 Open Journal of Apoptosis, 2013, 2, 1-11 http://dx.doi.org/10.4236/ojapo.2013.21001 Published Online January 2013 (http://www.scirp.org/journal/ojapo) Gsta4 Null Mouse Embryonic Fibroblasts Exhibit Enhanced Sensitivity to Oxidants: Role of 4-Hydroxyn onen al in O xidant Toxicity* Kevin E. McElhanon1,2, Chhanda Bose2,3, Rajendra Sharma1,2, Liping Wu1,2, Yogesh C. Awasthi4, Sharda P. Singh1,2# 1Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, USA 2Central Arkansas Veterans Healthcare System, Little Rock, USA 3Department of Internal Medicine, Nephrology Division, University of Arkansas for Medical Sciences, Little Rock, USA 4Department of Molecular Biology and Immunology, University of North Texas Health Science Center, Fort Worth, USA Email: #SinghShardaP@uams.edu Received November 28, 2012; revised December 29, 2012; accepted January 8, 2013 ABSTRACT The alpha class glutathione s-transferase (GST) isozyme GSTA4-4 (EC2.5.1.18) exhibits high catalytic efficiency to- wards 4-hydroxynon-2-enal (4-HNE), a major end product of oxidative stress induced lipid peroxidation. Exposure of cells and tissues to heat, radiation, and chemicals has been shown to induce oxidative stress resulting in elevated con- centrations of 4-HNE that can be detrimental to cell survival. Alternatively, at physiological levels 4-HNE acts as a signaling molecule conveying the occurrence of oxidative events initiating the activation of adaptive pathways. To ex- amine the impact of oxidative/electrophilic stress in a model with impaired 4-HNE metabolizing capability, we dis- rupted the Gsta4 gene that encodes GSTA4-4 in mice. The effect of electrophile and oxidants on embryonic fibroblasts (MEF) isolated from wild type (WT) and Gsta4 null mice were examined. Results indicate that in the absence of GSTA4-4, oxidant-induced toxicity is potentiated and correlates with elevated accumulation of 4-HNE adducts and DNA damage. Treatment of Gsta4 null MEF with 1,1,4-tris(acetyloxy)-2(E)-nonene [4-HNE(Ac)3], a pro-drug form of 4-HNE, resulted in the activation and phosphorylation of the c-jun-N-terminal kinase (JNK), extracellular-signal-regu- lated kinases (ERK 1/2) and p38 mitogen activated protein kinases (p38 MAPK) accompanied by enhanced cleavage of caspase-3. Interestingly, when recombinant mammalian or invertebrate GSTs were delivered to Gsta4 null MEF, acti- vation of stress-related kinases in 4-HNE(Ac)3 treated Gsta4 null MEF were inversely correlated with the catalytic effi- ciency of delivered GSTs towards 4-HNE. Our data suggest that GSTA4-4 plays a major role in protecting cells from the toxic effects of oxidant chemicals by attenuating the accumulation of 4-HNE. Keywords: Glutathione Transferase; Protein Adducts; Lipid Peroxidation, Oxidants, Stress Kinases 1. Introduction Lipid aldehydes generated duri ng li pid peroxidation (LPO) are highly reactive species capable of electro philic attack on DNA and proteins [1-4]. 4-HNE, one of the major end products of LPO, causes cytotoxicity and genotoxicity by inducing necrosis and pro-apoptotic signaling through multiple pathways at supraphysiological concentrations [5-7]. 4-HNE is also involved in regulation of gene ex- pression and c ell cycle signal ing in a concentrati on depen- dent manner [8-11]. We and others [12,13] have shown that 4-HNE causes activation and phosphorylation of the c-jun-N -te rminal k ina se (JN K ) an d p3 8 mito gen act i vate d protein kinases (p38 MA PK) in lung endothelial cells and contributes to apoptotic response in K562 cells. Our re- cent studies show that in addition to causing toxicity, 4- HNE induces defense m echanisms against oxidative stress and protects the neighboring cells from apoptosis [14]. Its concentration in cells is regulated by the alpha class gluta- thione transferases, particularly GSTA4-4, that catalyze its conjugation to glutathione (GSH) with high catalytic efficiency [15-17]. In order to understand the exact role of GSTA4-4 in protection of cells against acute 4-HNE to- xicity, we isolated MEF from previously generated Gsta4 null mice [18]. In this study, we compared the effects of electrophile/oxidants including 4-HNE(Ac)3, hydrogen peroxide (H2O2), and N,N’-dimethyl-4,4’-bipyridinium dichloride (paraquat) on Gsta4 null and WT MEF cells. 4-HNE(Ac)3 is a biologically inert pro-drug form of *Conflicts of interest: The authors report no conflicts of interest and are responsible for the content and writing of the paper. #Corresponding author. C opyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 2 4-HNE that is advantageous for in vitro treatment of cells [5,19]. 4-HNE is highly electrophilic and may interact with cell surface proteins and components of culture me- dium, whereas 4-HNE(Ac)3 is diffusible across the cell membrane and upon enzymatic reaction with intracellular hydrolases active 4-HNE is liberated, more closely mi- micking intracellular 4-HNE formation. Sensitivity of Gsta4 null cells towards 4-HNE was further correlated with the accumulation of 4-HNE-protein adducts, DNA fragmen- tation, and activation of stress related kinases. In addition, we tested if exogenous delivery of GSTA4-4 into Gsta4 null cells provides protection from 4-HNE toxicity by reversing the course of stress kinase activation. 2. Materials and Methods 2.1. Materials 4-HNE(Ac)3 was prepared by the method of Neely et al. [19]. DMEM cell culture medium, fetal bovine serum, Penicillin/streptomycin, pho sph ate buffered saline (PBS), proteinase K, 4% - 12% Bis-TrisNuPAGE gels, running and transfer buffers, and SYBR green were purchased from Invitrogen (Carlsbad, CA). BioTrek Protein De- livery Regent was purchased from Stratagene. Antibodies against caspase-3, JNK, ERK, and p38 MAPK were from Cell Signaling Technology (Beverly, MA) and MAPK inhibitors were purchased from EMD SERONO, INC. (Rockland, MA). Caspase-Glo® kit for the detection of caspase-3 , 8 , and 9 wer e purchased from Promega (Madi- son, WI). Kinase inhibitors were obtained from Calbio- chem, (EMD Biochemicals, Germany). CCK-8 kit was purchased from Dojindo Molecular Technologies, Inc. (Rockville, Maryland). Polyclonal antibodies against mGSTA4-4 and hGSTA1-1 were raised in chicken and rabbit respectively and antibody against DmGSTD1-1 was a kind gift from B. J. Cochrane, University of South Florida, Tam pa,FL. All other reagents a nd chemicals were purchased from Sigma-Aldrich (St. Louis, MO). 