 Journal of Behavioral and Brain Science, 2013, 3, 67-73 http://dx.doi.org/10.4236/jbbs.2013.31007 Published Online February 2013 (http://www.scirp.org/journal/jbbs) Effects of 17β-Estradiol on Cognitive Performance of Ovariectomized Female Rats Exposed to Space Radiation Bernard M. Rabin1*, Kirsty L. Carrihill-Knoll1, Lauren V. Long1, Steven C. Pitts1, Barbara Shukitt-Hale2 1Department of Psychology, University of Maryland, Baltimore County, Baltimore, USA 2Human Nutrition Research Center on Aging, USDA-ARS, Tufts University, Boston, USA Email: *rabin@umbc.edu Received December 14, 2012; revised January 15, 2013; accepted January 22, 2013 ABSTRACT On exploratory class missions to other planets astronauts will be exposed to types and doses of radiation that are not experienced in low earth orbit. While it is likely that the crew will consist of both male and female astronauts, there has been little research on the effects of exposure to space radiation on central nervous system function and cognitive per- formance in female subjects. Because estrogen can function as a neuroprotectant, the present experiments were de- signed to evaluate whether or not the presence or absence of estrogen at the time of irradiation would affect the suscep- tibility to the neurocognitive effects of exposure to 56Fe particles in female rats. Capsules containing 17β-estradiol or vehicle were implanted in ovariectomized rats three days prior to exposure 56Fe particles (50 - 200 cGy, 1000 MeV/n). Cognitive performance was evaluated using novel object recognition memory to measure learning and memory and operant responding on an ascending fix ed-ratio schedule to measure ch anges in motivation and in the responsiveness to environmental contingencies. The results indicated th e estrogen does not function as a neuroprotectant to minimize the cognitive effects of exposure to 56Fe particles. However, the presence/absence of estrogen at the time of irradiation could modulate the responsiveness of the subject to the disruptive effects of exposure to HZE particles on the perform- ance of specific cognitive tasks. Keywords: Cosmic Rays; Behavior; Sex Differences; Estrogen 1. Introduction On exploratory class missions to other planets astronauts will be exposed to types and do ses of radiation, including particles of high energy and charge (HZE particles) such as 56Fe, that are not experienced in low earth orbit [1-3] where there International Space Station and space shuttle operate. While it is likely that the crew will consist of both male and female astronauts, there has been little research on the effects of exposure to HZE particles on central nervous system function and cognitive perform- ance in female subjects. Both exposure to HZE particles [4-9] and the gonadal hormone environment [10-15] can affect neuronal function and cognitive performance. As such, it is possible that males and females may respond to HZE particle irradiation differently. Estrogen has been reported to provide protection against the development of neurodegenerative diseases, delaying the onset and progression of Alzheimer’s and Parkinson’s diseases and schizophrenia [16-19]. In addi- tion, estrogen protects against experimental ischemic stroke [16,19], chronic inflammatory diseases [20] and dopaminergic system dysfunction following the admini- stration of a variety of neurotoxins [21,22], including kainic acid and 6-hydroxydopamine. The mechanisms underlying the neuroprotective ef- fects of estrogen are not certain. It has been suggested that estrogen may function to activate free radical scav- enging systems [23], reducing oxidative stress and mini- mizing neuroinflammatory processes in the brain [24,25]. Alternatively, the neuroprotective effects of estrogen may possibly be mediated by actions on microglia [23-26], where estrogen can affect both apoptotic [27] and kinase signaling proce ss es [ 28,29]. Because exposure to HZE particles produces many of the same changes in neuronal function that characterize neurodegenerative diseases, including oxidative stress [7, 