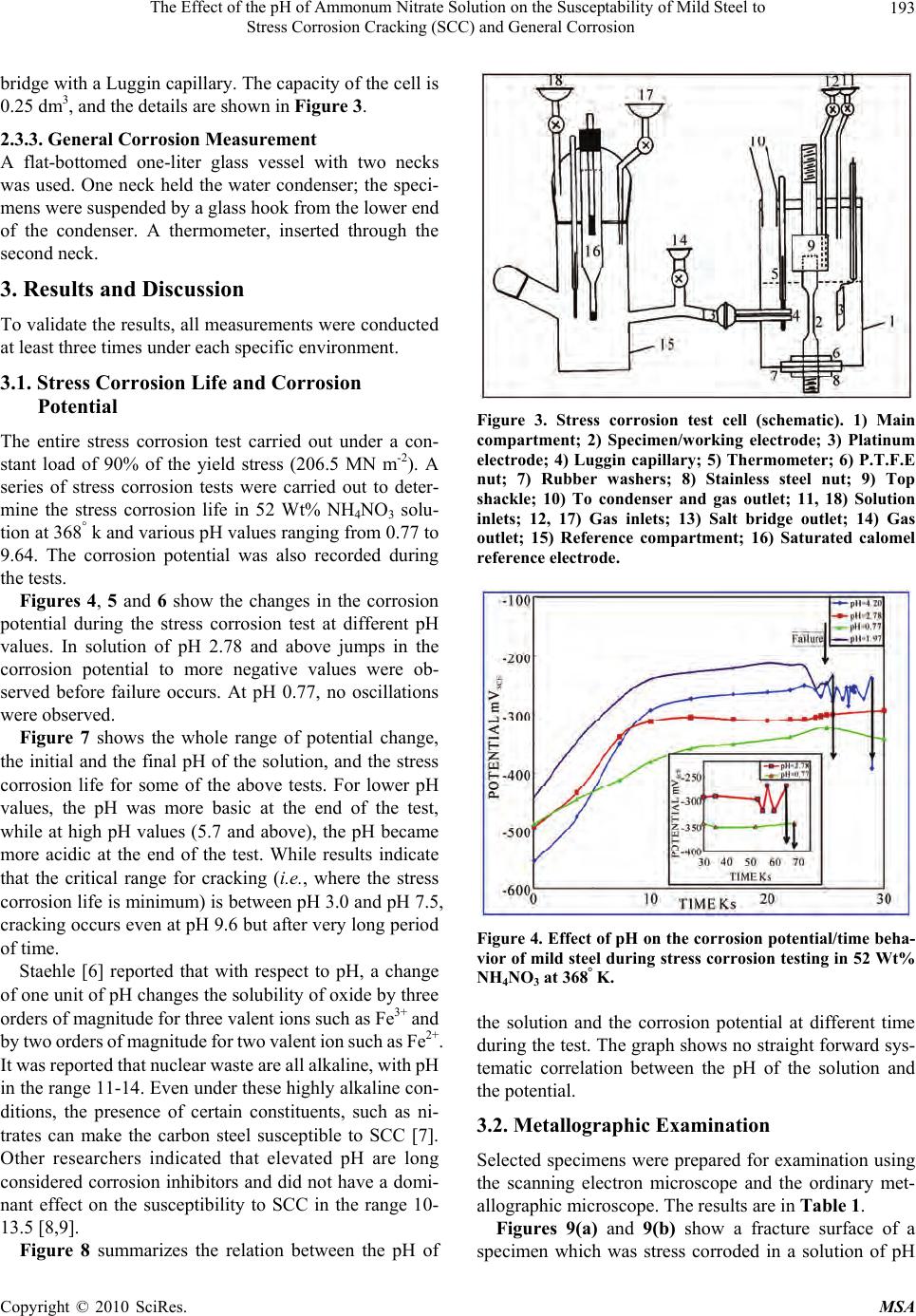 The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 193 bridge with a Luggin capillary. The capacity of the cell is 0.25 dm3, and the details are shown in Figure 3. 2.3.3. General Corrosion Measurement A flat-bottomed one-liter glass vessel with two necks was used. One neck held the water condenser; the speci- mens were suspended by a glass hook from the lower end of the condenser. A thermometer, inserted through the second neck. 3. Results and Discussion To validate the results, all measur ements were conducted at least three times under each specific environment. 3.1. Stress Corrosion Life and Corrosion Potential The entire stress corrosion test carried out under a con- stant load of 90% of the yield stress (206.5 MN m-2). A series of stress corrosion tests were carried out to deter- mine the stress corrosion life in 52 Wt% NH4NO3 solu- tion at 368° k and various pH values ranging from 0.77 to 9.64. The corrosion potential was also recorded during the tests. Figures 4, 5 and 6 show the changes in the corrosion potential during the stress corrosion test at different pH values. In solution of pH 2.78 and above jumps in the corrosion potential to more negative values were ob- served before failure occurs. At pH 0.77, no oscillations were observed. Figure 7 shows the whole range of potential change, the initial and the final pH of the solution, and the stress corrosion life for some of the above tests. For lower pH values, the pH was more basic at the end of the test, while at high pH values (5.7 and above), the pH became more acidic at the end of the test. While results indicate that the critical range for cracking (i.e., where the stress corrosion life is minimum) is between pH 3.0 and pH 7.5, cracking occurs even at pH 9.6 but after very long period of time. Staehle [6] reported that with respect to pH, a change of one unit of pH chang es the solubility of ox ide by three orders of magnitude for three valent ions such as Fe3+ and by two or ders of magnitude for two valent i on such as Fe2+. It was reported that nuclear waste are all alkaline, with pH in the range 11-14. Even under these highly alkaline con- ditions, the presence of certain constituents, such as ni- trates can make the carbon steel susceptible to SCC [7]. Other researchers indicated that elevated pH are long considered corrosion inhibitors and did not have a domi- nant effect on the susceptibility to SCC in the range 10- 13.5 [8,9]. Figure 8 summarizes the relation between the pH of Figure 3. Stress corrosion test cell (schematic). 