2.2. Mouse Embryonic Fibroblast Lines MEF were harvested according to standard protocol [20,21]. Briefly, uteri were obtained at 13.5 days of pregnancy from wild type (WT) and Gsta4 null mice in 129/Sv background [18] and embryos isolated. Heads and vis- cera were removed and bodies were minced then di- gested with 0.25% trypsin /1 mM EDTA (GIBCO) for 5 - 10 min at 37˚C. A single cell suspension was obtained by mixing digested bodies and complete DMEM medium. Spontaneously immortalized cell lines were used for most experiments at passage numbers less than 20. MEFs were cultured until 80% - 85% co nfluent and passag ed at a ratio of 1:2. Expression level of GSTA4-4 in WT and Gsta4 null MEF was verified by western blot using anti- mGSTA4-4 antibody. 2.3. Transient Transfection of Gsta4 Null MEF with Control and mGSTA4-4 Expression Vector Gsta4 null MEF were transiently transfected with pRC/ CMV/Gsta4 and co ntrol plasmi d constructed earlier in our lab [22] using Lipofectamine 2000 reagent (Life Techno- logies, Grand Island, NY). After 24 h, media containing transfection reagent and plasmid was replaced with com- plete DMEM and cells allowed to recover for an addi- tional 24 h before experiments. A portion of transiently transfected cells were examined by Western blot for the expression of mGSTA4-4 before cell viability assay (data not shown). 2.4. Cell Viability Assay Adherent MEF were trypsinized and pelleted by centri- fugation at 500 × g for 5 minutes at 2˚C - 8˚C and washed twice by suspending in 5 mL complete DMEM. Cell pellet was resuspendedat 2 × 105 cells/ml in DMEM and 100 µL/well were seeded in 96 well plates and allowed to recover for 16 - 18 hours before treatment. WT, Gsta4 null and transiently transfected Gsta4 null MEF cells were treated with 4-HNE(Ac)3 (0 - 25 µM), and in a separate experiment WT and Gsta4 null MEF cells were treated with paraq uat (0 - 250 µM), and H2O2 (0 - 700 µM) for 24 hours and analyzed for viability by MTT assay using CCK-8 kit. 2.5. Quantitation of 4-HNE-Protein Adducts by ELISA 4-HNE-protein adducts were quantitated by competitive ELISA [23] using a polyclonal antibody against 4-HNE- modified keyhole limpet hemocyanin, generously provid- ed by Dr. Dennis R. Petersen, University of Colorado, Denver. A debris free cell lysate from control and treated MEF or known amounts of 4-HNE-casein were preadsor- bed with anti 4-HNE antibody and added to a 96 well immunoassay microplate pre-coated with 4-HNE modi- fied casein (33 µg/ml per well). After incubation for 1 hr, plate was washed with PBS (containing 0.05% Tween 20 and 0.25 mg/ml casein), HRP conjugated secondary anti- body added to each well and incubated for an additional 30 min at RT. The plate was washed and color developed by adding 100 µl/well of TMB One Solution (Promega, Madison, WI). After blue color development (usually 4 - 5 min), the reaction was stopped by adding 1N HCl and plate read at 450 nm. Samples were quantified using the calibration curve constructed with known amounts of competitor (4HNE-casein). 2.6. Determination of Caspase Activity Activities of caspase-3, 8, and 9 in WT and Gsta4 null Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 3 MEF treated with/without 4-HNE(Ac)3 were analyzed us- ing Caspase-Glo® kit according to manufacturer’s in- structions. Briefly, 2 × 104 null or WT cells were grown for 24 h in white-walled 96 well plates compatible with luminometer (Molecular Devices, Sunnyvale, CA). After 24 h cells were treated with 20 µM 4-HNE(Ac)3 for 2 h with control cells receiving an equivalent amount of DMSO. Cleavage of caspase-3 was also confirmed by Western blot anal yses. For Wester n bl ot, 2 × 105 WT or Gsta4 null cells were plated in 100 mm dishes and after 24 h of incubation cells were treated with DMSO or 20 µM 4- HNE(Ac)3 for different time points (0 - 5 hr). Following treatment cells were lysed, clarified by centrifugation, and 25 µg protein/well separated on 4% - 12% NuPAGE gel. Anti-caspase-3 antibody was used to detect pro and cleaved caspas e-3 . 2.7. DNA Damage Determined by Comet Assay DNA dama ge was assessed in 4-HNE(Ac)3 treated/control WT and Gsta4 null MEF by “comet” assay (also called single cell gel electrophoresis; SCGE) according to me- thods described by Singh et al. [24] with slight modifica- tions. In brief, control and treated cells were suspende d i n liquid 0.5% low melting point agarose and spread on a glass microscope slide coated with 1% (w/v) agarose. Cells were ly sed ( 1% T ri t o n X -1 0 0 an d 1% sodium l a ury l sarcosinate for 1 h at 4 degree C in dark) and DNA al- lowed to unwind under alkaline conditions by covering the slides with a 300 mM NaOH, 1 mM EDTA (pH > 13.0) solution for 1 hr. Following unwinding, slides underwent electro-phoresis (25 volts (~0.74 V/cm) for 20 min), washed and stained with SYBR green for 5 min. Cells were scored for DNA damage [25] by computerized im- age analysis using CometScore™ Freeware (TriTek Cor- poration, Sumerduck, VA; http://autocomet.com). 2.8. Activation Analysis of Stress Kinases by Western Blot For the Western blot analyses, 2 × 105 cells were plated in 100 mm dishes and after 24 h of incubation treated with inhibitors of JN K (SP600125, 50 µM), ERK (UO1 26, 20 µM), and p38 MAPK (SB202190, 2 µM) for 1h before treatment wit h 20 µM 4-HNE(Ac)3. Afte r 2 h of treatment, control and tre at ed cel ls w ere h arvest ed, ext ract s pre pare d in RIPA buffer (Sigma-Aldrich, St. Louis, MO), and pro- tein content determined by the Bradford method [26]. 25 µg of cell extracts were separated by SDS-PAGE in pre- cast NuPage 4% - 12% Bis-Tris gels (Invitrogen, Carls- bad, CA), and t he electroblot pro bed with po lycl onal anti- bodies against phosphorylated JNK, ERK, and p38 MAPK. A peroxidase-coupled secondary antibody and SuperSignal West Pico (Thermo scientific, Rockford, IL) with chemiluminescent detection were used for visualiza- tion of bands on a Bio-Rad imaging system (Bio-Rad Laboratories, Hercul es, CA). 2.9. Effects of Kinase Inhibitors on MEF Viability To compare the effects of JNK, ERK and p38 MAPK inhibitors on the viability of WT and Gsta4 null MEF, 2 × 104 cells/well were plated in 96 well plates in complete growth medium. After 24 h the cells were separately treated with inhibitors of JNK (SP60 0125; 50 µM), ERK (UO126; 20 µM), and p38 (SB202190; 2 µM) for 1 h and then exposed to 20 µM 4-HNE(Ac)3. The viability of cells after 24 h was determined by MTT assay as described above. 