Paper Menu >>
Journal Menu >>
 Vol.2, No.10, 1221-1225 (2010) Health doi:10.4236/health.2010.210181 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Effect of calcium and diltiazem on phenylhydrazine-induced oxidative injury in goat erythrocytes Kaushik Das*, Jharna Bhattacharyya Division of Cell Biology and Physiology, Indian Institute of Chemical Biology, Kolkata, India; *Corresponding Author: kaushik.17@gmail.com Received 8 June 2010; revised 22 June 2010; accepted 7 July 2010. ABSTRACT Lipid peroxidation, hemolysis and thiol contents were studied in intact goat erythrocytes exposed to phenylhydrazine. An increase in lipid peroxi- dation, hemolysis and thiol content was observ- ed after phenylhydrazine treatment of erythrocy- te. Extracellular Ca2+ potentiates the phenylhy- drazine-induced lipid peroxidation and hemoly- sis of erythrocytes significantly. Ca2+ does not influence much the thiol content of phenylhydr- azine treated erythrocytes. No effect of Ca2+ on control lipid peroxidation, hemolysis and thiol contents of erythrocytes was observed. Diltiaz- em and EDTA inhibited the increased responses of lipid peroxidation and hemolysis due to Ca2+. However the thiol content was not much influen- ced by either diltiazem or EDTA. The results su- ggest that oxidative damage of erythrocyte cau- sed by phenyl hydrazine could be prevented by calcium channel antagonist, diltiazem, which may act as antioxidant also. Keywords: Ca2+; Diltiazem; Erythrocyte; Free Radical; Lipid Peroxidation; Phenylhydrazine 1. INTRODUCTION Phenylhydrazine (PHZ) and other oxygen generating sy- stems cause elevation of lipid peroxidation level in ery- throcytes [1]. PHZ through oxidative stress also altered methemoglobin level, catalase activity and turbidity of erythrocyte [1], which are typical biochemical markers of haemolytic anaemia. Oxidative stress is defined as str- uctural and/or functional injury produced in tissues by the uncontrolled formation of pro-oxidant free radicals. Oxi- dative stress usually develops when pro-oxidant action of an inducer exceeds the anti-oxidant capacity of the cell-defence system, altering its homeostatic capacity. PHZ, in presence of haemoglobin autooxidizes to form hydrogen peroxide, which leads to hemolysis, resulting in severe haemolytic anaemia [2] and generates reactive oxygen species [3] among which hydroxyl radical, OH· [4] is highly reactive and initiates the peroxidation of unsa- turated fatty acids in endogenous phospholipids. Oxida- tive stress due to overproduction of reactive species pl- ays a significant role in the pathogenesis of various dis- eases and involves numerous mechanisms. All biological molecules are prone to free radical attack. Lipid peroxi- dation is a chain reaction, involving numerous biprod- ucts caused by free radical damage. The modulators involved in the production of haemo- lytic anaemia caused by PHZ are not fully