30], inflammation [31], and changes in dopaminergic function [32-34], it is possible that estrogen may serve similar neuroprotective functions following exposure to HZE particles as it does for neurodegenerative diseases. The present experiments were designed to evaluate whe- ther or not the presence or absence of estrogen at the time *Corresponding a uthor. C opyright © 2013 SciRes. JBBS  B. M. RABIN ET AL. 68 of irradiation would affect susceptibility to the neuro- cognitive effects of exposure to 56Fe particles in female rats. 2. Experimental Procedure 2.1. Subjects The subjects were 100 ovariectomized (OVX) female Sprague-Dawley rats weighing 175 - 200 g obtained from Taconic Farms. All procedures were approved by the IACUCs of Brookhaven National Laboratory (BNL) and University of Maryland Baltimore County (UMBC). 2.2. Implantation of Estradiol/Vehicle Implantation of estradiol or vehicle was performed at BNL. The procedure was adapted from the one detailed by Strom and colleagues [35]. Thirty mm segments of silastic laboratory tubing (Inner/outer diameter: 1.575/ 3.175 mm, Dow Corning, VWR International, Buffalo Grove, IL) were filled with a solution of 180 pg l7β-es- tradiol/mL sesame oil or sesame oil alone (vehicle). The ends of the tubing were sealed with 5 mm pieces of woo- den applicator sticks, resulting in a vehicle- or 17β-estra- diol-filled column 20 mm in length. Before use, the cap- sules were stored overnight in a vial containing sesame oil with the same concentration of 17β-estradiol or vehi- cle as inside the capsules. To implant the capsules the rats were anesthetized with sodium pentobarbital (35 mg/kg, i.p.). A 5 mm inci- sion was made in the loose skin of the rat’s neck, and a pocket bluntly dissected caudally in which the silastic capsule was gently installed using forceps. The capsules were implanted subcutaneously in the neck because of its abundance of loose skin, and to minimize the risk of mechanical stress on the capsule. The incision was closed by a suture. Following surgery th e rats were monitored to make certain that they recovered from the anesthesia. Strom et al. [35] have used radioimmunoassay proce- dures to evaluate the levels of estradiol in serum follow- ing implantation of silastic capsules containing 17β-es- tradiol as detailed above. They reported that this proce- dure resulted in a relatively stable, physiolog ical level of estradiol in serum from the time of implantation and last- ing for 4 - 5 w eeks followin g implant a tion. 2.3. Radiation Because serum estradiol concentrations attained with the use of silastic capsule implants are relatively constant immediately following implantation [35] the rats were irradiated after a 48 hr recovery period. The rats were exposed to 56Fe particles at the NASA Space Radiation Laboratory (NSRL) at BNL. Dosimetry was provided by the staff of the NSRL using ionization chambers. The doses to which the rats were exposed were 50 (n = 20), 100 (n = 24), 150 (n = 24) and 200 (n = 20) cGy at a nominal dose rate of 25-100 cGy/min. The co ntrol rats (0 cGy, n = 22) were taken to the NSRL but were not irra- diated. Half the rats at each dose were implanted with silastic tubing containing 17β-estradiol and half were im- planted with tubing containing vehicle. For irradiation, the rats were placed in well-ventilated plastic tubes which were placed perpendicular to the beam. The animal was positioned with the center of its head in the beam. As such, some of the neck of the ani- mal was also exposed. Following irradiation the rats were shipped to UMBC for behavioral testing. 