Carbonation Resistance and Anticorrosive Properties of Organic Coatings for Concrete Structures 73
Table 6. Carbonation depth after one year of physical ex-
posure.
Nano HP Conv
1 2 3 1 2 3 1 2 3
Ref
Carbonation
depth (mm) 2 1 1 0 0 0 1 1 23
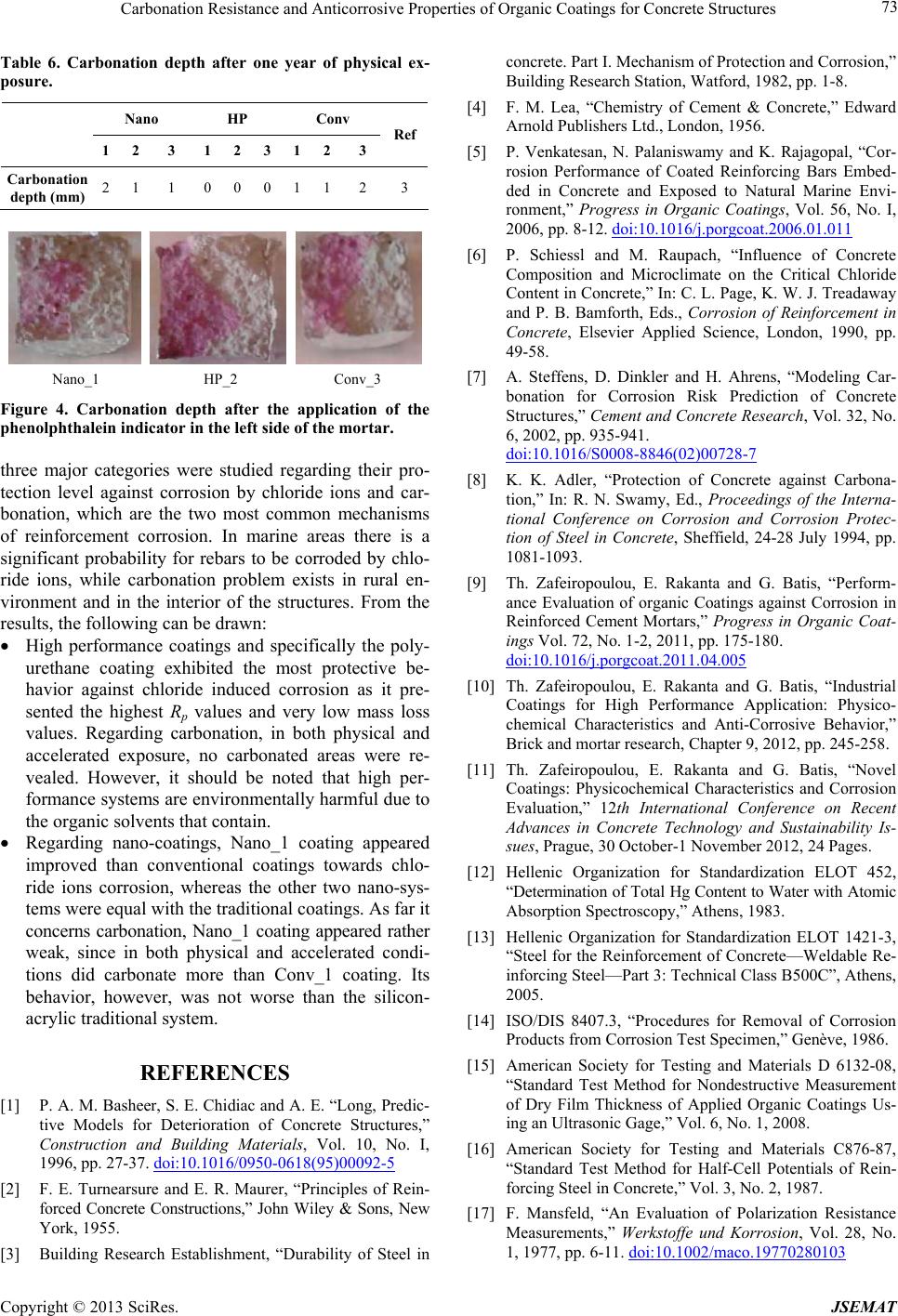
Nano_1 HP_2 Conv_3
Figure 4. Carbonation depth after the application of the
phenolphthalein indicator in the left side of the mortar.
three major categories were studied regarding their pro-
tection level against corrosion by chloride ions and car-
bonation, which are the two most common mechanisms
of reinforcement corrosion. In marine areas there is a
significant probability for rebars to be corroded by chlo-
ride ions, while carbonation problem exists in rural en-
vironment and in the interior of the structures. From the
results, the following can be drawn:
High performance coatings and specifically the poly-
urethane coating exhibited the most protective be-
havior against chloride induced corrosion as it pre-
sented the highest Rp values and very low mass loss
values. Regarding carbonation, in both physical and
accelerated exposure, no carbonated areas were re-
vealed. However, it should be noted that high per-
formance systems are environmentally harmful due to
the organic solvents that contain.
