Paper Menu >>
Journal Menu >>
 Journal of Environmental Protec tion, 2013, 4, 51-55 doi:10.4236/jep.2013.41b010 Published Online January 2013 (http://www.SciRP.org/journal/jep) Copyright © 2013 SciRes. JEP 51 Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite Teoh Wah Tzu, Takuma Tsuritani, Kazunori Sato Department of En vi ronment al Engineeri ng, Nagaoka Univers ity of Technology, Nagaoka, Japan. Email: teoh@vos.nagaokaut.ac.j p Received 2013 ABSTRACT The sorption of Pb(II), Cd(II), and Ni(II) toxic metal ions from aqueous solution by composite alginate-bentonite and alginate wa s investigated. The affinity and sorption cap acity of the toxic metal ions for both type of samples were eva- luated. The Langmuir maximum sorption capacity for each toxic metal ion increased for alginate-bentonite as compared to alginate. However, affinity for toxic metal ion remained unchanged for both alginate-bentonite and alginate in the order of Pb(II) > Cd(II) > Ni(II). Alginate-bentonite also shortens the d uration required f or complete sorptio n. Elemen- tary mapping anal ysis depicts the gradient di ffusion of toxic metal ions i nto the centre of alginate -bentonite beads indi- cated that sorption was contributed by surface adsorption and diffusion. Keywords: Toxic Metals; Al ginate; Bentonite; Wastewater 1. Introduction The presence o f toxic metal ions s uch as Pb (II), Cd(II), and Ni(II) in the aqua tic enviro nment is posi ng seriou s health threats to all livings. These toxic metals have long half-life and the toxicity is cumulated in human bodies for long duration. Industrialization and the growth of population is the main contributor in the release of these toxic pollutants. Since toxic metal ions in aquatic envi- ronment are of high mobility and able to spread to large area in short time, the removal of these toxic metal ions from aquatic environment is one of the challenges in science. Efforts to develop effective and efficient sorbent that fulfill both the cost as well as the performance are in grea t demand . Mineral clay such as bentonite, has been found to be capable of removing toxic metal ions in wastewater through sorption process [1]. The abundance of this naturally occurred low cost bentonite makes it a strong candidate for toxic metal ions removal application. However, the use of powder sorbent such as bentonite introduces prac- ticality problems since separation of solid and wastewa- ter is difficult and costly [2]. This problem can be over- come by powder immobilization technique which re- tained the bentonite in suitable polymer support. Utiliz- ing renewable bioresources such as alginate natural po- lysaccharide polymer to immobilize bentonite powder contribute to feasibility of developing low cost sorbent. Apart from the cost and availability, alginate is also known to have good affinity to adsorb divalent toxic metal ions [3 -4] si nce it i s ri c h i n ne ga ti ve l y c ha r ge f unc- tional group such as carboxylate group. Studi es of the alginate-bento ni t e were more focused on the application for drug delivery system and only very limited research focusing on toxic metal ions removal. In this study, the Pb(II), Cd(II), and Ni(II) sorption capacity as well as sorption rate of alginate bead and alginate- bentonite beads was evaluated using data which best- fitted with Langmuir isotherm model. The sorbents were characterized to understand the sorption mechanism. 