2.4. Behavioral Testing At UMBC the rats were given 7 - 10 days to recover from the effects of shipping before beginning behavioral testing. The behavioral procedures have been detailed in previous publications, so they will only be briefly de- scribed her e. 2.4.1. Object Reco gn ition [ 36] Subjects were tested in a dimly lit op en field (93 cm × 93 cm). The stimulus objects (which are no smaller than the size of the rat and no larger than two and a half times its size) vary in shape and color. After habituation to the apparatus, two identical (familiar) stimulus objects are placed in symmetrical locations in the open field. The rat is allowed to explore the stimuli until it accumulates 30 sec total object exploration (i.e., exploration of either ob- ject) or until 20 min have passed. After 24 hr delay, the rat is placed back in the field with one familiar and one novel object and allowed to explore both stimuli until it has accumulated 30 sec of object exploration on either object or until 20 min have passed. Normally rats will spend more time exploring the novel object; old rats and rats that have impaired recognition memory spend equal amounts of time with both the familiar and novel object. 2.4.2. Operant Responding [37] Food-deprived rats (maintained at 90% base weight) are first trained to press a lever on a continuous reinforce- ment schedule using an autoshaping procedure in which they are placed in an operant chamber for 12 hours. Most rats learn the response within a single session. The rats are then trained to respond on a fixed-ratio (FR) schedule by placing them in the chamber for 30 min and reward- ing them on FR-1, FR-5 ( every fifth response reward ed), FR-10, and FR-20 reinforcement schedules. For testing they are then given 30-min sessions in which they are rewarded on FR-1, FR-5, FR-10, FR-15, FR-20, FR-25, FR-30 and FR-35 reinforcement schedules on consecu- tive days. This task is a measure of an organism’s moti- Copyright © 2013 SciRes. JBBS 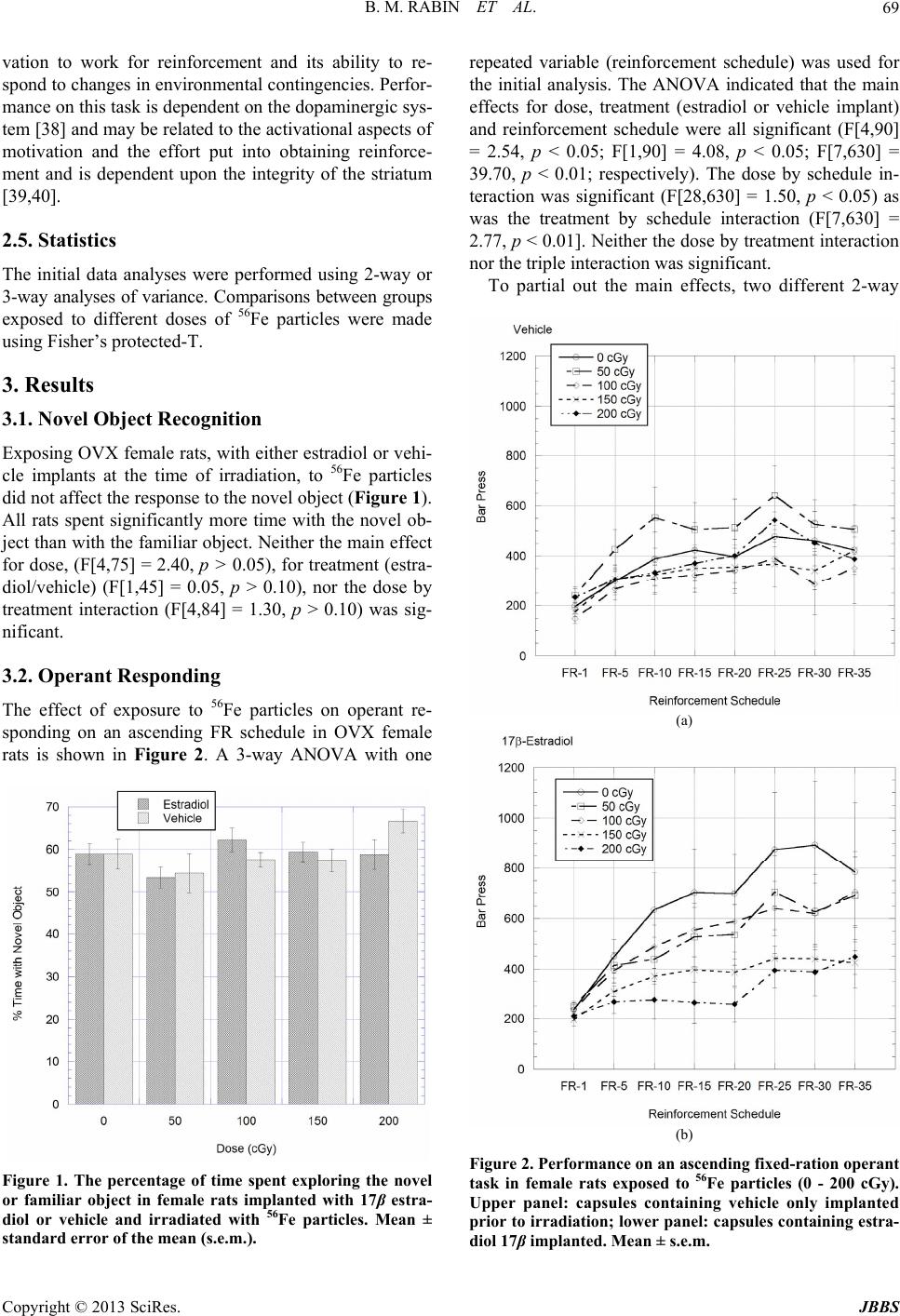 B. M. RABIN ET AL. 69 vation to work for reinforcement and its ability to re- spond to changes in env ironmental contingencies. Perfor- mance on this task is dependent on the dopaminergic sys- tem [38] and may be related to the activational aspects of motivation and the effort put into obtaining reinforce- ment and is dependent upon the integrity of the striatum [39,40]. 2.5. Statistics The initial data analyses were performed using 2-way or 3-way analyses of variance. Comparisons between groups exposed to different doses of 56Fe particles were made using Fisher’s protected-T. 3. Results 3.1. Novel Object Recognition Exposing OVX female rats, with either estradiol or vehi- cle implants at the time of irradiation, to 56Fe particles did not affect the response to the novel object (Figure 1). All rats spent significantly more time with the novel ob- ject than with the familiar object. Neither the main effect for dose, (F[4,75] = 2.40, p > 0.05), for treatment (estra- diol/vehicle) (F[1,45] = 0.05, p > 0.10), nor the dose by treatment interaction (F[4,84] = 1.30, p > 0.10) was sig- nificant. 3.2. Operant Responding The effect of exposure to 56Fe particles on operant re- sponding on an ascending FR schedule in OVX female rats is shown in Figure 2. A 3-way ANOVA with one Figure 1. The percentage of time spent exploring the novel or familiar object in female rats implanted with 17β estra- diol or vehicle and irradiated with 56Fe particles. Mean ± standard error of the mean (s.e.m.). repeated variable (reinforcement schedule) was used for the initial analysis. The ANOVA indicated that the main effects for dose, treatment (estradiol or vehicle implant) and reinforcement schedule were all significant (F[4,90] = 2.54, p < 0.05; F[1,90] = 4.08, p < 0.05; F[7,630] = 39.70, p < 0.01; respectively). The dose by schedule in- teraction was significant (F[28,630] = 1.50, p < 0.05) as was the treatment by schedule interaction (F[7,630] = 2.77, p < 0.01]. Neither the dose by treatment interaction nor the triple interaction was significant. To partial out the main effects, two different 2-way (a) (b) Figure 2. Performance on an ascending fixed-ration operant task in female rats exposed to 56Fe particles (0 - 200 cGy). Upper panel: capsules containing vehicle only implanted prior to irradiation; low er panel: capsules containing estra- diol 17β implanted. Mean ± s.e.m. Copyright © 2013 SciRes. JBBS  B. M. RABIN ET AL. 70 ANOVAs were run for each of the implant conditions using the MS error from the 3-way ANOVA [41]. For