1) Main compartment; 2) Specimen/working electrode; 3) Platinum electrode; 4) Luggin capillary; 5) Thermometer; 6) P.T.F.E nut; 7) Rubber washers; 8) Stainless steel nut; 9) Top shackle; 10) To condenser and gas outlet; 11, 18) Solution inlets; 12, 17) Gas inlets; 13) Salt bridge outlet; 14) Gas outlet; 15) Reference compartment; 16) Saturated calomel referenc e e lectrode. Figure 4. Effect of pH on the corrosion potential/time beha- vior of mild steel during stress corrosion testing in 52 Wt% NH4NO3 at 368° K. the solution and the corrosion potential at different time during the test. The grap h shows no straight forward sys- tematic correlation between the pH of the solution and the potential. 3.2. Metallographic Examination Selected specimens were prepared for examination using the scanning electron microscope and the ordinary met- allographic microscope. The results are in Table 1. Figures 9(a) and 9(b) show a fracture surface of a specimen which was stress corroded in a solution of pH 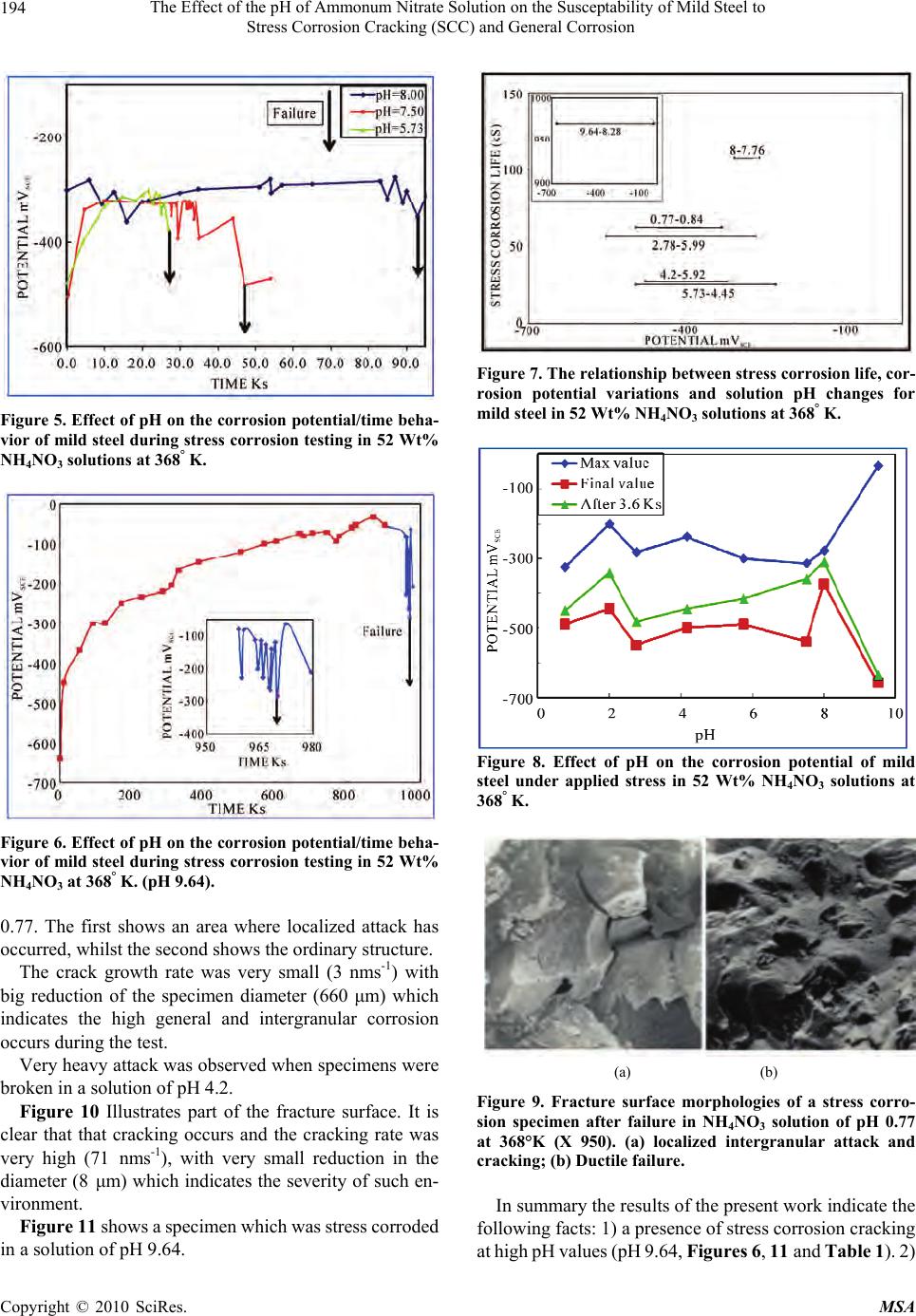 The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 194 Figure 5. Effect of pH on the corrosion potential/time beha- vior of mild steel during stress corrosion testing in 52 Wt% NH4NO3 solutions at 368° K. Figure 6. Effect of pH on the corrosion potential/time beha- vior of mild steel during stress corrosion testing in 52 Wt% NH4NO3 at 368° K. (pH 9.64). 0.77. The first shows an area where localized attack has occurred, whilst the second shows the ordinary structure. The crack growth rate was very small (3 nms-1) with big reduction of the specimen diameter (660 μm) which indicates the high general and intergranular corrosion occurs during the test. Very heavy attack was observed when specimens were broken in a solution of pH 4.2. Figure 10 Illustrates part of the fracture surface. It is clear that that cracking occurs and the cracking rate was very high (71 nms-1), with very small reduction in the diameter (8 μm) which indicates the severity of such en- vironment. Figure 11 shows a specime n which was stre ss corroded in a solution of pH 9.64. Figure 7. The relationship between stress corrosion life, cor- rosion potential variations and solution pH changes for mild steel in 52 Wt% NH4NO3 solutions at 368° K. Figure 8. Effect of pH on the corrosion potential of mild steel under applied stress in 52 Wt% NH4NO3 solutions at 368° K. (a) (b) Figure 9. Fracture surface morphologies of a stress corro- sion specimen after failure in NH4NO3 solution of pH 0.77 at 368°K (X 950). (a) localized intergranular attack and cracking; (b) Ductile failure. In summary the results of the present work indicate the following facts: 1) a presence of stress corrosion cracking at high pH values (pH 9. 