understood. Ca2+ has been implicated as an important contributory factor to cell damage caused by the resultant effect of oxidative stress in several diseases [5]. Ca2+ influx has been reported to increase in pathophysiological condi- tions where Ca2+-channel blockers have got an important regulatory role [5]. In some studies the role of lipid per- oxidation in cell death has implicated a concurrent invo- lvement of Ca2+. The component, which increases Ca2+ influx-increases lipid production biproducts also. A nu- mber of experimental systems have been used to exam- ine the interaction between lipid peroxidation and Ca2+ as mediators of functional membrane damage [6]. Volt- age dependant calcium channels (VDCCs) play a critical role in the regulation of levels of intracellular Ca2+ and thereby control an array of physiological processes in cells and VDCCs functioning could be prevented by using calcium channel blockers [7]. Thus regulating Ca2+ influx through VDCCs by using calcium channel block- ers, which act as antioxidants as well, could have some impact on the calcium mediated lipid peroxidation in several disease conditions. The present study is an attempt to study the role of ca- lcium and calcium channel blocker diltiazem, on lipid 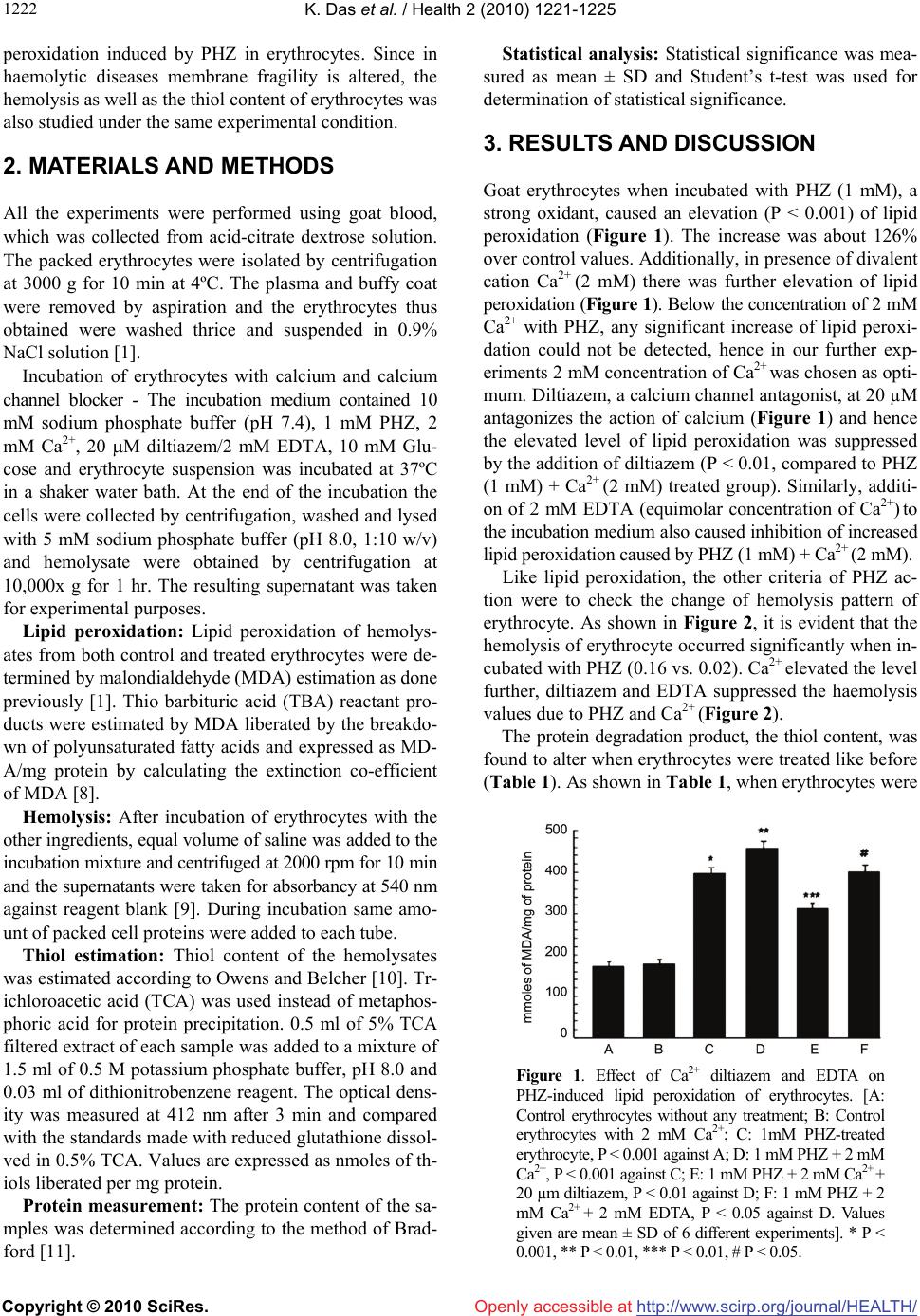 K. Das et al. / Health 2 (2010) 1221-1225 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1222 peroxidation induced by PHZ in erythrocytes. Since in haemolytic diseases membrane fragility is altered, the hemolysis as well as the thiol content of erythrocytes was also studied under the same experimental condition. 2. MATERIALS AND METHODS All the experiments were performed using goat blood, which was collected from acid-citrate dextrose solution. The packed erythrocytes were isolated by centrifugation at 3000 g for 10 min at 4ºC. The plasma and buffy coat were removed by aspiration and the erythrocytes thus obtained were washed thrice and suspended in 0.9% NaCl solution [1]. Incubation of erythrocytes with calcium and calcium channel blocker - The incubation medium contained 10 mM sodium phosphate buffer (pH 7.4), 1 mM PHZ, 2 mM Ca2+, 20 M diltiazem/2 mM EDTA, 10 mM Glu- cose and erythrocyte suspension was incubated at 37ºC in a shaker water bath. At the end of the incubation the cells were collected by centrifugation, washed and lysed with 5 mM sodium phosphate buffer (pH 8.0, 1:10 w/v) and hemolysate were obtained by centrifugation at 10,000x g for 1 hr. The resulting supernatant was taken for experimental purposes. Lipid peroxidation: Lipid peroxidation of hemolys- ates from both control and treated erythrocytes were de- termined by malondialdehyde (MDA) estimation as done previously [1]. Thio barbituric acid (TBA) reactant pro- ducts were estimated by MDA liberated by the breakdo- wn of polyunsaturated fatty acids and expressed as MD- A/mg protein by calculating the extinction co-efficient