2.10. Delivery of Purified GSTs into Gsta4 Null MEF Cells The BioTrek Protein Delivery Regent (Stratagene) was used to deliver purified recombinant mouse GSTA4-4, Drosophila DmGSTD1-1 [27], and human GSTA1-1 ex- pressed i n E. coli. Purified GSTs were dilut ed to 1 mg/mL with PBS and 100 µl of the dilu ted protein solution tran- sferred t o the tu be co ntaini ng ly ophil ized Bi oTre k reage nt, mixed thor ou g hl y , an d i ncubate d at R T f or 5 min. Serum- free medium was then added to a final volume of 500 µl. Gsta4 null MEF cells approximately 50% - 60% confluent were washed once with serum-free medium, 500 µl of fresh serum-free medium added to each well, and 500 µl of the BioTrek-protein mixture was added drop wise. After 2 h complet e medium was ad ded and ce lls incubate d at 37˚C and 5% CO2 in a humidified incubator for 16 h before treatment with 20 µM 4-HNE(Ac)3. Control WT and Gsta4 null MEF cells received reagent and/or treat- ment to match protein delivered Gsta4 null MEF cells. Cells were harvested 2 h af ter 4-HNE(Ac)3 treatment for Western blot analy ses. 3. Results 3.1. Expression of GSTA4-4 in WT and Gsta4 Null MEF Cells Expression of GSTA4-4 isozyme in WT and Gsta4 null MEF cells was analyzed by Western blot using specific antibody. The immunoblot presented in Figure 1(a) show- ed robust expression of mGSTA4-4 in WT MEF, whereas detectable expr essi on of t his isozyme was not obser ve d i n Gsta4 null MEF. 3.2. Gsta4 Null MEF Are More Sensitive to 4-HNE and Oxidant Toxicity In mammals, GSTA4-4 catalyzes conjugation additio n of Copyright © 2013 SciRes. OJApo 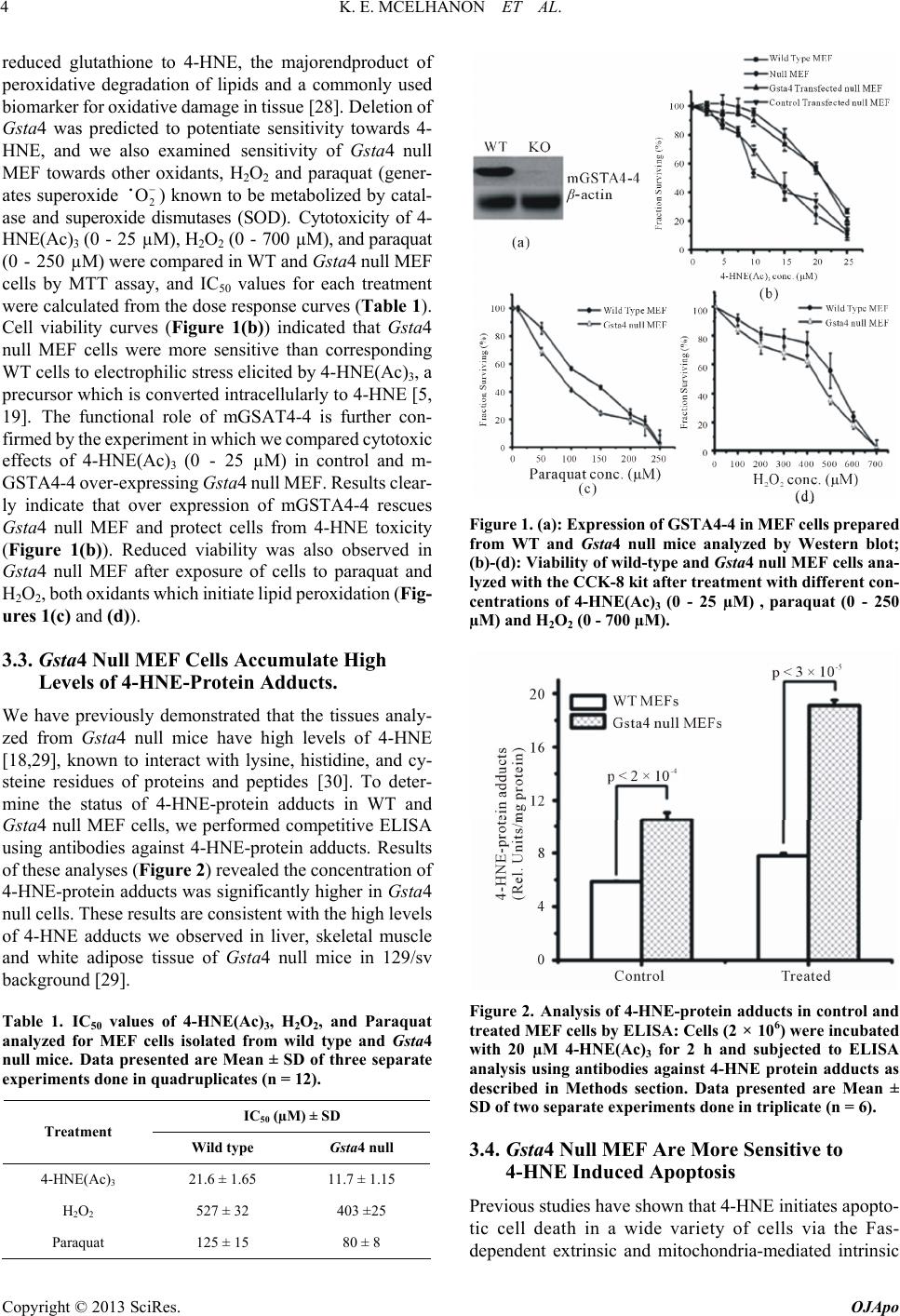 K. E. MCELHANON ET AL. 4 reduced glutathione to 4-HNE, the majorendproduct of peroxidative degradation of lipids and a commonly used biomarker f or oxidative dam age in tissue [28]. Deletion of Gsta4 was predicted to potentiate sensitivity towards 4- HNE, and we also examined sensitivity of Gsta4 null MEF towards other oxidants, H2O2 and paraquat (gener- ates superoxide 2) known to be metabolized by catal- ase and superoxide dismutases (SOD). Cytotoxicity of 4- HNE(Ac)3 (0 - 25 µM), H2O2 (0 - 700 µM), and paraquat (0 - 250 µM) were compared in WT and Gsta4 null MEF cells by MTT assay, and IC50 values for each treatment were calculated from the dose response curves (Table 1). Cell viability curves (Figure 1(b)) indicated that Gsta4 null MEF cells were more sensitive than corresponding WT cells to electrophilic stress elicited by 4-HNE(Ac)3, a precursor which is converted in tracellularly to 4 -HNE [5, 19]. The functional role of mGSAT4-4 is further con- firmed by the expe riment in which we compared cytotoxic effects of 4-HNE(Ac)3 (0 - 25 µM) in control and m- GSTA4-4 over-expressing Gsta4 null MEF. Results clear- ly indicate that over expression of mGSTA4-4 rescues Gsta4 null MEF and protect cells from 4-HNE toxicity (Figure 1(b)). Reduced viability was also observed in Gsta4 null MEF after exposure of cells to paraquat and H2O2, both oxidants which initiate lipid peroxidation (Fig- ures 1(c) and (d)). O 3.3. Gsta4 Null MEF Cells Accumulate High Levels of 4-HNE-Protein Adducts. We have previously demonstrated that the tissues analy- zed from Gsta4 null mice have high levels of 4-HNE [18,29], known to interact with lysine, histidine, and cy- steine residues of proteins and peptides [30]. To deter- mine the status of 4-HNE-protein adducts in WT and Gsta4 null MEF cells, we performed competitive ELISA using antibodies against 4-HNE-protein adducts. Results of these analyses (Figure 2) revealed the concentration of 4-HNE-protein adducts was significantly higher in Gsta4 null cells. These results are consistent with the high levels of 4-HNE adducts we observed in liver, skeletal muscle and white adipose tissue of Gsta4 null mice in 129/sv background [29]. Table 1. IC50 values of 4-HNE(Ac)3, H2O2, and Paraquat analyzed for MEF cells isolated from wild type and Gsta4 null mice. Data presented are Mean ± SD of three separate experiments done in quadruplicates (n = 12). IC50 (µM) ± SD Treatment Wild type Gsta4 null 4-HNE(Ac)3 21.6 ± 1.65 11.7 ± 1.15 H2O2 527 ± 32 403 ±25 Paraquat 125 ± 15 80 ± 8 Figure 1. ( a): E xp re ss ion o f GS TA 4-4 in MEF c el ls p rep are d from WT and Gsta4 null mice analyzed by Western blot; (b)-(d): Viability of wild-type and Gsta4 null MEF cells ana- lyzed with the CCK-8 kit after treatment with different con- centrations of 4-HNE(Ac)3 (0 - 25 µM) , paraquat (0 - 250 µM) and H2O2 (0 - 700 µM). Figure 2. Analysis of 4-HNE-protein adducts in control and treated MEF cells by ELISA: Cells (2 × 106) were incubated with 20 µM 4-HNE(Ac)3 for 2 h and subjected to ELISA analysis using antibodies against 4-HNE protein adducts as described in Methods section. Data presented are Mean ± SD of two separate e xperiments done in triplicate (n = 6). 3.4. Gsta4 Null MEF Are More Sensitive to 4-HNE Induced Apoptosis Previous studies have shown that 4-HNE initiates apopto- tic cell death in a wide variety of cells via the Fas- dependent extrinsic and mitochondria-mediated intrinsic Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 5 pathways [14,31,32]. While in Fas-dependent extrinsic apoptosis the initiator caspase-8 plays an important role, caspase-9 has been shown to be involved in the mito- chondria mediated apoptosis, h owever, both initiator cas- pases can lead to the activation of the executioner or effector caspases 3 and 7 [33]. Cytochrome C released from mitochondria facilitates the cleavage of pro-caspase- 3 (37 kDa) to smaller fragments of 20, 17, and 12 kDa [34]. Activation of caspase-3 analyzed by fluorescence assay indicated that in Gsta4-null MEF cells, 4-HNE and H2O2 mediated induction of caspase-3 was almost 2 fold higher than WT MEF cells, indicating enhanced sensi- tivity to 4-HNE-induced apoptosis (Figure 3(a)). This observation was further confirmed by Western blot ana- lysis (Figure 3(b)), which showed a time dependent in- crease in the cleavage of caspase-3 in null MEF cells after 4-HNE(Ac)3 treatment not detected in MEF WT cells. Likewise, the activation of caspase-8 and 9 was signi- ficantly increased in MEF null cells treated with 4-HNE (Figures 3(c) and (d)). 3.5. 4-HNE Causes DNA Damage Caspases have been identified as key pro-apoptotic pro- teins that can disrupt essential homeostatic processes and initiate an orderly disassembly of cells, including degra- dation of genomic DNA. Significant activation of cas- pases in Gsta4 null cells by 4-HNE(Ac)3 was further cor- related with strand breaks in DNA. The extent of DNA damage in treated and control MEF cells was analyzed by the alkaline comet assay [35]. As shown in Figure 4, MEFs treated with vehicle alone did not show any signi- ficant DNA damage. Substantial DNA fragmentation was observed in both WT and Gsta4 null MEFs upon expos ure to 20 µM 4-HNE(Ac)3 for 2 h, however, this damage was significantly more pronounced in Gsta4 nu l l cells. 