the animals given estradiol implants, the main effect for dose of radiation (F[4,90] = 2.77, p < 0.05) and for reinforce- ment schedule (F[7,28] = 17.63, p < 0.01) were signifi- cant. The dose by schedule interaction was also signifi- cant (F[28,630] = 1.62, p < 0.01) indicating that perfor- mance deteriorated as a greater number of responses were needed to produce reinforcement. In contrast, for the rats given vehicle implants, only the main effect for reinforcement schedule was significan t. Neither the main effect of dose nor the dose by schedule interactions was significant. Because visual inspection of Figure 2 suggests the possibility that there were differences in responding in the non-irradiated control animals as a function of estra- diol or vehicle implants, a final 2-way ANOVA was run to compare the performance of the non - irradia t e d an i mals (0 cGy) given either estradiol or vehicle implants. For both treatment groups (estradiol or vehicle) there was a significant increase in response rate as the reinforcement schedule increased (F[7,140] = 6.95, p < 0.01). However, there were no differences in performance as a function of treatment condition (F[1,20] = 1.78, p > 0.10), or in the treatment by schedule interaction (F[7,175] = 0.98, p > 0.10). These results indicate that the differences in re- sponding between animals given estradiol or vehicle at the time of radiation could not be due to differences in hormonal status only, but must reflect an interaction be- tween hormonal status and exposure to 56Fe particle. 4. Discussion While the results suggest that estrogen may exert both organizational and activational effects on cognitive per- formance following exposure to HZE particles, depend- ing upon the specific task, overall, the results do not support the or iginal hypo thesis that estrog en can function as a neuroprotectant to counteract the effects of exposure to 56Fe particles on cognitive performance. Rather, the presence of estrogen at the time of irradiation seems to be necessary for the particle-induced disruption of oper- ant responding. In addition, although male subjects were not run at the present time, comparison with the results of previous experiments u tilizing male rats [36,37] suggests that male and female rats may respond differently to ex- posure to HZE particles, depending upon the specific task. In contrast to operant performance none of the OVX rats, given either estradiol or vehicle at the time of irra- diation, showed a disruption of recognition memory fol- lowing exposure to 56Fe particles. All rats, both radiated and non-irradiated controls, spent more time interacting with the novel object than with the familiar object. Al- though the hormonal environment for these rats differed at the time of irradiation, depending upon whether the rats were given estradiol or vehicle implants, there were no differences in the hormonal environment at the time of testing because the estradiol levels measured in blood would have returned to normal OVX levels by the time they were tested on the novel object recognition task which occurred 4 - 6 weeks following surgery [35]. As such, these results indicate that the hormonal environ- ment (presence or absence of estradiol) at the time of ir- radiation was not a factor influencing the effects of ex- posure to 56Fe particles on recognition memory in female rats. These results differ from those obtained using male subjects which show a disruptio n of recognition memory following exposure to 56Fe particles [36] such that equal amounts of time are spent with both novel and familiar objects. The failure to observe a radiation-induced per- formance decrement in female rats, compared to male rats, following exposure to 56Fe particles may reflect dif- ferences in hormone levels at th e time of testing or in the organization of the brain as the result of differences in perinatal hormones [42]. Alternatively, the differences between males and females may reflect the fact that go- nadal hormones have can have different effects on cogni- tive performance in