of MDA [8]. Hemolysis: After incubation of erythrocytes with the other ingredients, equal volume of saline was added to the incubation mixture and centrifuged at 2000 rpm for 10 min and the supernatants were taken for absorbancy at 540 nm against reagent blank [9]. During incubation same amo- unt of packed cell proteins were added to each tube. Thiol estimation: Thiol content of the hemolysates was estimated according to Owens and Belcher [10]. Tr- ichloroacetic acid (TCA) was used instead of metaphos- phoric acid for protein precipitation. 0.5 ml of 5% TCA filtered extract of each sample was added to a mixture of 1.5 ml of 0.5 M potassium phosphate buffer, pH 8.0 and 0.03 ml of dithionitrobenzene reagent. The optical dens- ity was measured at 412 nm after 3 min and compared with the standards made with reduced glutathione dissol- ved in 0.5% TCA. Values are expressed as nmoles of th- iols liberated per mg protein. Protein measurement: The protein content of the sa- mples was determined according to the method of Brad- ford [11]. Statistical analysis: Statistical significance was mea- sured as mean ± SD and Student’s t-test was used for determination of statistical significance. 3. RESULTS AND DISCUSSION Goat erythrocytes when incubated with PHZ (1 mM), a strong oxidant, caused an elevation (P < 0.001) of lipid peroxidation (Figure 1). The increase was about 126% over control values. Additionally, in presence of divalent cation Ca2+ (2 mM) there was further elevation of lipid peroxidation (Figure 1). Below the concentration of 2 mM Ca2+ with PHZ, any significant increase of lipid peroxi- dation could not be detected, hence in our further exp- eriments 2 mM concentration of Ca2+ was chosen as opti- mum. Diltiazem, a calcium channel antagonist, at 20 µM antagonizes the action of calcium (Figure 1) and hence the elevated level of lipid peroxidation was suppressed by the addition of diltiazem (P < 0.01, compared to PHZ (1 mM) + Ca2+ (2 mM) treated group). Similarly, additi- on of 2 mM EDTA (equimolar concentration of Ca2+) to the incubation medium also caused inhibition of increased lipid peroxidation caused by PHZ (1 mM) + Ca2+ (2 mM). Like lipid peroxidation, the other criteria of PHZ ac- tion were to check the change of hemolysis pattern of erythrocyte. As shown in Figure 2, it is evident that the hemolysis of erythrocyte occurred significantly when in- cubated with PHZ (0.16 vs. 0.02). Ca2+ elevated the level further, diltiazem and EDTA suppressed the haemolysis values due to PHZ and Ca2+ (Figure 2). The protein degradation product, the thiol content, was found to alter when erythrocytes were treated like before (Table 1). As shown in Table 1, when erythrocytes were Figure 1. Effect of Ca2+ diltiazem and EDTA on PHZ-induced lipid peroxidation of erythrocytes. [A: Control erythrocytes without any treatment; B: Control erythrocytes with 2 mM Ca2+; C: 1mM PHZ-treated erythrocyte, P < 0.001 against A; D: 1 mM PHZ + 2 mM Ca2+, P < 0.001 against C; E: 1 mM PHZ + 2 mM Ca2+ + 20 µm diltiazem, P < 0.01 against D; F: 1 mM PHZ + 2 mM Ca2+ + 2 mM EDTA, P < 0.05 against D. Values given are mean ± SD of 6 different experiments]. * P < 0.001, ** P < 0.01, *** P < 0.01, # P < 0.05. 