3.6. Activation and Phosphorylation of ERK, JNK, and p38 MAPK Is Elevated in Gsta4 Null MEF Treatment of hepatocytes or endothelial cells with 4-HNE results in phosphorylation of ERK, JNK, and p38 MAPKs [12,36], furthermore, pretreatment of bovine lung mic- rovascular endothelial cells with inhibitors of MEK1/2, JNK, or p38 MAPK only partially attenuated 4-HNE- mediated barrier function and cytoskeletal remodeling [12]. Western blot anal y si s (Figure 5) indicated that even though 4-HNE treatment resulted in the activation and phosphorylation of ERK, JNK, and p38 MAPK in both WT and null MEF, the activation of these kinases was only moderately more pronounced in null cells, which could be attributed to elevated basal levels of 4-HNE. Results showed that 4-HNE induced activation of these protein kinases correlated with the increased sensitivity of null MEF to oxidant ind uced apoptosis. To further corre- Figure 3. 4-HNE or H2O2 induced activation of caspases in MEF cells; (a) Activation of caspase-3 in MEF cells treated with H2O2 (100 µM) and 4-HNE(Ac)3 (20 µM) measur ed by ELISA; (b) Western blot analyses of 4-HNE induced activa- tion of caspase3. Cells were treated with 4-HNE(Ac)3 (20 µM) for 0 h - 5 hr, harvested, washed, and ly sed in RIPA buffer. 25 µg of protein from control and treated samples were resolved by SDS-PAGE and immunobloted on nitrocellu- lose membrane. Immunoblots were probed with caspase3 antibodies; (c) and (d). 4-HNE induced activation of cas- pase-8 and 9 in MEF cells: WT and null MEF cells (5000 cells) wer e treated with 4-HNE(Ac)3 (20 µM) for 2 h. Control and 4-HNE treated cells were analyzed for the activation of caspase-8 and 9 by ELISA as describe d in the Methods sec- tion. Data presented are Mean ± SD of two separate exper- iments done in quadruplicates (n = 8). late 4-HNE mediated induction of MAP kinases and in- creased cytotoxicity in Gsta4 null MEF, we pre-treated cells with selective and specific inhibitors of ERK (UO126) [37], JNK (SP600125) [38], and p38 MAPK (SB 202190) [39]. Pretreatment of MEF with kinase inhibitors blocked the phosphorylation of stress related kinases in cells treated with 4-HNE(Ac)3 (Figure 5) and partially abro- gate the cytotoxic effect in Gsta4 null MEF (Figure 6). Results clearly suggests that MAP kinases play an im- portant role in 4-HNE mediated tox icity and cell death in MEF and absence of GSTA4-4 potentiates the cytotoxic effects of 4-HNE. 3.7. Delivery of GSTA4-4 into MEF Null Cells Provides Protection from 4-HNE Induced Apoptosis In order to ascertain the protective role of GSTA4-4 against oxidant toxicity, we delivered mGSTA4-4, Droso- phila DmGSTD1-1, and hGSTA1-1 (catalytic efficiency towards 4-HNE diminishes respectively) into Gsta4 null Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 6 Figure 4. DNA damage determined by alkaline comet assay. MEFs from wild-type and mGsta4 null mice were treated for 4 hr with vehicle or with 4-HNE(Ac)3. 60 cells from each tre- atment were scored for DNA damage [25] using Comet- Score (http://autocomet.com). Box: 25 - 75 percentile; whis- kers: 10 - 90 percentile; horizontal line: median, circle: mean. Untreated WT and mGsta4 null cells do not differ (p > 0.4), but cells treated with 20 µM 4-HNE(Ac)3 are signi- ficantly different (p = 0.005, Wilcoxon rank-sum test). Figure 5. Activation of MAP kinases in MEF cells after treatment with kinase inhibitors and 4-HNE(Ac)3. Cells (2 × 106) were treated separately with the SP600125 (JNK in- hibitor), UO126 (ERK inhibitor) and SB202190 (p38 inhi- bitor) and exposed to 4-HNE(Ac)3 (20 µM) for 2 hr. Cells treated only with 4-HNE(Ac)3 and vehicle alone were used as controls. E xtra cts of con trol an d tre ated c ells we re su bje cted to Western blot analyses and probed separately with anti- bodies against pERK [anti-pp44/42(Thr202/Tyr204)], p38 [anti-phospho-Thr180/Tyr204] and pJNK [anti-pJNK(Thr183/ Tyr185)]. Antibodies against β actin were used to ascertain equal loading of proteins. Appropriate lanes in the Western blot have been marked. MEF using Stratagene Bio Trek delivery system [40]. After verifying successful delivery of GSTs by Western blot (Figure 7(a)), we compared the effect of 4-HNE on the viability of GST isozyme delivered null MEF cells. Results of these studies indicated that while-cells deliv- ered with mGSTA4-4 isozyme showed significant resis- tance to 4-HNE(Ac)3, DmGSTD1-1 and hGSTA1-1 deliv- ered cells did not show significant alteration in viability upon treatment with 4-HNE(Ac)3 (data not shown). Ef- fects of 4-HNE on th e activation of JNK and p38 MAPK were also compared in GST isozyme delivered null MEF cells. Western blot analyses (Figure 7(b)) revealed that delivery of mammalian and invertebrate GSTs into Gsta4 null MEF cells indeed resulted in the attenuation of 4- HNE mediated activation of JNK, ERK1/2, and p38 MAPK in a manner dependent on the catalytic efficiency of each towards 4-HNE. Murine GSTA4-4, which has the highest catalytic efficiency towards 4-HNE (1500 S-1. mM-1 [41]), shows the highest reversal of stress kinase activati on; foll owed by Dm GSTD1-1 (399 S-1. mM-1 [27]) and hGSTA1-1 (58.8 S-1. mM-1 [42]). Reversal by 4- HNE-metabolizing GSTs indicates that the increased act- ivation of stress-related kinases in Gsta4 null MEFs is most likely due to 4-HNE and the activation of these pro- tein kinases contributes to 4-HNE-induced apoptotic sig- naling. 4. Discussion. It is widely recognized that GSTs play a major role in the Figure 6. Effect of inhibitors of p38 (a ); ERK ( b) and J NK (c) on viability of MEF cells:WT and null cells ( 2 × 104) were seeded in a 96 well plate and treated with fixed concen- tration of inhibitors for 1 h then treated with 10 µM of 4- HNE(Ac)3 for 24 h. Control cells received equal volume of DMSO. After completion of treatment cell viability was as- sayed by MTT as described in the Methods section. Data shown are Mean ± SD of two experiments with eight re- plicate wells. Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 7 Figure 7. (a) Verification of GST isozyme uptake in Gsta4 null MEF cells after delivery with BioTrek reagent: mGsta4- 4, hGSTA1-1, and DmGSTD1-1 were delivered into Gsta4 null MEF cells using BioTrek (BT) reagent as described in Methods section. Delivery of each isozyme in Gsta4 null MEF was confirmed by Western blot analyses of GST tran- sfected and control cell extracts (BT reagent treated and untreated). (b) Effect on stress kinase activation by exo- genous introduction of GST protein into Gsta4 null MEF cells: Lane 1: untreated wild-type cells. Lane 2: wild-type cells treated with protein delivery reagent only. Lane 3: wild-type cells treated with protein delivery reagent and 20 µM 4-HNE(Ac)3. Lanes 4-10: mGsta4 null cells. Lane 4: untreated. Lane 5: treated with 20 µM 4-HNE(Ac)3. Lane 6: treated with delivery reagent only. Lane 7: treated with delivery reagent and 20 µM 4-HNE(Ac )3. Lanes 8-10: treat- ed with 20 µM 4-HNE(Ac)3 with delivery reagent plus 100 µg/dish of mGSTA4-4 (lane 8), hGSTA1-1 (lane 9), or DmGSTD1-1 (lane 10), respectively. The core comparison: delivery of no protein (lane 7) or the three GSTs into KO cells (lane 8-10) exposed to 4-HNE(Ac)3. regulation of intracellular 4-HNE levels and in defense mechanisms against oxidative stress. The alpha class GST isozymes GSTA1-1, GSTA2-2 , and GSTA3-3 efficiently catalyze the GSH dependent reduction of fatty acid hy- droperoxides [28,43] and limit the formation of 4-HNE, while the isozyme GSTA4-4 exhibits high catalytic effici- ency for conjugating 4-HNE to GSH for its metabolism and disposition [41]. Thus, the alpha class GSTs limit the cellular accumulation of 4-HNE and related unsaturated lipid aldehydes asso ciated with oxidative damage to cells via GSH peroxidase and GSH con jugating activities. The Gsta4 null mouse model generated by us [18] has pro- vided significant in sight into the physiolog ical and patho- logical roles of 4-HNE. The ob served hypersensitivity of Gsta4 null MEF cells to oxidants and the correlation of this sensitivity with increased accumulation of 4-HNE- protein adducts is consistent with previous findings [44, 45], suggesting that oxidative damage to cells leads to significant increases in 4-HNE adducts and overexpres- sion or induction of GSTA4-4 prevents such accretion [13]. Rapid release of reactive oxygen species such as superoxide radical and hydrogen peroxide during oxid- ative stress leads to formation of the lipid peroxidation product 4-HNE, which could be responsible for the im- pairment of downfield protective mechanisms in the cel- lular system [46]. Increased sensitivity of Gsta4 null MEF towards unrelated substrates (H2O2 and paraquat) of GSTA4-4 during in vitro treatment is possibly due to excessive production of 4-HNE by treated cells. The rad- ical-initiated reaction with polyunsaturated fatty acids is unique since it results in a chain reaction [47], thus, 4- HNE formation will be accelerated despite the protective effects of catalase and supero xide dism uta se in Gsta4 null cells. For this reason, cytotoxic effects of other oxidants are not surprising. These results clearly indicate that GS- TA4-4 is an important enzyme and it plays a pivotal protective role du ri n g c hr o nic and acute oxidative stress. Apoptosis is characterized by cell shrinkage, chrom atin condensation, and blebbing of cellular components pro- ducing apoptotic bodies [48-50]. The process can be sum- marized as initiation by an apoptosis inducing agent, cleavage of pro-caspases constituting a family of aspar- tate-specific cysteine proteases resulting in a caspase cas- cade, and culminating with the cleavage of proteins by executioner ca spases and cel l death [51,52]. Supraphysio- logical concentrations of 4-HNE are known to induce apoptosis in most cell types studied to date and is induced via the death receptor Fas-mediated extrinsic and the mitochondria mediated intrinsic apoptotic p athway [52,53]. Increased apoptosis accompanied by increased 4-HNE- protein a dducts an d DNA dama ge in Gsta4 null MEF cells supports the pro-apoptotic role of 4-HNE. Furthermore, an accelerated activat ion of caspase -3, caspase- 8, and cas- pase-9 is consistent with our previous findings [14,31] suggesting that 4-HNE induces apoptosis via both the ex- trinsic and intrinsic pathways in a cell type independent manner. 4-HNE(Ac)3 activated caspase-3 in Gsta4 null cells possibly targets key enzymes responsible for DNA repair and fragmentation to execute apoptosis [51]. The observed increase in DNA fragmentation assessed by comet assay in treated Gsta4 null cells positively corre- lates with the execution er role of caspase-3. ERK, JNK, and p38 MAPK are vital and central ele- ments of the MAPK family, mediating multiple signaling cascades initiated by stress, cytokines, and growth factors. ERK is the final enzyme of the MAPK pathway which transmits signals into the nucleus and chronic activation of ERK can i n duce apoptosis [54]. Consi st e nt wi t h earl i e r studies [12,31,45], treatment with 4-HNE resulted in the Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 8 activation and phosphorylatio n of JNK, ERK1/2, and p38 MAPK in MEF cells. Higher basal levels of activated/ phosphorylated JNK, ERK1/2, and p38 MAPK observed in Gsta4 null MEFs accompanied by increased 4-HNE levels further correlates the role of 4-HNE in the activa- tion of these ki nases. Even thou gh stres s ki nase act ivation is known to play an important role in the mechanisms of apoptosis, cell survival/proliferation, and inflammation, their ultimate effect on cellular fate is still controversial. For example, activation of different isoforms of JNK under various stimuli has been shown to affect both apo- ptotic and pro-survival signaling [32,55]. Our results showing that pretreatment of MEF with inhibitors of MAPK and JNK only partially abrogated the sensitivity of MEF null cells to 4-HNE(Ac)3 (Figure 6) sugge st 4- HN E also exerts its toxicity through undefined mechanisms in addition to MAPK and JNK mediated apop tosis. Functional differences between WT and cells lacking GSTA4-4 could be triggered by di fferi ng l e vel s of 4-HNE, by a change in the concentration of mGSTA 4-4 subst rates other than 4-HNE, or by non-catalytic functions of mGS- TA4-4, such as direct binding to proteins. GSTs of the Pi and Mu classes are known to act as stress sensors by se- questering signaling kinases under normal condition s and releasing them in response to stress [56-62]. GSTs may also serve an anti-apoptotic role, one example is a novel plant GST shown to suppress Bax, and thus affect apop- tosis [63-65]. To distinguish between the various modes of GST action, we directly introduce mGSTA4-4 (which has 4-HNE-conjugating ability), the phylogenetically di- stant Drosophila DmGSTD1-1 (which is also capable of