2. Materials and Method 2.1. Materials Sodium alginate (EP grade) and bentonite powder (EP grade) were used without any further purification process. 0.2 M calcium chloride (CaCl2) solution was prepared from calcium chloride salt (GR grade). Pb(II), Cd(II) and Ni(II) solutions for sorption experiments were prepared from lead nitrate (purity over 99%), cadmium nitrate tetrahydrate (purity over 99%), and nickel nitrate hex- ahydrate (purity over 99%). The toxic metal ions con- centration was determined by Inductively Coupled Plas- ma Atomic Emissio n Spectroscopy/ICP -AES (Shi madz u, ICP-7510), standard calibration was carried out using 100 mg/L Pb(II), 100 mg/L Cd(II), and 100 mg/L Ni(II) standard solutions that diluted to produce 1 mg/L, 10 mg/L and 100 mg/L solution. All the above mentioned chemicals were supplied by Nacalai Tesque.  Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite Copyright © 2013 SciRes. JEP 52 2.2. Alginate-Bentonite Beads Pre paration and Characterization Alginate-bentonite beads were prepared by adding 4.0 g of bentonite into 100 ml of distilled water, the mixture was stirred for 0.5 hour and 1.5 g of sodium alginate was slowly added. The solution was stirred until a uniform creamy color alginate-bentonite solution was formed. To form alginate-bentonite beads with consistent diameter, the solution was dispensed into 0.2 M CaCl2 solution from a syringe (1.2 mm in diameter) using an automated dispensing pump (As One, SPE-1) at fixed height. The beads were immersed in the CaCl2 solution for 3 hours to enhance the polymerization process and subsequently rinsed with distilled water. The gel beads were dried at ambient atmosphere until constant weight was obtained. To prepare of alginate beads, the procedure is similar except bentonite was excluded in the preparation. The morphology of the dried alginate and alginate- bentonite beads before and after sorption experiments were analyze using scanning electron microscope/SEM (Shi madzu, EPMA-2000). To investigate the diffusion of toxic metal ions into the alginate-bentonite beads, ele- mental mapping analysis was carried out on a cross-sec- tioned of post-sorption alginate-bentonite bead. All the samples were sputtered with gold prior to the SEM ob- serva tion and elemental mapping analysis. 