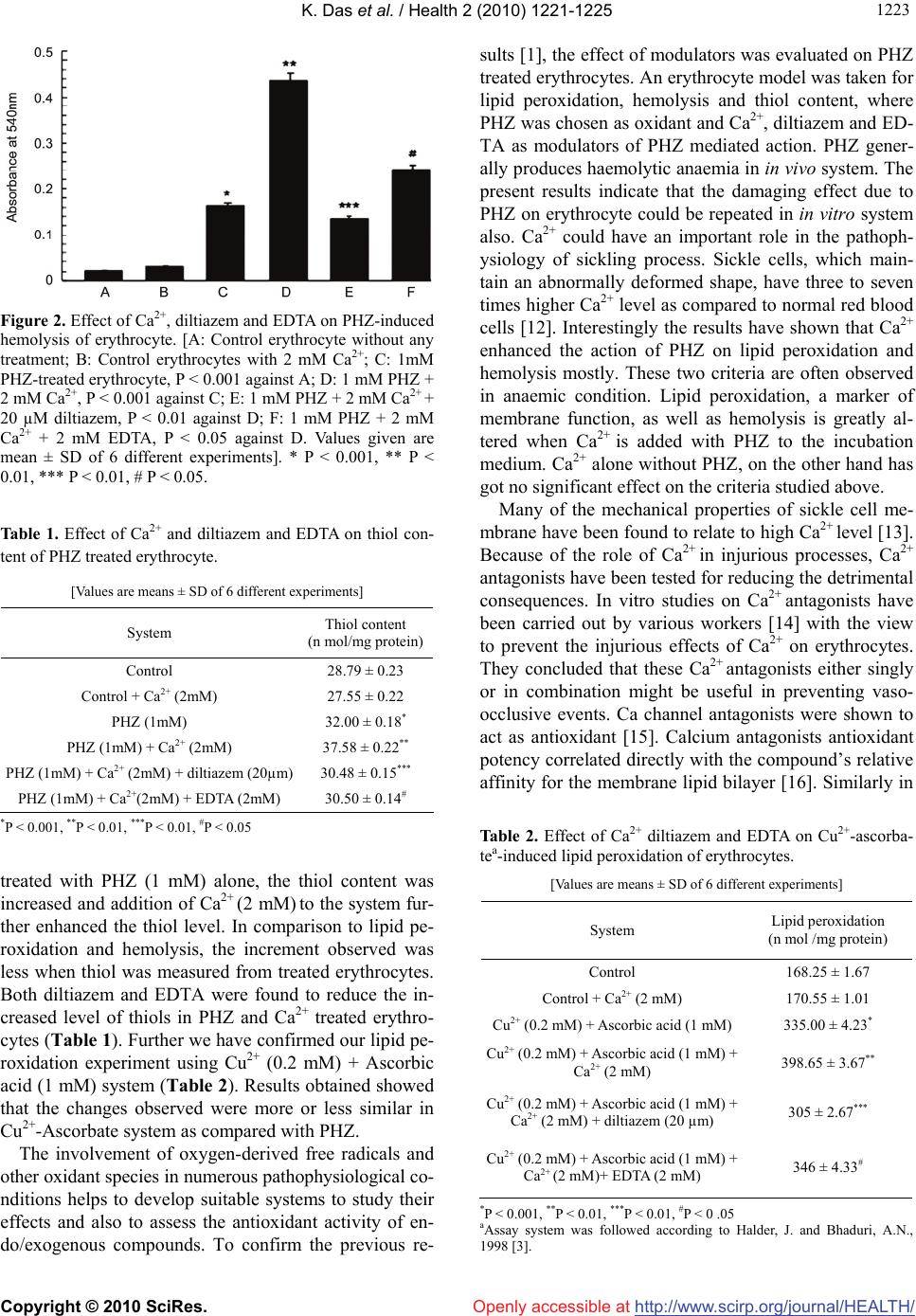 K. Das et al. / Health 2 (2010) 1221-1225 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 122 1223 Figure 2. Effect of Ca2+, diltiazem and EDTA on PHZ-induced hemolysis of erythrocyte. [A: Control erythrocyte without any treatment; B: Control erythrocytes with 2 mM Ca2+; C: 1mM PHZ-treated erythrocyte, P < 0.001 against A; D: 1 mM PHZ + 2 mM Ca2+, P < 0.001 against C; E: 1 mM PHZ + 2 mM Ca2+ + 20 µM diltiazem, P < 0.01 against D; F: 1 mM PHZ + 2 mM Ca2+ + 2 mM EDTA, P < 0.05 against D. Values given are mean ± SD of 6 different experiments]. * P < 0.001, ** P < 0.01, *** P < 0.01, # P < 0.05. Table 1. Effect of Ca2+ and diltiazem and EDTA on thiol con- tent of PHZ treated erythrocyte. [Values are means ± SD of 6 different experiments] System Thiol content (n mol/mg protein) Control 28.79 ± 0.23 Control + Ca2+ (2mM) 27.55 ± 0.22 PHZ (1mM) 32.00 ± 0.18* PHZ (1mM) + Ca2+ (2mM) 37.58 ± 0.22** PHZ (1mM) + Ca2+ (2mM) + diltiazem (20µm) 30.48 ± 0.15*** PHZ (1mM) + Ca2+(2mM) + EDTA (2mM) 30.50 ± 0.14# *P < 0.001, **P < 0.01, ***P < 0.01, #P < 0.05 treated with PHZ (1 mM) alone, the thiol content was increased and addition of Ca2+ (2 mM) to the system fur- ther enhanced the thiol level. In comparison to lipid pe- roxidation and hemolysis, the increment observed was less when thiol was measured from treated erythrocytes. Both diltiazem and EDTA were found to reduce the in- creased level of thiols in PHZ and Ca2+ treated erythro- cytes (Table 1). Further we have confirmed our lipid pe- roxidation experiment using Cu2+ (0.2 mM) + Ascorbic acid (1 mM) system (Table 2). Results obtained showed that the changes observed were more or less similar in Cu2+-Ascorbate system as compared with PHZ. The involvement of oxygen-derived free radicals and other oxidant species in numerous pathophysiological co- nditions helps to develop suitable systems to study their effects and also to assess the antioxidant activity of en- do/exogenous compounds. To confirm the previous re- sults [1], the effect of modulators was evaluated on PHZ treated erythrocytes. An erythrocyte model was taken for lipid peroxidation, hemolysis and thiol content, where PHZ was chosen as oxidant and Ca2+, diltiazem and ED- TA as modulators of PHZ mediated action. PHZ gener- ally produces haemolytic anaemia in in vivo system. The present results indicate that the damaging effect due to PHZ on erythrocyte could be repeated in in vitro system also. Ca2+ could have an important role in the pathoph- ysiology of sickling process. Sickle cells, which main- tain an abnormally deformed