64, Figures 6, 11 and Ta ble 1). 2) 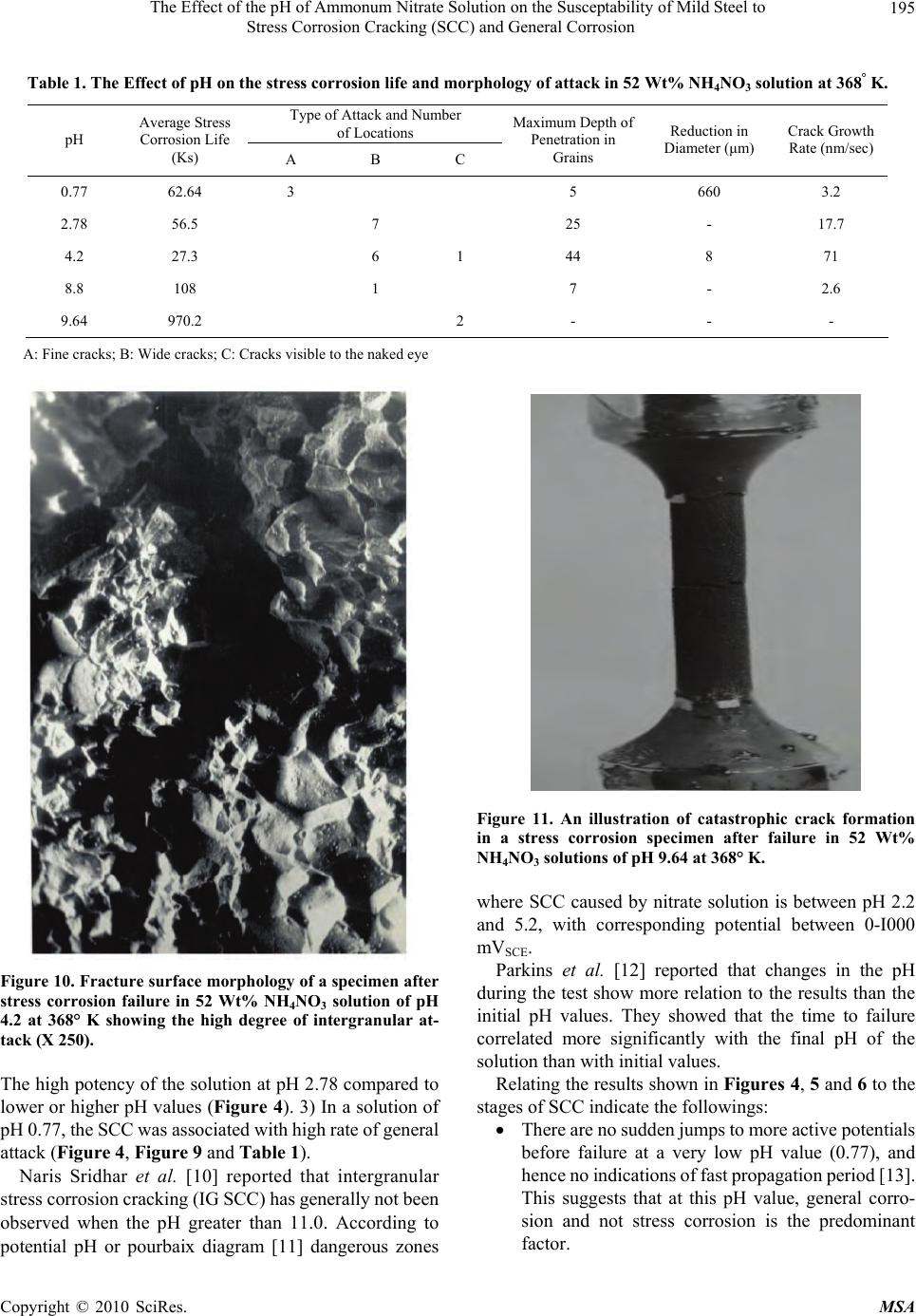 The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 195 Table 1. The Effect of pH on the stress corrosion life and morphology of attack in 52 Wt% NH4NO3 solution at 368° K. Type of Attack and Number of Locations pH Average Stress Corrosion Life (Ks) A B C Maximum Depth of Penetration in Grains Reduction in Diameter (μm) Crack Growth Rate (nm/sec) 0.77 62.64 3 5 660 3.2 2.78 56.5 7 25 - 17.7 4.2 27.3 6 1 44 8 71 8.8 108 1 7 - 2.6 9.64 970.2 2 - - - A: Fine cracks; B: Wide cracks; C: Cracks visible to the naked eye Figure 10. Fracture surface morphology of a specimen after stress corrosion failure in 52 Wt% NH4NO3 solution of pH 4.2 at 368° K showing the high degree of intergranular at- tack (X 250). The high potency of the solution at pH 2.78 compared to lower or higher pH values (Figure 4). 3) In a solution of pH 0.77, the S CC wa s associ ated with high rate of gen eral attack (Figure 4, Figure 9 and Table 1). Naris Sridhar et al. [10] reported that intergranular stress corr osi on crac king ( IG S CC) has gene rall y not b een observed when the pH greater than 11.0. According to potential pH or pourbaix diagram [11] dangerous zones Figure 11. An illustration of catastrophic crack formation in a stress corrosion specimen after failure in 52 Wt% NH4NO3 solutions of pH 9.64 at 368° K. where SCC caused by nitrate solution is between pH 2.2 and 5.2, with corresponding potential between 0-I000 mVSCE. Parkins et al. [12] reported that changes in the pH during the test show more relation to the results than the initial pH values. They showed that the time to failure correlated more significantly with the final pH of the solution than with initial values. Relating the results sho wn in Figures 4, 5 and 6 to the stages of SCC indicate the followings: There are no sudden jumps to more active potentials before failure at a very low pH value (0.77), and hence no indi cations of fast propagati on period [13]. This suggests that at this pH value, general corro- sion and not stress corrosion is the predominant factor.  The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 196 In the pH range 2.0 – 8.0, oscillations in potential are evident, occurring over a period representing 20 – 25% of the total life. This suggests th at variations in pH above and below th e natura l pH of 4.2 do not affect the percentage of the total time taken up by the fast propagat i on period [13]. At relatively high pH (9.64), the duration of the fast propagation period is relatively unchanged, but it occupies only about 1% of the total life. This can be attributed to the very low rate of attack at such high pH value. 