2.3. Sorption Experiment Batch isotherm sorption experiments were conducted by placing 0.02 g of sorbent, i.e. alginate beads or alginate- bentonite bead in respe cti ve 50 ml gla ss bottle c ontaining 30 ml of various concentration of Pb(II), Cd(II), and Ni(II) solution. The toxic metal ions concentration used in this experiment was 0.2 mM, 0.4 mM, 0.6 mM, 0.8 mM, 1.0 mM, 1.5 mM. The bottles were agitated at 100 rpm usi ng a water bath s haker (BT 100, Ya ma to Scientific) for 24 hours; the temperature of the waterbath was set at 303 K. Final concentration of toxic metals ion after the sorption process was determined by ICP-AES. To study the sorption against time, the samples were placed in 100 ml of 1 mM Pb(II), Cd(II), and Ni(II) solu- tion respectively; 3 ml of the toxic metal ions solution was sampled at the specified interval, namely 0.5 hour (h), 1 h, 2 h, 3 h, 6 h, 12 h, and 24 h. T he concentratio n of Pb(II), Cd(II), and Ni(II) at interval time was deter- mined by ICP-AES. 3. Discussions 3.1. Characterization of the Beads SEM was employed to determine the surface properties of the samples before and after the toxic metal ions sorp- tion. The surface morphology of alginate and algi- nate-bentonite bead was given in Figure 1. SEM analysis a b c d Figure 1. Surface morphology for: (a) alginate bef ore sorp- tion, (b) alg inate beads af ter s orptio n, (c) alginate-bentonite befo re s o rption, and (d) a lginate-bentonite after sorption. a b c d 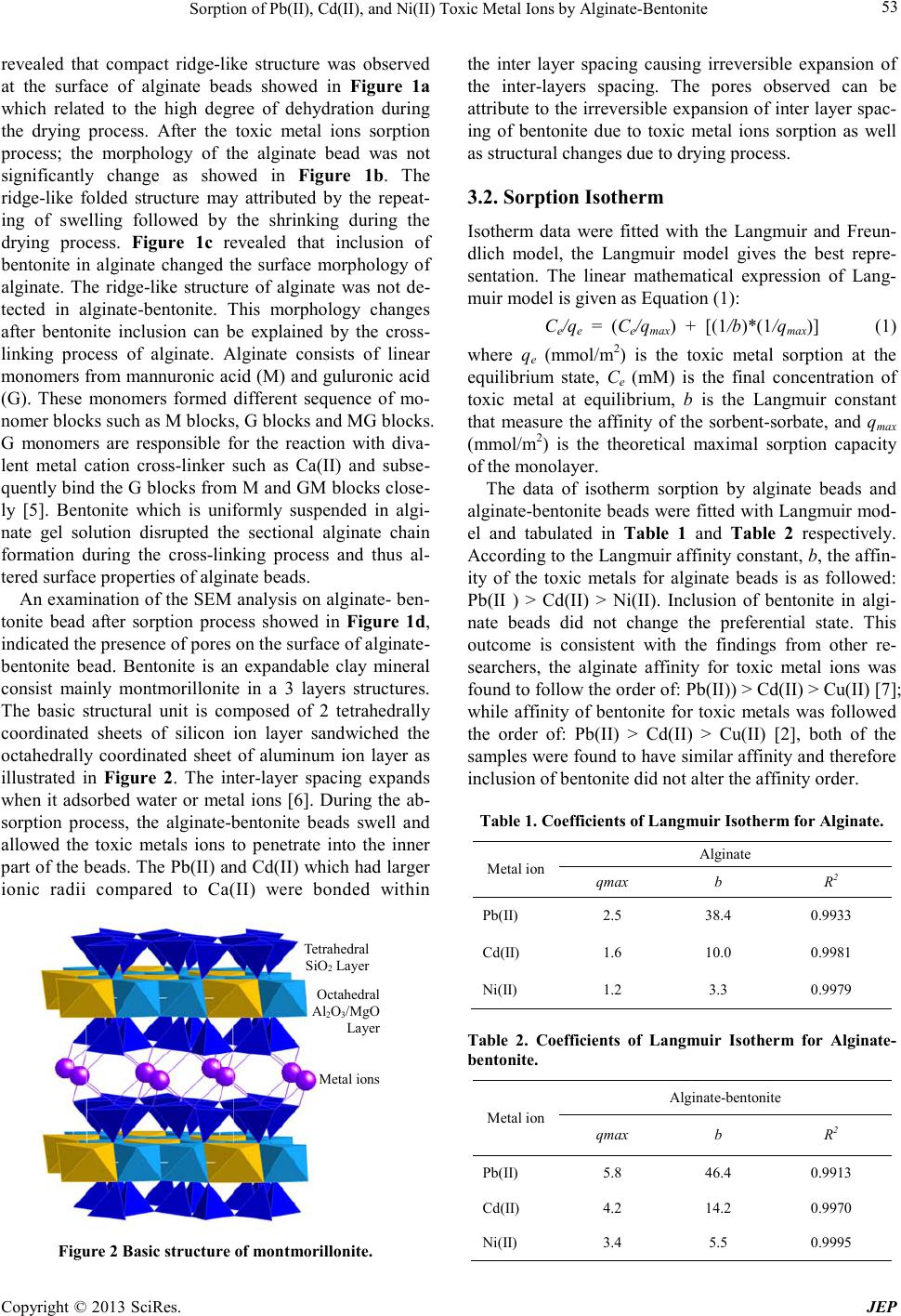 Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite Copyright © 2013 SciRes. JEP 53 revealed that compact ridge-like structure was observed at the surface of alginate beads showed in Figure 1a which related to the high degree of dehydration during the drying process. After the toxic metal ions sorption process; the morphology of the alginate bead was not significantly change as showed in Figure 1b. The ridge-like folded structure may attributed by the repeat- ing of swelling followed by the shrinking during the drying process. Figure 1c revealed that inclusion of bentonite in alginate changed the surface morphology of alginate. The ridge-like structure of alginate was not de- tected in alginate-bentonite. This morphology changes after bentonite inclusion can be explained by the cross- linking process of alginate. Alginate consists of linear mono mers fro m ma nnuroni c aci d (M) and guluro nic ac id (G). These monomers formed different sequence of mo- nomer blocks such as M blocks, G blocks and MG blocks. G monomers are responsible for the reaction with diva- lent metal cation cross-linker such as Ca(II) and subse- quentl y bind the G blocks from M and GM blocks close- ly [5]. Bentonite which is uniformly suspended in algi- nate gel solution disrupted the sectional alginate chain formation during the cross-linking process and thus al- tered surface properties of alginate beads. An e xaminat ion o f the S EM a nalysi s on al ginate - ben- tonite bead after sorption process showed in Figure 1d, indicated the presence of pores on the surface of alginate- bentonite bead. Bentonite is an expandable clay mineral consist mainly montmorillonite in a 3 layers structures. The basic structural unit is composed of 2 tetrahedrally coordinated sheets of silicon ion layer sandwiched the octahedrally coordinated sheet of aluminum ion layer as illustrated in Figure 2. The inter-layer spacing expands when it adsorbed water or metal ions [6]. During the ab- sorption