shape, have three to seven times higher Ca2+ level as compared to normal red blood cells [12]. Interestingly the results have shown that Ca2+ enhanced the action of PHZ on lipid peroxidation and hemolysis mostly. These two criteria are often observed in anaemic condition. Lipid peroxidation, a marker of membrane function, as well as hemolysis is greatly al- tered when Ca2+ is added with PHZ to the incubation medium. Ca2+ alone without PHZ, on the other hand has got no significant effect on the criteria studied above. Many of the mechanical properties of sickle cell me- mbrane have been found to relate to high Ca2+ level [13]. Because of the role of Ca2+ in injurious processes, Ca2+ antagonists have been tested for reducing the detrimental consequences. In vitro studies on Ca2+ antagonists have been carried out by various workers [14] with the view to prevent the injurious effects of Ca2+ on erythrocytes. They concluded that these Ca2+ antagonists either singly or in combination might be useful in preventing vaso- occlusive events. Ca channel antagonists were shown to act as antioxidant [15]. Calcium antagonists antioxidant potency correlated directly with the compound’s relative affinity for the membrane lipid bilayer [16]. Similarly in Table 2. Effect of Ca2+ diltiazem and EDTA on Cu2+-ascorba- tea-induced lipid peroxidation of erythrocytes. [Values are means ± SD of 6 different experiments] System Lipid peroxidation (n mol /mg protein) Control 168.25 ± 1.67 Control + Ca2+ (2 mM) 170.55 ± 1.01 Cu2+ (0.2 mM) + Ascorbic acid (1 mM) 335.00 ± 4.23* Cu2+ (0.2 mM) + Ascorbic acid (1 mM) + Ca2+ (2 mM) 398.65 ± 3.67** Cu2+ (0.2 mM) + Ascorbic acid (1 mM) + Ca2+ (2 mM) + diltiazem (20 µm) 305 ± 2.67*** Cu2+ (0.2 mM) + Ascorbic acid (1 mM) + Ca2+ (2 mM)+ EDTA (2 mM) 346 ± 4.33# *P < 0.001, **P < 0.01, ***P < 0.01, #P < 0 .05 aAssay system was followed according to Halder, J. and Bhaduri, A.N., 1998 [3].  K. Das et al. / Health 2 (2010) 1221-1225 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1224 our studies the results have shown that micromolar conc- entration of Ca2+ antagonist, diltiazem, decreased the le- vel of lipid peroxidation, hemolysis and thiol content of erythrocyte when treated with PHZ and Ca2+. Metal che- lator EDTA, on the other hand was not found to be as ef- fective as diltiazem in preventing these damaging proc- esses. Earlier reports [17] using erythrocyte as a model have shown increased erythrocytic protein degradation when erythrocytes were treated with PHZ. Therefore, it is exp- ected that more the degradation, more thiol groups wou- ld be liberated. Practically this was found in the present experiments, where in vitro addition of PHZ plus Ca2+ caused increased SH level (though not to a greater extent) and diltiazem was as effective as before. EDTA could not markedly prevent the degradative phenomenon cau- sed by PHZ plus Ca2+. Lipid peroxidation in