3.3. Corrosion Rate and Corrosion Potential Measurement on Unstressed Specimens Figure 12 shows the effect of pH of the so lution on both the general corrosion rate and the stress corrosion life. This figure indicates that the increase in the stress corro- sion life at lower pH values was associated with high general corrosion, while at higher pH values (greater than 7.5) it was associated with low general corrosion. The variation of the corrosion potential with time for unstressed specimens was measured for period of ~70 ks at different pH values, ranging from 1.05 to 9.13 (Figure 13 and 14). The potential changed to the more noble di- rection as the test proceeded over almost the whole range of the investigated pH except at pH 9.13. At this pH the potential at the beginning was less noble, and after ap- proximately 30 ks more noble values were observed. From Figures 12, 13 and 14, the following are observed: High general corrosion at lower pH values is ac- companied by a more active potential. The non dependence of pH on the corrosion rate between pH values of 4.2 and 6.9 is associated with unchanged corrosion potential. The decrease in the corrosion rate with increasing pH in the range 6.0 – 8.5 is characterized by erratic changes in the corrosion potential, e.g. it is com- paratively noble at pH 7 and yet more active at higher values. The abov e behav ior prob ably refl ected the differen ce of the solubility of the corrosion product in solutions of different pH values. Figure 15 shows the effect of stress on the maximum corrosion potential during testing in 52 Wt% NH4NO3 at 368° K at different pH values. The stress appears to cause a shift in the corrosion potential to more active values in the range of the critical pH. 3.4. Crack Growth Rate From the microscopic examination and the results of stress corrosion life in different pH values reported in Table 1, the crack growth rate was calculated by dividing Figure 12. Effect of pH on the corrosion rate and stress corrosion life of mild steel in 52 Wt% NH4NO3 solutions at 368° K. Figure 13. Effect of pH on the corrosion potential/time be- havior of mild steel in 52 Wt% NH4NO3 solutions at 368° K. Figure 14. Effect of pH on the corrosion potential/time be- havior of mild steel in 52 Wt% NH4NO3 solutions at 368° K.  