process, the alginate-bentonite beads swell and allowed the toxic metals ions to penetrate into the inner part of the beads. The Pb(II) and Cd(II) which had larger ionic radii compared to Ca(II) were bonded within Octahedral Al2O3/MgO La y er Metal ions Tetrahedral SiO2 Layer Figure 2 Basic struct ure of montmorillonit e. the inter layer spacing causing irreversible expansion of the inter-layers spacing. The pores observed can be attribute to the irr eversible e xpansion of inter la yer spac- ing of bentonite due to toxic metal ions sorption as well as structural changes due to drying process. 3.2. Sorption Isotherm Isotherm data were fitted with the Langmuir and Freun- dlich model, the Langmuir model gives the best repre- sentation. The linear mathematical expression of Lang- muir model is given as Equation (1): Ce/qe = (Ce/qmax) + [(1/b)*(1/qmax)] (1) where qe (mmol/m2) is the toxic metal sorption at the equilibrium state, Ce (mM) is the final concentration of toxic metal at equilibrium, b is the Langmuir constant that measure the affinity of the sorbent-sorbate, and qmax (mmol/m2) is the theoretical maximal sorption capacity of the monolayer. The data of isotherm sorption by alginate beads and alginate-bentonite beads were fitted with Langmuir mod- el and tabulated in Table 1 and Table 2 respectively. Accor din g to t he La ng muir a f finit y co nsta nt, b, the affin- ity of the toxic metals for alginate beads is as followed: Pb(II ) > Cd(II) > Ni(II). Inclusion of bentonite in algi- nate beads did not change the preferential state. This outcome is consistent with the findings from other re- searchers, the alginate affinity for toxic metal ions was found to follow the order of: Pb (II)) > Cd(II) > Cu(II) [7]; while affinity of bentonite for toxic metals was follo wed the order of: Pb(II) > Cd(II) > Cu(II) [2], both of the samples were found to have similar affinity and therefore inclusion of be nto nite did not a lte r the affinity order. Table 1. Coefficients of Langmuir Isotherm for Alginate. Metal ion Alginate qmax b R2 Pb(II) 2.5 38.4 0.9933 Cd(II) 1.6 10.0 0.9981 Ni(II) 1.2 3.3 0.9979 Table 2. Coefficients of Langmuir Isotherm for Alginate- bentonite. Metal ion Alginate-bentonite qmax b R2 Pb(II) 5.8 46.4 0.9913 Cd(II) 4.2 14.2 0.9970 Ni(II) 3.4 5.5 0.9995 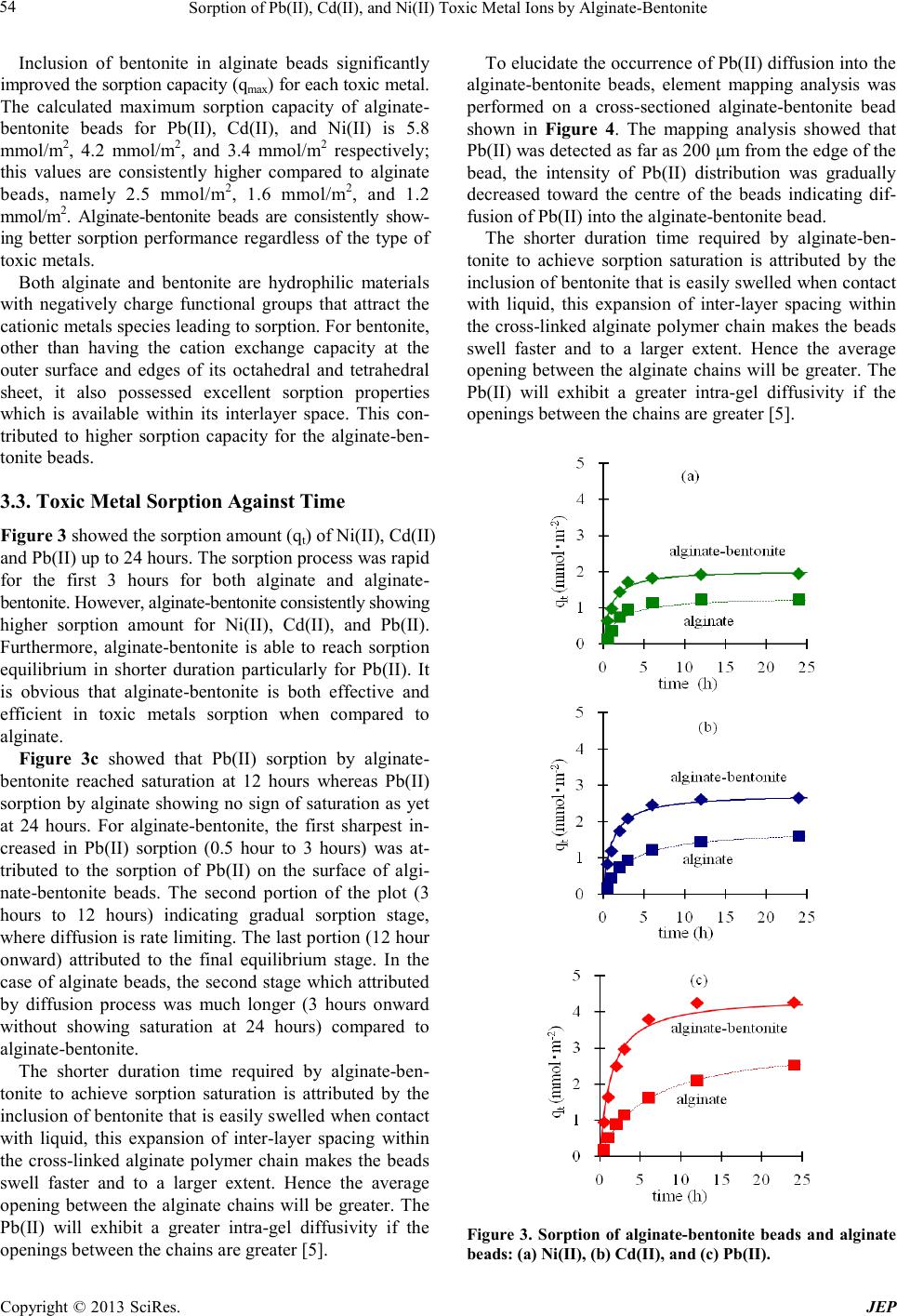 Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite Copyright © 2013 SciRes. JEP 54 Inclusion of bentonite in alginate beads significantly improved the sorption capacity (qmax) for each toxic metal. The calculated maximum sorption capacity of alginate- bentonite beads for Pb(II), Cd(II), and Ni(II) is 5.8 mmol/m2, 4.2 mmol/m2, and 3.4 mmol/m2 respectively; this values are consistently higher compared to alginate beads, namely 2.5 mmol/m2, 1.6 mmol/m2, and 1.2 mmo l / m2. Alginate-bentonite beads are consistently show- ing better sorption performance regardless of the type of toxic metals. Both alginate and bentonite are hydrophilic materials with negatively charge functional groups that attract the cationic metals sp ecies lead i ng to so rptio n. For b entonite, other than having the cation exchange capacity at the outer surface and edges of its octahedral and tetrahedral sheet, it also possessed excellent sorption properties which is available within its interlayer space. This con- tributed to higher sorption capacity for the alginate-ben- tonite beads. 