conjugating 4-HNE, albeit with a lesser catalytic effi- ciency than mGSTA4-4) [27], and hGSTA1-1 (which is closely related to mGSTA4-4 but lacks significant activity toward 4-HNE) into Gsta4 null cells. mGSTA4-4 and, to a lesser extent, consistent with its lower activity, DmG- STD1-1 were able to abrogate the activation of p38 MAPK and JNK (Figure 7) by 4-HNE(Ac)3, whereas hGSTA1-1 had no effect. Drosophila DmGSTD1-1 be- longs to a different family of GSTs than mGSTA4-4 [27], and is unlikely to enter into specific protein-protein in- teractions in a mammalian system. Thus, the only known property of the three enzymes that correlates with the abil- ity to prevent stress kinase activation is conjugation of 4- HNE, indicating that 4-HNE mediates kinase activation. Together, results of present studies clearly demonstrate that 4-HNE significantly contributes to the cytotoxicity and that GSTA4-4 plays a crucial ro le in nullifying acute 4-HNE toxicity by converting it to non-electrophilic glu- tathione conjugate g lutathionyl-HNE (GS-HNE). 5. Concluding Remarks. GSTA4-4 is a key enzyme that regulates 4-HNE concen- tration in mammalian cellular systems. Our findings clear- ly suggest that the impairment of 4-HNE conjugation in Gsta4 null MEF increases the sensitivity toward s 4-HNE by activating caspases and stress-activated kinases. Fur- thermore, the positive relationships between DNA da- mage, increased 4-HNE-protei n adduct accumulation, and apoptosis in Gsta4 null MEF upon treatment with 4- HNE(Ac)3 were demonstrated. These results suggest that 4-HNE ind uced apoptosis of Gsta4 null MEF is associated with the enhanced accumulation of 4-HNE-protein ad- ducts, DNA damage, and the activation of caspases-3, 8 and 9. We also demonstrated that exogenous delivery of GST isozymes with medium to high catalytic efficiency towards 4-HNE prevents stress-related kinase activation upon treatment with HNE(Ac)3. Thus, we conclude that GSTA4-4 is a major 4-HNE metabolizing enzyme in the mouse, which protects cells from 4-HNE toxicity during acute oxidative stress. 6. Acknowledgements. This work was supported in part by National Institutes of Health grants R01 AG028088 and AG032643 (to Piotr Zimniak and Sharda Singh), Pilot and Exploratory Stud- ies Program grant from Claude Pepper Older Americans Independence Center (to Sharda P. Singh), and a grant from Patricia Rogers Joslin Foundation for Pancreatic Cancer Rsearch (to Yogesh C. Awasthi). REFERENCES [1] M. Perluigi, R. Coccia and D. A. Butterfield, “4-Hydro- xy-2-nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies,” Antioxidants and Redox Signaling, Vol. 17, No. 11, 2012, pp. 1590- 1609. doi:10.1089/ars.2011.4406 [2] A. Winczura, D. Zdzalik and B. Tudek, “Damage of DNA and Proteins by Major Lipid Peroxidation Products in Genome Stability,” Free Radical Research, Vol. 46, No. 4, 2012, pp. 442-459. doi:10.3109/10715762.2012.658516 [3] G. P. Voulgaridou, I. Anestopoulos, R. Franco, M. I. Panayiotidis and A. Pappa, “DNA Damage Induced by Endogenous Aldehydes: Current State of Knowledge,” Mutation Research, Vol. 711, No. 1-2, 2011, pp. 13-27. doi:10.1016/j.mrfmmm.2011.03.006 [4] H. Esterbauer, R. J. Schaur and H. Zollner, “Chemistry and Biochemistry of 4-Hydroxynonenal, Malonaldehyde and Re lat ed Al dehy de s,” Free Radical Biology and Medi- cine, Vol. 11, No. 1, 1991, pp. 81-128. doi:10.1016/0891-5849(91)90192-6 [5] S. P. Singh, T. Chen, L. Chen, N. Mei, E. McLain, V. Samokyszyn, et al., “Mutagenic Effects of 4-Hydroxy- nonenal Triacetate, a Chemically Protected form of the Lipid Peroxidation Product 4-Hydroxynonenal, as Assay- ed in L5178Y/Tk+/-Mouse Lymphoma Cells,” Journal of Pharmacology and Experimental Therapeutics, Vol. 313, No. 2, 2005, pp. 855-861. doi:10.1124/jpet.104.080754 Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 9 [6] K. S. Fritz and D. R. Petersen, “An Overview of the Chemistry and Biology of Reactive Aldehydes,” Free Radical Biology and Medicine, 2012. (In Press) doi:10.1016/j.freeradbiomed.2012.06.025 [7] Y. C. Awasthi, R. Sharma, J. Z. Cheng, Y. Yang, A. Sharma, S. S. Singhal, et al., “Role of 4-Hydroxynonenal in Stress-Mediated Apoptosis Signaling,” Molecular As- pect s of Medici ne, Vol. 24, No. 4-5, 2003, pp. 219-230. doi:10.1016/S0098-2997(03)00017-7 [8] P. V. Usatyuk and V. Natarajan, “Hydroxyalkenals and Oxidized Phospholipids Modulation of Endothelial Cy- toskeleton, Focal Adhesion and Adherens Junction Pro- teins in Regulating Endothelial Barrier Function,” Mi- crovascular Research, Vol. 83, No. 1, 2012, pp. 45-55. doi:10.1016/j.mvr.2011.04.012 [9] Y. Yang, A. Sharma, R. Sharma, B. Patrick, S. S. Singhal, P. Zimniak, et al., “Cells Preconditioned with Mild, Tran- sient UVA Irradiation Acquire Resistance to Oxidative Stress and UVA-Induced Apoptosis: Role of 4-Hydro- xynonenal in UVA-Mediated Signaling for Apoptosis,” Journal of Biological Chemistry, Vol. 278, No. 4, 2003, pp. 41380-41388. doi:10.1074/jbc.M305766200 [10] S. O. Abarikwu, A. B. Pant and E. O. Farombi, “4-Hy- droxynonenal Induces Mitochondrial-Mediated Apoptosis and Oxidative Stress in SH-SY5Y Human Neuronal Cells,” Basic & Clinical Pharmacology & Toxicology, Vol. 110, No. 5, 2012, pp. 441-448. doi:10.1111/j.1742-7843.2011.00834.x [11] W. Black, Y. Chen, A. Matsumoto, D. C. Thompson, N. Lassen, A. Pappa, et al., “Molecular Mechanisms of ALDH3A1-Mediated Cellular Protection against 4-Hy- droxy-2-nonenal,” Free Radical Biology and Medicine, Vol. 52, No. 9, 2012, pp. 1937-1944. doi:10.1016/j.freeradbiomed.2012.02.050 [12] P. V. Usatyuk and V. Natarajan, “Role of Mitogen-Acti- vated Protein Kinases in 4-Hydroxy-2-nonenal-Induced Actin Remodeling and Barrier Function in Endothelial Cells,” Journal of Biological Chemistry, Vol. 279, No. , 2004, pp. 11789-11797. doi:10.1074/jbc.M311184200 [13] J. Z. Cheng, S. S. Singhal, M. Saini, J. Singhal, J. T. Piper, F. J. Van Kuijk, et al., “Effects of mGST A4 Transfection on 4-Hydroxynonenal-Mediated Apoptosis and Differen- tiation of K562 Human Erythroleukemia Cells,” Archives of Biochemistry and Biophysics, Vol. 372, No. 1, 1999, pp. 29-36. doi:10.1006/abbi.1999.1479 [14] R. Sharma, A. Sharma, S. Dwivedi, P. Z imniak, S. Awas- thi and Y. C. Awasthi, “4-Hydroxynonenal Self-Limits Fas-Mediated DISC-Independent Apoptosis by Promot- ing Export of Daxx from the Nucleus to the Cytosol and Its Binding to Fas,” Bioche mistry, Vol. 47, No. 1, 2008, pp. 143-156. doi:10.1021/bi701559f [15] P. Zimniak, M. A. Eckles, M. Saxena and Y. C. Awasthi, “A Subgroup of Class Alpha Glutathione