biological membranes causes impairment of membrane function [18] decreases fluid- ity and inactivation of membrane bound enzymes and receptors [19]. In most cases haemoglobin acts as the ca- talyst for lipid peroxidation [4] and the erythrocyte me- mbrane becomes an important target for damage [20]. Su- peroxide and hydroxyl radical might participate in PHZ- induced toxicity [2]. Administration of PHZ to animals can induce peroxidative lipid damage of erythrocyte me- mbrane resulting in the accumulation of MDA [21]. Ma- ny toxicants like PHZ induce hemolysis by the alteration of cytoskeletal protein [22]. Further detailed examina- tion is necessary to clarify the mechanism of action of PHZ. Results from the present experiments suggest that selecting erythrocyte, as an in vitro model, could be used to ascertain the mechanism of haemolytic anaemia and prevention by calcium channel blocker which could act as antioxidant. 4. ACKNOWLEDGEMENTS Thanks are due to the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of a research fellowship to Kaushik Das. We also acknowledge Late Dr. Asoke G Datta for his valuable advice and constructive criticism till the last moment of his life. REFERENCES [1] Biswas, S., Bhattacharyya, J. and Datta, A.G. (2005) Ox- idant induced injury of erythrocyte-role of green tea leaf and ascorbic acid. Molecular and Cellular Biochemistry, 276(1-2), 205-210. [2] Latunde-Dada, G.O., Vulpe, C.D., Anderson, G.J., Sim- pson, R.J. and McKie, A.T. (2004) Tissue-specific chan- ges in iron metabolism genes in mice following phenyl- hydrazine-induced haemolysis. Biochimica et Biophysica Acta, 1690(2), 169-176. [3] Halder, J. and Bhaduri, A.N. (1998) Protective role of bla- ck tea against oxidative damage of human red blood cells. Biochem. Biophysical Research Communications, 244(3), 903-907. [4] Shetlar, M.D. and Hill, H.A. (1985) Reactions of hemog- lobin with phenylhydrazine: A review of selected aspects. Environmental Health Perspectives, 64, 265-281. [5] Bhattacharyya, J., Thompson, K.D. and Sayeed, M.M. (1993) Skeletal muscle Ca2+ flux and catabolic response during sepsis. American Journal of Physiology, 265(3), 487-493. [6] Braughler, J.M., Dyncan, L.A. and Goodman, T. (1985) Calcium enhances in vitro free radical induced damage to brain synaptosomes mitochondria and cultured spinal cord neurons. Journal of Neurochemistry, 45(4), 1288- 1293. [7] Lu, C., Chan, S.L., Fu, W. and Mattson, M.P. (2002) The lipid peroxidation product 4-Hydroxyneal facilitates op- ening of voltage dependent Ca2+ channels in neurons by increasing protein tyrosine phosphorylation. Journal of Biological Chemistry, 277(27), 24368-24375. [8] Buege, J.A. and Aust, S.D. (1978) Microsomal lipid per- oxidation. Methods in Enzymology, 52, 302-310. [9] Zhang, A., Zhu, Q.Y., Luk, Y.S., Ho, K.Y., Fung, K.P. and Chen, Z.Y. (1997) Inhibitory effect of jasmine green tea epicatechin isomers on