males and female [43-45]. Operant responding on an ascending FR schedule, a measure of the responsiveness of the organism to envi- ronmental contingencies, showed distinct changes in the patterns of responding as a function of hormonal status at the time of irradiation. The ovariectomized females given vehicle-only implants showed no effect of exposure to 56Fe particles on their response pattern: there were no differences in response patterns between the non-irradi- ated control rats and the irradiated rats. However, the OVX females given estradiol implants showed an effect of irradiation on cognitive performance following irra- diation. Compared to the non-irradiated controls, the OVX rats with estradiol implants at the time of irradia- tion failed to show a corresponding increase in respond- ing as the reinforcement schedule increased following exposure to 150 or 200 cGy of 56Fe particles. In this re- gard, the performance of the female rats with estradiol implants at the time of irradiation was similar to that of male rats which showed a disruption of operan t respond- ing following exposure to 200 cGy of 56Fe particles [37]. The mechanisms underlying the interaction between exposure to 56Fe particles and estradiol in OVX rats which result in the disruption of operant responding are not certain. Performance on this specific task is depend- ent upon the integrity of the dopaminergic system [38]. Gender differences have been reported in the functioning of the dopaminergic system in the striatum, with estrogen enhancing the release of dopamine in female, but not male rats [46]. Estrogen has been reported to have dif- Copyright © 2013 SciRes. JBBS  B. M. RABIN ET AL. 71 ferent effects on striatal- and hippocampal-mediated learn- ing [10], such that estrogen facilitates hippocampal-me- diated learning while it interferes with striatally- mediated learning. It has been further suggested that the interaction between estrogen and dopamine influences the use of striatally- or hippocampally-mediated strategies in the performance of a cognitive task [13]. Similar task-dependent sex differences following ex- posure to HZE particles have been reported by Villasana et al. [8]. Exposure to 56Fe particles impaired perform- ance of hippocampal-dependent contextual fear condi- tioning task in female mice, but led to improved perfor- mance in male mice. There was no effect of exposure to 56Fe particles in the performance of a cued fear condi- tioning task, which is not mediated by the hippocampus, in either male or female mice. Overall, the results of these experiments indicate that the relationship between the gonadal hormone environ- ment and cognitive performance following exposure to 56Fe particles is complex. The effects of gonadal hor- mones on neuronal function can be classified as “activa- tional” or “organizational” [42,47]. Activational effects refer to contemporary correlations between the current hormonal environment and cognitive performance. Or- ganizational effects refer to developmental correlations between the perinatal hormonal environment which in- fluences the org anization of the brain, and which may, at some later time, affect cognitive performance. Both types of effects are observed following exposure to 56Fe parti- cles. Estradiol- and vehicle-implanted rats showed no differences in performance on the novel object recogni- tion task, indicating that the hormonal environment, pre- sence or absence of estrogen at the time of irradiation, had no effect on subsequent performance. However, the observation that neither the estradiol or vehicle implanted females, in contrast to males [36], spent more time with the novel object following irradiation suggests that there may be differences in brain organization which affect performance on this task. In contrast, activational effects were observed in the effects of exposure to 56Fe particles on operant performance. In summary, the results of these experiments indicate that estrogen, despite its capacity to function as a neuro- protectant following a variety of toxic insults [19,21,22] or neurodegenerative disorders [16,18], may not fulfill that role following ex posu re to HZE particles. Rather, the results suggest that the activational effects of estrogen may be to produce a performance deficit with selected cognitive tasks, sp ecifically operant responding on an as- cending fixed ratio reinforcement schedule which is a measure of the responsiveness of the organism to changes in environmental contingencies. As such, the presence of estrogen at the time of irradiation seems to function to make females respond to exposure to 56Fe particles in a manner similar to males. The role of testosterone in male subjects re mains to be establ ished . 5. Acknowledgements This research was supported by NASA grant NNX08 AM66G. REFERENCES [1] F. A. Cucinotta, W. Schimmerling, P. B. Saganti, J. W. Wilson, L. E. Peterson, G. D. Badhwar and J. F. Dicello, “Space Radiation Cancer Risks and Uncertainties for Mars Missions,” Radiation Research, Vol. 156, No. 5, 2001, pp. 682-688. doi:10.1667/0033-7587(2001)156[0682:SRCRAU]2.0.C O;2 [2] A. A. Edwards, “RBE of Radiations in Space and the Implications for Space Travel,” Physica Medica, Vol. 27, 2001, pp. 147-152. [3] W. Schimmerling, F. A. Cucinotta and J. W. Wilson, “Radiation Risk and Human Space Exploration” Ad- vances in Space Research, Vol. 31, No. 1, 2003, pp. 27- 34. doi:10.1016/S0273-1177(02)00653-1 [4] G. Casadesus, B. Shukitt-Hale, H. M. Stellwagen, M. A. Smith, B. M. Rabin and J. A. Joseph, “Hippocampal Neurogenesis and PSA-NCAM Expression Following Exposure to 56Fe Particles Mimics That Seen during Ag- ing in Rats,” Experimental Gerontology, Vol. 40, No. 3, 2005, pp. 249-254. doi:10.1016/j.exger.2004.09.007 [5] N. Denisova, B. Shukitt-Hale, B, M. Rabin and J. A. Jo- seph, “Brain Signaling and Behavioral Responses In- duced by Exposure to 56Fe Radiation,” Radiation Re- search, Vol. 158, No. 6, 2002, pp. 725-734. doi:10.1667/0033-7587(2002)158[0725:BSABRI]2.0.CO; 2 [6] J. A. Joseph, B. Shukitt-Hale, J. McEwen and B. M. Ra- bin, “CNS-Induced Deficits of Heavy Particle Irradiation in Space: The Aging Connection,” Advances in Space Research, Vol. 25, No. 10, 2000, pp. 2057-2064. doi:10.1016/S0273-1177(99)01013-3 [7] B. Shukitt-Hale, G. Casadesus, A. Carey, B. M. Rabin and J. A. Joseph, “Exposure to 56Fe Irradiation Acceler- ates Normal Brain Aging and Produces Deficits in Learn- ing and Memory,” Advances in Space Research, Vol. 39, No. 6, 2007, pp. 1087-1092. doi:10.1016/j.asr.2006.11.005 [8] L. Villasana, J. Rosenberg and J. Raber, “Sex -Dependent Effects of 56Fe Irradiation on Contextual Fear Condition- ing in C57BL/6J Mice,” Hippocampus, Vol, 20, 2010, pp. 19-23. [9] R. Vlkolinsky, T. Krucker, A. L. Smith, T. C. Lamp, G. A. Nelson and A. Obenhaus, “Effects of Lipopolysaccha- ride on 56Fe-Particle Radiation-Induced Impairment of Synaptic Plasticity in the Mouse Hippocampus,” Radia- tion Research, Vol. 168, No. 4, 2007, pp. 462-470. doi:10.1667/RR1038.1 [10] D. M. Davis, T. K. Jacobsen, S. Aliakbari and S. J. Y. Copyright © 2013 SciRes. JBBS  B. M. RABIN ET AL. 72 Mizumori, “Differential Effects of Estrogen on Hippo- campal- and Stiratal-Dependent Learning,” Neurobiology of Learning and Memory, Vol. 84, No. 2, 2005, pp. 132- 137. doi:10.1016/j.nlm.2005.06.004 [11] L. A. M. Galea, “Gonadal Hormone Modulation of Neu- rogenesis in the Dentate Gyrus of Adult Male and Female Rodents,” Brain Research Reviews, Vol. 57, No. 2, 2008, pp. 332-341. doi:10.1016/j.brainresrev.2007.05.008 [12] J. Prange-Kiel and G. M. Rune, “Direct and Indirect Ef- fects of Estrogen on Rat Hippocampus,” Neuroscience, Vol. 138, No. 3, 2006, pp. 756-772. doi:10.1016/j.neuroscience.2005.05.061 [13] M. G. Quinal, D. Hussain and E. G. Brake, “Use of Cog- nitive Strategies in Rats: The Role of Estradiol and Its In- teraction with Dopamine,” Hormones and Behavior, Vol. 53, No. 1, 2008, pp. 185-191. doi:10.1016/j.yhbeh.2007.09.015 [14] F. Van Haaren, A. van Hest and R. P. W. Heinsbroek, “Behavioral Differences between Male and Female Rats: Effects of Gonadal Hormones in Learning and