The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 197 Figure 15. Effect of pH on the maximum corrosion potential value attained for mild steel in 52 Wt% NH4NO3 solutions at 368° K. the maximum measured crack depth by the total time to failure. The obtained value gives an estimate of the rate since it does not take into account the time to initiate cracks. The growth rate of any observed cracking is as- sumed to be constant throughout the exposure period. These estimates provide a simple semi quantitative di- agnostic to classify the SCC propensity. The following points regar ding the crack growt h rate a re concl uded from Table 1. In the solut ion of pH 0.77 the crack growth was only 3.2 nms-1 bu t there was a big reduction (1 8%) in the diameter which clearly indicates that general cor- rosion was dominating. The crack g rowth rate at pH 2.78 is 17.7 nms-1 while it is 71 nms-1 at pH 4.2; thus it is obvious that the latter environment is more prone to SCC. In the solution o f pH 8.8 the crack growth rate was 2.6 nms-1 and this is probably because of the longer initiation period. 3.5. Potentiodynamic Polarization Figure 16 shows the p otenti ody nam ic pol arizat ion c urves for different pH values of 52 Wt% NH4NO3 solution at 368 K ranging from 2.04 to 8.35. The scanning started approximately 200 mv more ne- gative than the open circuit potential (OPC) in the noble direction t o m ore tha n +1 500 mv usi ng s weep rate of 0.33 mVs -1. Several distinct characteristics for solution of different pH are as below: For pH 2.04 1) An active dissolution regime be- tween −500 mVSCE and −150 mVSCE, 2) First pas- sive plateau at a current density of 2 × 103 Am-2 between potential −150mVSCE and zero mVSCE, 3) Figure 16. Effect of pH on the potentiodynamic anodic po- larization behavior of mild steel in 52 Wt% NH4NO3 solu- tion at 368 K (sweep rate 0.33 mVs-1). Broad active to passive transition peak starting at zero mV with corresponding current density of 0.85 × 103 Am-2, 4) Second dissolution regime between + 500 mV and 600 mV, 5) Second passive p lateau at current density of 1.3 × 103 Am-2 between 600 mV and 1300 mVSCE, 6) A transpassive regime starting at 1300 mVSCE. For pH 4. 2 1) An acti ve diss olu ti on r e gime between −500 mVSCE and −350 mVSCE, 2) First active-pas- sive transition starting at −350 mV with a corre- sponding current density of 1 × 103 Am-2, 3) An active dissolution regime between −300 mVSCE to −150 mVSCE, 4) A second active-passive transition starting at −150 m VSCE with a corresponding current density of 3 × 103 Am-2, 5) Transition peak at po- tential 0 mV wi th a corres ponding c urrent de nsity of 0.6 × 103 Am-2, 6) A passive plateau at a current density of 0.4 × 103 Am-2 between 500 mVSCE and 1400 mVSCE, 7) The initiation of the transpassive regime at 1450 