S-Transferases. Cloning of cDNA for Mouse Lung Glutathione S-Tran- sferase GST 5.7,” FEBS Letters, Vol. 313, No. 2, 1992, pp. 173-176. doi:10.1016/0014-5793(92)81438-R [16] L. M. Balogh, I. Le Trong, K. A. Kripps, L. M. Shireman, R. E. Stenkamp, W. Zhang, et al., “Substrate Specificity Combined with Stereopromiscuity in Glutathione Trans- ferase A4-4-Dependent Metabolism of 4-Hydroxynone- nal,” Biochemistry, Vol. 49, No. 7, 2010, pp. 1541-1548. doi:10.1021/bi902038u [17] J. Z. Cheng, Y. Yang, S. P. Singh, S. S. Singhal, S. Awasthi, S. S. Pan, et al., “Two distinct 4-Hydroxy-none- nal Metabolizing Glutathione S-Transferase Isozymes Are Differentially Expressed in Human Tissues,” Bio- chemical and Biophysical Research Communications, Vol. 282, No. 5, 2001, pp. 1268-1274. doi:10.1006/bbrc.2001.4707 [18] M. R. Engle, S. P. Singh, P. J. Czernik, D. Gaddy, D. C. Montague, J. D. Ceci, et al., “Physiological Role of mGSTA4-4, a Glutathione S-Transferase Metabolizing 4-Hydroxynonenal: Generation and Analysis of mGsta4 Null Mouse,” Toxicology and Applied Pharmacology, Vol. 194, No. 3, 2004, pp. 296-308. doi:10.1016/j.taap.2003.10.001 [19] M. D. Neely, V. Amarnath, C. Weitlauf and T. J. Monti ne, “Synthesis and Cellular Effects of an Intracellularly Ac- tivated Analogue of 4-Hydroxynonenal,” Chemical Re- search in Toxicology, Vol. 15, No. 1, 2002, pp. 40-47. doi:10.1021/tx010115w [20] J. Xu, Preparation, “Preparation, Culture, and Immortali- zation of Mouse Embryonic Fibroblasts,” John Wiley & Sons, Inc., Hoboken, 2001. [21] R. B. B. Hogan , F. Cost anti ni and E. Lacy, “Manipulating the Mouse Embryo: A Laboratory Manual,” Cold Spring Habor Laboratory Press, Cold Spring Habor, New York, 1994. [22] L. Zimniak, S. Awasthi, S. K. Srivastava and P. Zimniak, “Increased Resistance to Oxidative Stress in Transfected Cultured Cells Overexpressing Glutathione S-Transferase mGSTA4-4,” Toxicology and Applied Pharmacology, Vol. 143, No. 1, 1997, pp. 221-229. doi:10.1006/taap.1996.8070 [23] K. Satoh, S. Yamada, Y. Koike, Y. Igarashi, S. Toyokuni, T. Kumano, et al., “A 1-Hour Enzyme-Linked Immuno- sorbent Assay for Quantitation of Acrolein- and Hydro- xynonenal-Modified Proteins by Epitope-Bound Casein Matrix Method,” Analytical Biochemistry, Vol. 270, No. 2, 1999, pp. 323-328. doi:10.1006/abio.1999.4073 [24] N. P. Singh, M. T. McCoy, R. R. Tice and E. L. Schnei- der, “A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells,” Experimental Cell Research, Vol. 175, No. 1, 1988, pp. 184-191. doi:10.1016/0014-4827(88)90265-0 [25] A. R. Collins, “The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations,” Mo- lecular Biotechnology, Vol. 26, No. 3, 2004, pp. 249-261. doi:10.1385/MB:26:3:249 [26] M. M. Bradford, “A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding,” Analytical Bio- chemistry, Vol. 72, No. 1-2, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3 [27] R. Sawicki, S. P. Singh, A. K. Mondal, H. Benes and P. Zimniak, “Cloning, Expression and Biochemical Charac- terization of One Epsilon-Class (GST-3) and Ten Delta- Class (GST-1) Glutathione S-Transferases from Droso- Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. 10 phila Melanogaster, and Identification of Additional Nine Members of the Epsilon Class,” Biochemical Journal, Vol. 370, Supplement 2, 2003, pp. 661-669. doi:10.1042/BJ20021287 [28] R. Sharma, B. Ellis and A. Sharma, “Role of Alpha Class Glutathione Transferases (GSTs) in Chemoprevention: GSTA1 and A4 Overexpressi ng Human Leukemia (HL60) Cells Resist Sulforaphane and Curcumin Induced Toxic- ity,” Phytotherapy Research, Vol. 25, No. 4, 2011, pp. 563-568. doi:10.1002/ptr.3297 [29] S. P. Singh, M. Niemczyk, D. Saini, Y. C. Awasthi, L. Zimniak and P. Zimniak, “Role of the Electrophilic Lipid Peroxidation Product 4-Hydroxynonenal in the Devel- opment and Maintenance of Obesity in Mice,” Biochem- istry, Vol. 47, No. 12, 2008, pp. 3900-3911. doi:10.1021/bi702124u [30] K. Uchida, S. Toyokuni, K. Nishikawa, S. Kawakishi, H. Oda, H. Hiai, et al., “Michael Addition-Type 4-Hydroxy- 2-Nonenal Adducts in Modified Low-Density Lipopro- teins: Markers for Atherosclerosis,” Biochemistry, Vol. 33, No. 41, 1994, pp. 12487-12494. doi:10.1021/bi00207a016 [31] J. Li, R. Shar ma, B. Patrick, A. Sharma, P. V. Jeyabal, P. M. Reddy, et al., “Regulation of CD95 (Fas) Expression and Fas-Mediated Apoptotic Signaling in HLE B-3 Cells by 4-Hydroxynonenal,” Biochemistry, Vol. 45, No. 40, 2006, pp. 12253-12264. doi:10.1021/bi060780+ [32] A. Sharma, R. Sharma, P. Chaudhary, R. Vatsyayan, V. Pearce, P. V. Jeyabal, et al., “4-Hydroxynonenal Induces p53-Mediated Apoptosis in Retinal Pigment Epithelial Cells,” Archives of Biochemistry and Biophysics, Vol. 480, No. 2, 2008, pp. 85-94. doi:10.1016/j.abb.2008.09.016 [33] S. J. Riedl and Y. Shi, “Molecular Mechanisms of Cas- pase Regulation during Apoptosis,” Nature Reviews Mo- lecular Cell Biology, Vol. 5, No. 11, 2004, pp. 897-907. doi:10.1038/nrm1496 [34] N. N. Danial and S. J. Korsmeyer, “Cell Death: Critical Control Points,” Cell, Vol. 116, No. 2, 2004, pp. 205-219. doi:10.1016/S0092-8674(04)00046-7 [35] D. W. Fairbairn, P. L. Olive and K. L. O’Neill, “The Comet Assay: A Comprehensive Review,” Mutation Re- search, Vol. 339, No. 1, 1995, pp. 37-59. doi:10.1016/0165-1110(94)00013-3 [36] B. P. Sampey, D. L. Carbone, J. A. Doorn, D. A. Drech- sel and D. R. Petersen, “4-Hydroxy-2-nonenal Adduction of Extracellular Signal-Regulated Kinase (ERK) and the Inhibition of Hepatocyte ERK-EST-Like Protein-1-Acti- vating Protein-1 Signal Transduction,” Molecular Phar- macology, Vol. 71, No. 3, 2007, pp. 871-883. doi:10.1124/mol.106.029686 [37] M. F. Favata, K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, et al., “Identification of a Novel Inhibitor of Mitogen-Activated Protein Kinase Kinase,” Journal of Biological Chemistry, Vol. 273, No. 29, 1998, pp. 18623-18632. doi:10.1074/jbc.273.29.18623 [38] B. L. Bennett, D. T. Sasaki, B. W. Murray, E. C. O’Leary, S. T. Sakata, W. Xu, et al., “SP600125, an Anthrapyra- zolone Inhibitor of Jun N-Terminal Kinase,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 98, No. 24, 2001, pp. 13681-13686. doi:10.1073/pnas.251194298 [39] C. L. Manthey, S. W. Wang, S. D. Kinney and Z. B. Yao, “SB202190, a Selective Inhibitor of P38 Mitogen-Acti- vated Protein Kinase, is a Powerful Regulator of LPS- Induced mRNAs in Monocytes,” Journal of Leukocyte Biology, Vol. 64, No. 3, 1998, pp. 409-417. [40] O. Zelphati, Y. Wang, S. Kitada, J. C. Reed, P. L. Felgner and J. Corbeil, “Intracellular delivery