3.3. Toxic Metal Sorption Against Time Figure 3 showe d the sorption amount (qt) of Ni(II), Cd(II) and Pb(II) up to 24 hours. The sorption process was rapid for the first 3 hours for both alginate and alginate- bentonite. However, alginate-bentonite consistently showing higher sorption amount for Ni(II), Cd(II), and Pb(II). Furthermore, alginate-bentonite is able to reach sorption equilibrium in shorter duration particularly for Pb(II). It is obvious that alginate-bentonite is both effective and efficient in toxic metals sorption when compared to alginate. Figure 3c showed that Pb(II) sorption by alginate- bentonite reached saturation at 12 hours whereas Pb(II) sorption by alginate sho wing no sign of saturation as yet at 24 hours. For alginate-bentonite, the first sharpest in- creased in Pb(II) sorption (0.5 hour to 3 hours) was at- tributed to the sorption of Pb(II) on the surface of algi- nate-bentonite beads. The second portion of the plot (3 hours to 12 hours) indicating gradual sorption stage, where diffusion is rate limiting. The last portion (12 hour onward) attributed to the final equilibrium stage. In the case of alginate beads, the second stage which attributed by diffusion process was much longer (3 hours onward without showing saturation at 24 hours) compared to alginate-bentonite . The shorter duration time required by alginate-ben- tonite to achieve sorption saturation is attributed by the inclusion of be ntonite that is easily s welled when contact with liquid, this expansion of inter-layer spacing within the cross-linked alginate polymer chain makes the beads swell faster and to a larger extent. Hence the average opening between the alginate chains will be greater. The Pb(II) will exhibit a greater intra-gel diffusivity if the openings between the chains are greater [5]. To elucidate the occurrence of Pb(II) diffusion into the alginate-bentonite beads, element mapping analysis was performed on a cross-sectioned alginate-bentonite bead shown in Figure 4. The mapping analysis showed that Pb(II) was detected as far as 200 μm from the edge of the bead, the intensity of Pb(II) distribution was gradually decreased toward the centre of the beads indicating dif- fusion of Pb(II) into the alginate-bentonite bead. The shorter duration time required by alginate-ben- tonite to achieve sorption saturation is attributed by the inclusion of be ntonite that is easily s welled when contact with liquid, this expansion of inter-layer spacing within the cross-linked alginate polymer chain makes the beads swell faster and to a larger extent. Hence the average opening between the alginate chains will be greater. The Pb(II) will exhibit a greater intra-gel diffusivity if the openings between the chains are greater [5]. Figure 3. Sorption of alginate-bentonite beads and alginate beads: (a) Ni(II), (b) Cd(II) , and (c) Pb(II).  Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite Copyright © 2013 SciRes. JEP 55 100 μm 200 μm Figure 4. Ele menta l map pi ng a naly sis at t he cr oss -sectioned of post-sorption alginate-bentonit e bead. To elucidate the occurrence of Pb(II) diffusion into the alginate-bentonite beads, element mapping analysis was performed on a cross-sectioned alginate-bentonite bead shown in Figure 4. The mapping analysis showed that Pb(II) was detected as far as 200 μm from the edge of the bead, the intensity of Pb(II) distribution was gradually decreased toward the centre of the beads indicating dif- fusion of Pb(II) into the alginate-bentonite bead. 4. Conclusion Alginate-bentonite beads had evidently shown effective- ness and efficiency for the sorption of Pb(II), Cd(II) and Ni(II) toxic metals compared to alginate. The toxic met- als affinity is in the order of Pb(II) > Cd(II) > Ni(II). Langmuir sorption capacity coefficient shows that inclu- sion of bentonite in alginate increased the surface active sites for toxic metals sorption. Mapping analysis con- firmed the diffusion of Pb(II) into the beads, attributed by the expandable inter-layer spacing of bentonite that enlarged the openings between the chains of alginate polymer. Combination of this low cost bentonite and alginate sorbents had proven to show synergy effects in the sorption of toxic metals from aquatic environment. 5. Acknowledgements The authors gratefully acknowledge the support of re- search grant from Nagaoka University of T echno log y. REFERENCES [1] S. Kubilay, R. Gurkan, A. Savran, T. Sahan, “Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solution by adsorption onto natural bentonite”, Adsorption, Vol. 13, 2007, pp . 41-51. [2] G. Bereket, A.Z. Aroguz, M.A. Ozel, “Removal of P b(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by ad- sorption on bentonite”, J. Collo id Inter fa ce Sci ., Vol. 187, 1997, pp . 338-343. [3] F .A. Ab u Al-Ru b, M.H. El -Naas, F. Benyahia, I. Ashour, “Biosorption of nickel on blank alginate beads, free and immobilized algal cells”, Process Biochem., Vol. 39, 2004, pp . 1767-1773. [4] H. Katircioglu, A. Aslim, A.R. Turker, T. Atici, Y. Beyatli, “Removal of cadmium(II) ion from aqueous sys- tem by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from fresh- water (Morgan Lake)”, Bioresource Technol.,Vol. 99, 2008, pp . 4185-4191. [5] B. Amsden, N. Turner, “Diffusion Characteristic of cal- cium alginat e gel”, Biotechnol. Bioeng., Vol. 65, 1999, pp. 605-610. [6] E. Eren, A. Afsin, “An investigation of Cu(II) adsorption by raw and acid activated bentonite: A combined poten- tiometric, thermodynamic, XRD, IR, DTA study”, J. Ha- zard Mater., V ol. 15 1, 2008, pp. 682-691. [7] S. K. Papageorgiou, F. K. Katsaros, E. P. Kouvelos , J. W. Nolan, H. L. Deit, N. K. Kanellopoulos, “Heavy metal sorption by calcium alginate beads from Laminaria digi- tata”, J. Hazard Mater. , B13 7, 2006, pp. 1765-1772. |