mVSCE. For pH 6.97 1) An active dissolution regime be- tween −500 mVSCE and −150 mVSCE, 2) A passive plateau at a current density of 3.5 × 103 Am-2 be- tween −100 mVSCE and +500 mVSCE, 3) Sudden decrease in the current density to 0.2 × 103 Am-2 at −520 mVSCE, 4) passi ve plateau at cu rrent de nsity of 0.2 Am-2 between 550 mVSCE and 1200 mVSCE, 5) The initiation of transpassive regime at 1200 mVSCE. For pH 8. 35 1) A slow diss olution fr om −250 mVSCE to 800 mVSCE to a maxim um current de nsity of 0.1 × 103 Am-2, 2) A nother disso lut ion beh avi or from 800 mVSCE to 1200 mVSCE to a maximum current den- sity of 0.9 × 103 Am-2, 3) Passive plateau at a cur- rent density of 1 × 103 Am-2 between 1200 mVSCE  The Effect of the pH of Ammonum Nitrate Solution on the Susceptability of Mild Steel to Stress Corrosion Cracking (SCC) and General Corrosion Copyright © 2010 SciRes. MSA 198 and 1600 mVSCE, 4) The initiation of transpassive regime at 1600 mVSCE. From above it is clear that the potentiodynamic po- larization curves again emphasize the validity of the gra- vimetric measurement and show the influence of pH on the anodic dissolution characteristics of mild steel. These results were found to be in acco rd with previou s research [14]. 4. Conclusions At very low pH values, stress corrosion cracking is asso- ciated with very high rate of general corrosion. In the region of p H 2.0 to 4.2 the stre ss corrosion life is rel atively unchanged and general corrosion rate decreases with increasing of the pH level. Between pH 4.2 and 6.0, the corrosion rate and stress corrosion life are alm ost constant. In the region of pH 6.0 to 7.5, the stress corrosion life increases slightly and the corrosion rate decreases. Above pH 7.5, there is a noticeable increase in the stress corro- sion life while the general corrosion rate shows a marked decrease. Experim ental observation suggests that an oxide film of critical physical properties is fo rmed at immersion. This film suffers localized breakdown at the grain boun- dary. These limited grain boundary micro fissures will only propagate if aided by stress which facilitates the continuing action of the corrosion process. Subsequently, the precipitation of a layer of stifling corrosion product re-occurs and the cyclic process is repeated until failure. The local dissolution rate at the crack tip is accelerated with test time, which would be attributed to the continu- ous increase in the stress concentration. This reflects the interaction of stress and anodic dissolution during the SCC process. REFERENCES [1] ASM International, “Metals Handbook (Desk Edition),” Chapter 32 (Failure Analysis), American Society for Metals, 1997. [2] C. Sean Brossia, et al., “A Study of Stress Corrosion Cracking and Localized Corrosion of Carbon Steel in Ni- trate Based Nuclear Waste,” NACE Conference, Georgia, 2009. [3] R. N. Parkins and R. Usher, “The Effect of Nitrate Solu- tion in Producing Stress Corrosion Cracking in Mild Steel,” Proceeding of the 1st International Congress on Metallic Corrosion, London, 1961, p. 289. [4] R. N. Parkins, “Environmental Aspect of Stress Corrosion Cracking in Low Strength Ferrite Steels,” Proceeding of the International Conference on Stress Corrosion Crack- ing and Hydrogen Embrittlement of Iron Base Alloys, Firming, 1973. [5] Y. Z. Wang, R. W. Revie and M. T. Shahatas, “Early Stages of Stress Corrosion Cracks Development of X65 Pipeline Steel in Near Neutral pH Solution,” Materials for Resource Recovery and Transport, L. Collins, Ed., The Metallurgical Society of CIM, Montreal, 1998, p. 71. [6] R. W. Stachle, “Framework for Predicting Stress Corro- sion Cracking,” Proceedings of Environmentally Assisted Cracking; Predictive Methods for Risk Assessment and Evaluation of Materials, Equipments and Structure, Or- lando, 2000. [7] K. D. Boomer, J. Beavers, et al., “A Study of Corrosion and Stress Corrosion Cracking of Carbon Steel Nuclear Waste Storage tanks,” Material Science and Technology Conference and Exhibition, Michigan, 2007. [8] F. Gui, C. S. Brossia, et al., “On the Anodic Polarization Behavior of Carbon Steel in Hanford Nuclear Wastes,” Corrosion, National Association of Catering Executives, Houston, 2007. [9] C. S. Brossia, C. Scott, et al., “Inhibition of Stress Corro- sion Cracking of Carbon Steel Storage Tanks at Han- ford,” Corrosion, National Association of Catering Ex- ecutives, Houston, 2007. [10] N. Sridhar, et al., “Grain Boundary Chemistry and Weak Link Behavior of Polycrystalline YBa2Cu4O8,” Proceed- ing of Environmentally Assisted Cracking Predictive Methods for Risk Assessment and Evaluation of Materials, Equipments and Structure, Vol. 241, Orlando, 2000. [11] R. G. J. Leferink and W. M. M. Huijbregts, “Nitrate Stress Corrosion Cracking in Waste heat Recovery Boil- ers,” Anti – Corrosion Methods and Materials, Vol. 49, No. 2, 2002, pp. 118-126. [12] R. N. parkins, et al., “Stress Corrosion Test Method,” British Corrosion Journal, Vol. 7, 1972, p. 154. [13] F. S. Mohammed, “Stages of Corrosion Cracking of Mild Steel in Nitrate Solution,” 3rd International Material Conference, College of Engineering, Australia, 2008. [14] Corrosion Source, Last Revised December 2005. http:// www.corrosionsource.com/handbook/testing/scc.htm |