Regarding nano-coatings, Nano_1 coating appeared
improved than conventional coatings towards chlo-
ride ions corrosion, whereas the other two nano-sys-
tems were equal with the traditional coatings. As far it
concerns carbonation, Nano_1 coating appeared rather
weak, since in both physical and accelerated condi-
tions did carbonate more than Conv_1 coating. Its
behavior, however, was not worse than the silicon-
acrylic traditional system.
REFERENCES
[1] P. A. M. Basheer, S. E. Chidiac and A. E. “Long, Predic-
tive Models for Deterioration of Concrete Structures,”
Construction and Building Materials, Vol. 10, No. I,
1996, pp. 27-37. doi:10.1016/0950-0618(95)00092-5
[2] F. E. Turnearsure and E. R. Maurer, “Principles of Rein-
forced Concrete Constructions,” John Wiley & Sons, New
York, 1955.
[3] Building Research Establishment, “Durability of Steel in
concrete. Part I. Mechanism of Protection and Corrosion,”
Building Research Station, Watford, 1982, pp. 1-8.
[4] F. M. Lea, “Chemistry of Cement & Concrete,” Edward
Arnold Publishers Ltd., London, 1956.
[5] P. Venkatesan, N. Palaniswamy and K. Rajagopal, “Cor-
rosion Performance of Coated Reinforcing Bars Embed-
ded in Concrete and Exposed to Natural Marine Envi-
ronment,” Progress in Organic Coatings, Vol. 56, No. I,
2006, pp. 8-12. doi:10.1016/j.porgcoat.2006.01.011
[6] P. Schiessl and M. Raupach, “Influence of Concrete
Composition and Microclimate on the Critical Chloride
Content in Concrete,” In: C. L. Page, K. W. J. Treadaway
and P. B. Bamforth, Eds., Corrosion of Reinforcement in
Concrete, Elsevier Applied Science, London, 1990, pp.
49-58.
[7] A. Steffens, D. Dinkler and H. Ahrens, “Modeling Car-
bonation for Corrosion Risk Prediction of Concrete
Structures,” Cement and Concrete Research, Vol. 32, No.
6, 2002, pp. 935-941.
doi:10.1016/S0008-8846(02)00728-7
[8] K. K. Adler, “Protection of Concrete against Carbona-
tion,” In: R. N. Swamy, Ed., Proceedings of the Interna-
tional Conference on Corrosion and Corrosion Protec-
tion of Steel in Concrete, Sheffield, 24-28 July 1994, pp.
1081-1093.
[9] Th. Zafeiropoulou, E. Rakanta and G. Batis, “Perform-
ance Evaluation of organic Coatings against Corrosion in
Reinforced Cement Mortars,” Progress in Organic Coat-
ings Vol. 72, No. 1-2, 2011, pp. 175-180.
doi:10.1016/j.porgcoat.2011.04.005
[10] Th. Zafeiropoulou, E. Rakanta and G. Batis, “Industrial
Coatings for High Performance Application: Physico-
chemical Characteristics and Anti-Corrosive Behavior,”
Brick and mortar research, Chapter 9, 2012, pp. 245-258.
[11] Th. Zafeiropoulou, E. Rakanta and G. Batis, “Novel
Coatings: Physicochemical Characteristics and Corrosion
Evaluation,” 12th International Conference on Recent
Advances in Concrete Technology and Sustainability Is-
sues, Prague, 30 October-1 November 2012, 24 Pages.
[12] Hellenic Organization for Standardization ELOT 452,
“Determination of Total Hg Content to Water with Atomic
Absorption Spectroscopy,” Athens, 1983.
[13] Hellenic Organization for Standardization ELOT 1421-3,
“Steel for the Reinforcement of Concrete—Weldable Re-
inforcing Steel—Part 3: Technical Class B500C”, Athens,
2005.
[14] ISO/DIS 8407.3, “Procedures for Removal of Corrosion
Products from Corrosion Test Specimen,” Genève, 1986.
[15] American Society for Testing and Materials D 6132-08,
“Standard Test Method for Nondestructive Measurement
of Dry Film Thickness of Applied Organic Coatings Us-
ing an Ultrasonic Gage,” Vol. 6, No. 1, 2008.
[16] American Society for Testing and Materials C876-87,
“Standard Test Method for Half-Cell Potentials of Rein-
forcing Steel in Concrete,” Vol. 3, No. 2, 1987.
[17] F. Mansfeld, “An Evaluation of Polarization Resistance
Measurements,” Werkstoffe und Korrosion, Vol. 28, No.
1, 1977, pp. 6-11. doi:10.1002/maco.19770280103
Copyright © 2013 SciRes. JSEMAT