Memory,” Neuroscience and Biobehavioral Reviews, Vol. 14, No. 1, 1990, pp. 23-33. doi:10.1016/S0149-7634(05)80157-5 [15] M. Wallace, V. Luine, A. Arekkanos and M. Frankfurt, “Ovariectomized Rats Show Decreased Recognition Me- mory and Spine Density in the Hippocampus and Pre- frontal Cortex,” Brain Research, Vol. 1176, No. 1, 2006, pp. 176-182. doi:10.1016/j.brainres.2006.07.064 [16] D. E. Brann, K. Dhandapani, C. Wakade, V. B. Mahesh and M. M. Khan, “Neurotrophic and Neuroprotective Ac- tions of Estrogen: Basic Mechanisms and Clinical Impli- cations,” Steroids, Vol. 72, No. 5, 2007, pp. 381-405. doi:10.1016/j.steroids.2007.02.003 [17] L. M. Garcia-Segura, I. Azcoitiaa and L. L. DonCarlos, “Neuroprotection by Estradiol,” Progress in Neurobiol- ogy, Vol. 63, No. 3, 2001, pp. 29-60. doi:10.1016/j.physbeh.2005.04.007 [18] G. E. Gilles and S. McArthur, “Independent Influences of Sex Steroids of Systemic and Central Origin in a Rat Model of Parkinson’s Disease: A Contribution to Sex- Specific Neuroprotection by Estrogens,” Hormones and Behavior, Vol. 57, No. 1, 2010, pp. 23-34. doi:10.1016/j.yhbeh.2009.06.002 [19] P. S. Green and J. W. Simpkins, “Neuroprotective Effects of Estrogens: Potential Mechanisms of Action,” Interna- tional Journal of Developmental Neuroscience, Vol. 18, No. 4-5, 2000, pp. 347-358. doi:10.1016/S0736-5748(00)00017-4 [20] L. Charalampopoulos, E. Remboutsika, A. N. Margoris and A. Gravanis, “Neurosteroids as Modulators of Neu- rogenesis and Neuronal Survival,” Trends in Endocrinol- ogy and Metabolism, Vol. 19, No. 8, 2008, pp. 300-307. doi:10.1016/j.tem.2008.07.004 [21] D. E. Dluzen, “Estrogen Decreases Corpus Striatal Neu- rotoxicity in Response to 6-Hydroxydopamine,” Brain Research, Vol. 767, No. 2, 1997, pp. 340-344. doi:10.1016/S0006-8993(97)00630-6 [22] D. E. Dluzen, “Neuroprotective Effects of Estrogen upon the Nigrostratal Dopaminergic System,” Journal of Neu- rocytology, Vol. 29, No. 5-6, 2000, pp. 387-399. doi:10.1023/A:1007117424491 [23] J. Nilson, “Estradiol and Neurodegenerative Oxidative Stress,” Frontiers in Neuroendocrinology, Vol. 29, No. 4, 2008, pp. 463-475. doi:10.1016/j.yfrne.2007.12.005 [24] M. Kipp, S. Karakaya, J. Pawlak, G. Araujo-Wright, S. Arnold and C. Beyer, “Estrogen and the Development and Protection of Nigrostriatal Dopaminergic Neurons: Con- certed Action of a Multitude of Signals, Protective Mole- cules, and Growth Factors,” Frontiers in Neuroendocrin- ology, Vol. 27, No. 4, 2006, pp. 376-390. doi:10.1016/j.yfrne.2006.07.001 [25] E. Vegeto, V. Benedusi and A. Maggi, “Estrogen Anti-In- flammatory Activity in Brain: A Therapeutic Opportunity for Menopause and Neurodegenerative Diseases,” Fron- tiers in Neuroendocrinology, Vol. 29, No. 4, 2008, pp. 507-519. doi:10.1016/j.yfrne.2008.04.001 [26] J. W. Simpkins and J. A. Dykens, “Mitochondrial Mecha- nisms of Estrogen Neuroprotection,” Brain Research Re- views, Vol. 57, No. 2, 2008, pp. 421-430. doi:10.1016/j.brainresrev.2007.04.007 [27] J. D. Yager and J. Q. Chen, “Mitochondrial Estrogen Receptors—New Insights into Specific Functions,” Trends in Endocrinology & Metabolism, Vol. 18, No. 3, 2007, pp. 89-91. doi:10.1016/j.tem.2007.02.006 [28] M. Cordey, U. Gundimeda, R. Gopalakrishna and C. J. Pike, “Estrogen Activates Protein Kinase C in Neurons: Role in Neuroprotection,” Journal of Neurochemistry, Vol. 84, No. 6, 2003, pp. 1340-1348. doi:10.1046/j.1471-4159.2003.01631.x [29] K. M. Dhandapani and D. W. Brann, “Role of Astrocytes in Estrogen-Mediated Neuroprotection,” Experimental Ge- rontology, Vol. 42, No. 1-2, 2007, pp. 70-75. doi:10.1016/j.exger.2006.06.032 [30] C. L. Limoli, E. Giedsinski, H. Baure, R. Rola and J. R. Fike, “Redox Changes Induced Hippocampal Precursor Cells by Heavy Ion Irradiation,” Radiation and Environ- mental Biophysics, Vol. 46, No. 2, 2007, pp. 167-172. doi:10.1007/s00411-006-0077-9 [31] R. Rola, V. Sarkissian, A. Obenhaus, G. A. Nelson, S. Otsuka, C. L. Limoli and J. R. Fike, “High-LET Radia- tion Induces Inflammation and Persistent Changes in Markers of Hippocampal Neurogenesis,” Radiation Re- search, Vol. 