of proteins with a new lipid-mediated delivery system,” Journal of Biologi- cal Chemistry, Vol. 276, No. 37, 2001, pp. 35103-35110. doi:10.1074/jbc.M104920200 [41] P. Zimniak, S. S. Singhal, S. K. Srivastava, S. Awasthi, R. Sharma, J. B. Hayden, et al., “Estimation of Genomic Complexity, Heterologous Expression, and Enzymatic Characterization of Mouse Glutathione S-transferase mGS- TA4-4 (GST 5.7),” Journal of Biological Chemistry, Vol. 269, No. 2, 1994, pp. 992-1000. http://www.ncbi.nlm.nih.gov/pubmed/7904605 [42] T. Zhao, S. S. Singhal, J. T. Piper, J. Cheng, U. Pandya, J. Clark-Wronski, et al., “The Role of Human Glutathione S-Transferases hGSTA1-1 and hGSTA2-2 in Protection Against Oxidative Stress,” Archives of Biochemistry and Biophysics, Vol. 367, No. 2, 1999, pp. 216-224. doi:10.1006/abbi.1999.1277 [43] R. Sharma, G. S. Ansari and Y. Awasthi, “Physiological Substrates of Glutathione S-Transferases,” Informa Heal- thcare, 2006, pp. 179-203. [44] M. Dhiman, M. P. Zago, S. Nunez, A. Amoroso, H. Re- menteria, P. Dousset, et al., “Cardiac-Oxidized Antigens Are Targets of Immune Recognition by Antibodies and Potential Molecular Determinants in Chagas Disease Pathogenesis,” PLos One, Vol. 7, No. 1, 2012, p. e28449. doi:10.1371/journal.pone.0028449 [45] B. P. Sampey, B. J. Stewart and D. R. Petersen, “Etha- nol-Induced Modulation of Hepatocellular Extracellular Signal-Regulated Kinase-1/2 Activity via 4-Hydroxy- nonenal,” Jou rnal of Biologic al Chemistry, Vol. 282, No. 3, 2007, pp. 1925-1937. doi:10.1074/jbc.M610602200 [46] R. S. Harry, L. A. Hiatt, D. W. Kimmel, C. K. Carney , K. C. Halfpenny, D. E. Cliffel, et al., “Metabolic Impact of 4-Hydroxynonenal on Macrophage-Like RAW 264.7 Function and Activation,” Chemical Research in Toxi- cology, Vol. 25, No. 8, 2012, pp. 643-1651. doi:10.1021/tx3001048 [47] J. M. C. Gutteridge and B. Halliwell, “The Measurement and Mechanism of Lipid Peroxidation in Biological Sys- tems,” Trends in Biochemical Sciences, Vol. 15, No. 4, 1990, pp. 129-135. doi:10.1016/0968-0004(90)90206-Q [48] K. M. Boyle, J. P. Irwin, B. R. Humes and S. W. Runge, “Apoptosis in C3H-10T1/2 Cells: Roles of Intracellular pH, Protein Kinase C, and the Na+/H+ Antiporter,” Jour- nal of Cellular Biochemistry, Vol. 67, No. 2, 1997, pp. 231-240. http://www.ncbi.nlm.nih.gov/pubmed/9328828 [49] J. E. Chipuk and D. R. Green, “Dissecting p53-Dependent Apoptosis,” Cell Death and Differentiation, Vol. 13, No. 6, 2006, pp. 994-1002. doi:10.1038/sj.cdd.4401908 Copyright © 2013 SciRes. OJApo  K. E. MCELHANON ET AL. Copyright © 2013 SciRes. OJApo 11 [50] R. Simstein, M. Burow, A. Parker, C. Weldon and B. Beckman, “Apoptosis, Chemoresistance, and Breast Can- cer: Insights from the MCF-7 Cell Model System,” Ex- perimental Biology and Medicine (Maywood), Vol. 228, No. 9, 2003, pp. 995-1003. http://www.ncbi.nlm.nih.gov/pubmed/14530507 [51] T. J. Fan, L. H. Han, R. S. Cong and J. Liang, “Caspase Family Proteases and Apoptosis,” Acta Biochimica et Biophysica Sinica (Shanghai), Vol. 37, No. 11, 2005, pp. 719-727. http://www.ncbi.nlm.nih.gov/pubmed/16270150 [52] S. Fulda and K. M. Debatin, “Apoptosis Signaling in Tumor Therapy,” Annals of the New York Academy of Sciences, Vol. 1028, No. 1, 2004, pp. 150-156. doi:10.1196/annals.1322.016 [53] S. Fulda and K. M. Debatin, “Extrinsic versus Intrinsic Apoptosis Pathways in Anticancer Chemotherapy,” On- cogene, Vol. 25, No. 34, 2006, pp. 4798-4811. doi:10.1038/sj.onc.1209608 [54] E. C. Cheung and R. S. Slack, “Emerging Role for ERK as a Key Regulator of Neuronal Apoptosis,” Science’s STKE, Vol. 2004, No. 251, 2004, p. pe45. doi:10.1126/stke.2512004pe45 [55] J. Liu and A. Lin, “Role of JNK Activation in Apoptosis: A Double-Edged Sword,” Cell Research, Vol. 15, No. 1, 2005, pp. 36-42. doi:10.1038/sj.cr.7290262 [56] M. Castro-Caldas, A. N. Carvalho, E. Rodrigues, C. Hen- derson, C. R. Wolf and M. J. Gama, “Glutathione S-Trans- ferase pi Mediates MPTP-Induced c-Jun N-Terminal Kinase Activation in the Nigrostriatal Pathway,” Molecu- lar Neurobiology, Vol. 45, No. 3, 2012, pp. 466-477. doi:10.1007/s12035-012-8266-9 [57] V. Adler, Z. Yin, S. Y. Fuchs, M. Benezra, L. Rosario, K. D. Tew, et al., “Regulation of JNK Signaling by GSTp,” EMBO Journal, Vol. 18, No. 5, 1999, pp. 1321-1334. doi:10.1093/emboj/18.5.1321 [58] Z. Yin, V. N. Ivanov, H. Habelhah, K. Tew and Z. Ronai, “Glutathione S-Transferase p Elicits Protection against H2O2-Induced Cell Death via Coordinated Regulation of Stress Kinases,” Cancer Research, Vol. 60, No. 15, 2000, pp. 4053-4057. http://www.ncbi.nlm.nih.gov/pubmed/10945608 [59] K. Ryoo, S. H. Huh, Y. H. Lee, K. W. Yoon, S. G. Cho and E. J. Choi, “Negative Regulation of MEKK1-Induced Signaling by Glutathione S-Transferase Mu,” Journal of Biological Chemistry, Vol. 279, No. 42, 2004, pp. 43589- 43594. doi:10.1074/jbc.M404359200 [60] S. G. Cho, Y. H. Lee, H. S. Park, K. Ry oo, K. W. Kang, J. Park, et al., “Glutathione S-Transferase Mu Modulates the Stress-Activated Signals by Suppressing Apoptosis Signal-Regulating Kinase 1,” Journal of Biological Che- mistry, Vol. 276, No. 16, 2001, pp. 12749-12755. doi:10.1074/jbc.M005561200 [61] S. Dorion, H. Lambert and J. Landry, “Activation of the p38 Signaling Pathway by Heat Shock Involves the Dis- sociation of Glutathione S-Transferase Mu from Ask1,” Journal of Biological Chemistry, Vol. 277, No. 34, 2002, pp. 30792-30797. doi:10.1074/jbc.M203642200 [62] P. Bhattacharya and A. F. Keating, “Protective Role for Ovarian Glutathione S-Transferase Isoform pi during 7, 12-Dimethylbenz[a]anthracene-Induced Ovotoxicity,” Tox- icology and Applied Pharmacology, Vol. 260, No. 2, 2012, pp. 201-208. doi:10.1016/j.taap.2012.02.014 [63] I. Dimitrova, G. G. Toby, E. Tili, R. Strich, S. C. Kam- pranis and A. M. Makris, “Expression of Bax in Yeast Affects Not Only the Mitochondria but Also Vacuolar Integrity and Intracellular Protein Traffic,” FEBS Letters, Vol. 566, No. 1, 2004, pp. 100-104. doi:10.1016/j.febslet.2004.04.012 [64] S. C. Kampranis, R. Damianova, M. Atallah, G. Toby, G. Kondi, P. N. Tsichlis, et al., “A Novel Plant Glutathione S-Transferase/Peroxidase Suppresses Bax Lethality in Yeast,” Journal of Biological Chemistry, Vol. 275, No. 38, 2000, pp. 29207-29216. doi:10.1074/jbc.M002359200 [65] K. G. Kilili, N. Atanassova, A. Vardanyan, N. Clatot, K. Al-Sabarna, P. N. Kanellopoulos, et al., “Differential Roles of Tau Class Glutathione S-Transferases in Oxida- tive Stress,” Journal of Biological Chemistry, Vol. 279, No. 23, 2004, pp. 24540-24551. doi:10.1074/jbc.M309882200
|