free radical induced lysis of red blood cells. Life Sciences, 61(4), 383-394. [10] Owens, C.W. and Belcher, R.V. (1965) A colorimetric micro-method for the determination of glutathione. Bio- chemical Journal, 94(3), 705-711. [11] Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein util- izing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. [12] Williamson, P., Puchulu, E., Westerman, M. and Sc- hlegel, R.A. (1990) Erythrocyte membrane abnormalities in sickle cell disease. Biotechnology and Applied Bio- chemistry, 12(5), 523-528. [13] Nash, G.B., Boghossian, S., Parmar, J., Dormandy, J.A. and Bevan, D. (1989) Alteration of the mechanical prop- erties of sickle cells by repetitive deoxygenation: role of calcium and the effects of calcium blockers. Journal of British Society of Haematology, 72(2), 260-264. [14] Ohnishi, S.T., Horiuchi, K.Y., Horiuchi, K., Jurman, M.E. and Sadanaga, K.K. (1986) Nitrendipine, nifedipine, and verapamil inhibit the in vitro formation of irreversibly sickled cells. Pharmacology, 32(5), 248-256. [15] Rojstaczer, N. and Triggle, D.J. (1994) Calcium channel antagonists as antioxidants. Cardiovascular Drug Re- views, 12(1), 70-84. [16] Mason, R.P., Mak, I.T., Trumbore, M.W. and Mason, P.E. (1999) Antioxidant properties of Ca antagonists re- lated to membrane biophysical interactions. American Journal of Cardiology, 84(4A), 16-22. [17] Chowdhury, T.D., Das, N., Chattopadhyay, A. and Datta, A.G. (1999) Effect of oxidative stress and erythropoietin on cytoskeletal protein and lipid organization in human erythrocytes. Polish Journal of Pharmacology & Phar- macy, 51(4), 341-350. [18] Gutteridge, J.M. and Halliwell, B. (1990) The measure- ment and mechanism of lipid peroxidation in biological systems. Trends in Biochemical Sciences, 15(4), 129- 135.  K. Das et al. / Health 2 (2010) 1221-1225 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 122 1225 [19] Watanabe, H., Kobayashi, A., Yamamoto, T., Suzuki, S., Hayashi, H. and Yamazaki, N. (1990) Alterations of hu- man erythrocyte membrane fluidity by oxygen-derived free radicals and calcium. Free Radical Biology and Medicine, 8(6), 507-514. [20] Deuticke, B., Heller, K.B. and Haest, C.W.M. (1987) Progressive oxidative membrane damage in erythrocytes afterpulse treatment with t-butyl-hydroperoxide. Bio- chimica et Biophysica Acta, 899(1), 113-124. [21] Jain, S.K. (1985) In vivo externalization of phosphati- dylserine and phosphatidylethanolamine in the mem- brane bilayer and hyper-coagulability by the lipid per- oxidation of erythrocytes in rats. Journal of Clinical In- vestigation, 76(1), 281-286. [22] Zachary, S.B., Jason, D.M., David, J.J. and David, C.M. (2005) Primaquine-induced hemolytic anaemia: role of membrane lipid peroxidation and cytoskeletal protein al- terations in the hemotoxicityof 5-hydroxyprimaquine. Journal of Pharmacology and Experimental Therapeu- tics, 314(2), 838-845. |