164, No. 4, 2005, pp. 556-560. doi:10.1667/RR3412.1 [32] A. N. Carey, B. Shukitt-Hale, B. M Rabin and J. A. Jo- seph, “Interaction between Age and Exposure to 56Fe Par- ticles on Behavior and Neurochemistry,” Advances in Space Research, Vol. 39, No. 6, 2007, pp. 987-993. doi:10.1016/j.asr.2006.11.012 [33] J. A. Joseph, W. A. Hunt, B. M. Rabin and T. K. Dalton, “Possible ‘Accelerated Aging’ Induced by 56Fe Heavy Par - ticle Irradiation: Implications for Manned Space Flights,” Radiation Research, Vol. 130, No. 1, 1991, pp. 88-93. doi:10.2307/3578484 [34] J. A. Joseph, W. A. Hunt, B. M. Rabin, T. K. Dalton and A. H. Harris, “Deficits in Striatal Muscarinic Receptor Sensitivity Induced by 56Fe Heavy Particle Irradiation: Copyright © 2013 SciRes. JBBS  B. M. RABIN ET AL. Copyright © 2013 SciRes. JBBS 73 Further ‘Age-Radiation’ Parallels,” Radiation Research, Vol. 135, No. 2, 1993, pp. 257-261. doi:10.2307/3578303 [35] J. O. Strom, E. Theodorsson and A. Theodorsson, “Order of Magnitude Differences Between Methods for Main- taining Physiological 17B-Oestradiol Concentration in Ovariectomized Rats,” Scandinavian Journal of Clinical and Laboratory Investigation, Vol. 68, No. 8, 2008, pp. 814-822. doi:10.1080/00365510802409703 [36] B. M. Rabin, K. L. Carrihill-Knoll, M. Hinchman, B. Shukitt-Hale, J. A. Joseph and B. C. Foster, “Effects of Heavy Particle Irradiation and Diet on Object Recogni- tion Memory in Rats,” Advances in Space Research, Vol. 43, No. 8, 2009, pp. 1193-1199. doi:10.1016/j.asr.2009.01.015 [37] B. M. Rabin, J. A. Joseph and B. Shukitt-Hale, “A Longi- tudinal Study of Operant Responding in Rats Irradiated When 2 Months Old,” Radiation Research, Vol. 164, No. 4, 2005, pp. 552-555. doi:10.1667/RR3349.1 [38] M. D. Lindner, M. A. Plone, J. M. Francis, T. J. Blane, J. D. Salmone and D. F. Emerich, “Rats with Partial Striatal Dopamine Depletions Ex hibit R obust an d Long-la sting B e- havioral Deficits in a Simple Fixed-Ratio Bar Pressing Task,” Behavioural Brain Research, Vol. 86, No. 1, 1997, pp. 25-40. doi:10.1016/S0166-4328(96)02240-1 [39] J. D. Salamone, “The Involvement of Nucleus Accum- bens Dopamine in Appetitive and Aversive Motivation,” Behavioural Brain Research, Vol. 61, No. 2, 1994, 117- 133. doi:10.1016/0166-4328(94)90153-8 [40] J. D. Salamone and M. Correa, “Motivational Reviews of Reinforcement: Implications for Understanding the Be- havioral Functions of Nucleus Accumbens Dopamine,” Behavioural Brain Research, Vol. 137, No. 1-2, 2002, pp. 3-25. doi:10.1016/S0166-4328(02)00282-6 [41] G. Keppel, “Design and Analysis: A Researcher’s Hand- book,” 4th Edition, Prentice Hall, Upper Saddle River, 1991. [42] M. M. McCarthy, “How It’s Made: Organisational Ef- fects of Hormones on the Developing Brain,” Journal of Neuroendocrinology, Vol. 22, No. 7, 2010, pp. 736-742. doi:10.1111/j.1365-2826.2010.02021.x [43] T. Aubele, R. Kaufman, F. Montalmant and M. F. Kritzer, “Effects of Gonadectomy and Hormone Replacement on a Spontaneous Novel Object Recognition Task in Adult Male Rats,” Hormones and Behavior, Vol. 54, No. 2, 2008, pp. 244-252. doi:10.1016/j.yhbeh.2008.04.001 [44] R. B. Gibbs, “Testosterone and Estradiol Produce Dif- ferent Effects on Cognitive Performance in Male Rats,” Hormones and Behavior, Vol. 48, No. 3, 2005, pp. 266- 277. doi:10.1016/j.yhbeh.2005.03.005 [45] J. S. Sutcliffe, K. M. Ma rshall and J. C. Neill, “Influence of Gender on Working and Spatial Memory in the Novel Object Recognition Task in the Rat,” Behavioural Brain Research, Vol. 177, No. 1, 2007, pp. 117-125. doi:10.1016/j.bbr.2006.10.029 [46] J. B. Becker, “Gender Differences in Dopaminergic Func- tion in Striatum and Nucleus Accumbens,” Pharmacology Biochemistry and Behavior, Vol. 64, No. 4, 1999, pp. 803-812. doi:10.1016/S0091-3057(99)00168-9 [47] D. Mitsushima, K. Takase, T. Funabashi and F. Kimura, “Gonadal Steroids Maintain 24 h Acetylcholine Release in the Hippocampus: Organizational and Activational Ef- fects in Behaving Rats,” Journal of Neuroscience, Vol. 29, No. 12, 2009, pp. 3808-3815. doi:10.1523/JNEUROSCI.5301-08.2009
|