Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35324,5 pages DOI:10.4236/fns.2013.48A027
Lipid Oxidation in Mechanically Deboned Chicken Meat: Effect of the Addition of Different Agents
![]()
Department of Food Technology, Federal Technological University of Paraná (UTFPR), Francisco Beltrão, Brazil.
Email: *alexandre@utfpr.edu.br
Copyright © 2013 Juliana Bigolin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 3rd, 2013; revised July 3rd, 2013; accepted July 10th, 2013
Keywords: MDCM; Lipid Oxidation; Agents
ABSTRACT
The study evaluated the effect of sodium chloride (1.5%), sodium erythorbate (0.5% and 1.0%) and ascorbic acid (0.1% and 0.2%) on inhibiting lipid oxidation in mechanically deboned chicken meat (MDCM). The peroxide, acidity, pH, color and odor values of the samples were determined on the 1st, 3rd and 5th days. Treatments with sodium erythorbate and ascorbic acid had significant influence (p ≤ 0.05) on the peroxide, acidity and pH values. Ascorbic acid and erythorbate sodium were especially effective in reducing lipid oxidation in mechanically deboned chicken meat.
TABLE I. 1. Introduction
TABLE II.
Mechanically deboned chicken meat (MDCM) is a widely used raw material for the production of meat patties. According to the Sindiavipar Journal [1], in 2010 the state of Paraná—Brazil, exported more than 1 million tons of chicken meat to more than 120 countries worldwide, accounting for over 1.69 billion dollars.
On account of technological modernization, MDCM has branched out mainly due to its simplicity to obtain and process industrialized products [2].
The mechanically separated poultry meat emerged in the late 1950s in the United States. MDCM emerged in order to satisfy consumer preferences for chicken cuts and fillets instead of whole chickens, thus giving rise to finding ways to take advantage of chicken backs, necks and bones from deboning techniques [3].
Mechanically separated poultry meat is a widely used industrial process that enables using non-prime raw materials or with no commercial value. Because it is a lowcost raw material, MDCM is widely used as a protein source in the formulation of industrial products [4]. Due to the high lipid content in its composition, it is very susceptible to oxidative reactions. These reactions occur from the metabolic transformations of fatty acids in the meat. Under the current law, the maximum fat allowed in MDCM is of 30% [5].
In addition to the high lipid content, the obtention method is another aspect that contributes to the occurrence of lipid oxidation in MDCM as in the processing the incorporation of minerals occurs from the fragmented bones. Furthermore, the grinding during process increases the product’s contact surface with light and oxygen, the oxidation accelerating agents. The lifespan of MDCM is of 24 hours at a temperature below 4˚C, 72 hours if kept at 0˚C and 90 days if stored at a temperature of −18˚C [5].
Oxidation is a natural process in meat and its derivatives, and its occurrence is potentiated in the presence of oxidative agents. An alternative for retarding lipid oxidation in mechanically separated meats is the addition of antioxidants and preservatives, but this practice is not allowed by the current law. Thomas [6] defines antioxidants as any substance that, when present at low concentrations compared with those of an oxidizable substrate, significantly retards or inhibits oxidation of the substrate. Antioxidants can act by different mechanisms protecting the target lipids from the onset of oxidation or impeding the propagation phase as described in Mariutti and Bragagnolo [7]. Sodium erythorbate and ascorbic acid are widely used antioxidants in the food industry, while sodium chloride is used as a preservative, which also provides flavor to meat and meat products. According to Rafecas and others [8], deciding on an antioxidant should take into account factors such as laws, cost and consumer preference for natural antioxidants.
This study evaluated the inhibition of lipid oxidation in mechanically deboned chicken meat with the addition of sodium chloride, sodium erythorbate and ascorbic acid, therefore the pH, acidity, peroxide index, color and odor of the MDCMs were evaluated.
2. Material and Methods
2.1. Samples
Mechanically separated meat samples were used in this work, which were obtained from poultry processing plants located in the state of Paraná, employing mechanical deboning methods (Poss Limited, mod. PDE 2500).
After collecting the MDCM samples, they were immediately sent to the Physical-Chemical Laboratory of the production unit and divided into the following treatments: control treatment—in natura MDCM sample; 1.5% sodium chloride treatment (SC); 0.5% sodium erythorbate treatment (SE1); 1.0% sodium erythorbate treatment (SE2); 0.1% ascorbic acid treatment (AA1); 0.2% ascorbic acid treatment (AA2).
The following were used: Sodium chloride, minimum purity of 99.8% (Romani), ascorbic acid, minimum purity of 99% (Makeni Chemicals) and sodium erythorbate, minimum purity of 98% (ICL Brasil).
The samples were appropriately homogenized, separated into smaller samples, supplemented with the agents 1.5% sodium chloride, 0.5% and 1.0% sodium erythorbate and 0.1% to 0.2% ascorbic acid, and then identified and placed in refrigerator at 0˚C, for 72 hours in order to simulate the conservation process of MDCM during the production, transport, packaging and industrialization process of the final product.
The analyses were performed 24 hours (1st day), 72 hours (3rd day) and 120 hours (5th day) after the production process of MDCM.
2.2. pH Determination
For the pH determination, 50 grams of sample were used and 20 ml of distilled water were added as described by BRASIL [9], and the reading was performed using a potentiometer (Mettler Toledo, mod DL 25).
2.3. Determination of Acidity
The method used was based on the extraction of fat using a mixture of ethyl ether: ethyl alcohol 2:1, with continuous stirring for 2 hours or 8 hours of rest and neutralization titration with NaOH 0.1 mol/L and phenolphthalein as an indicator, and the values were expressed as mg of NaOH/g of fat [10].
2.4. Determination of the Peroxide Value
The fat extraction was performed by mixing ethyl ether: petroleum ether 1:1 and the iodometric test procedure, and the values were expressed in mEq/Kg fat [11].
2.5. Overall Evaluation
The overall evaluation was performed using the visual and olfactory perception of the characteristics of the MDCMs.
2.6. Statistical Analysis
The statistical data analysis was performed by Analysis of Variance (ANOVA) and the results were submitted to Tukey’s test, with reliability ≥95%, using the Statistica 6.0 software program for Windows (StatsoftTM, Inc., Tulsa, USA).
3. Results and Discussion
Regarding the color of MDCM, the addition of sodium chloride caused, between the 1st and 3rd day, the formation of dark pigments on the mechanically deboned chicken meat of the SC treatment, and also of the Control treatment. The sodium erythorbate treatments in concentrations of 0.5% and 1.0% exhibited noticeable influence on the color parameter, between the 1st and 5th day of the product’s shelf life. In contrast, the treatments with 0.1% and 0.2% ascorbic acid had little effect on the color, and the color was characteristic only in the first shelf life day, with the characteristic color only in the first shelf life day, while between the 3rd and 5th days, the samples displayed a pink color with dark spots. However, the control treatment displayed a change in color, a pink-brown color after the 2nd shelf life day.
Meat quality changes can be perceived by changes in taste, color, texture, nutritional value and the production of potentially toxic compounds. Regarding the color of the meat, Liu and others [12] argue that there is a hypothesis that certain free radicals produced during lipid oxidation act directly on the pigment, resulting in its oxidation or damaging the pigment’s reduction systems. Generally, the surface of the meat exposed to oxygen is bright red because the myoglobin is oxygenated, but this color can deteriorate during storage and exposure to light due to the oxidation of lipids and pigments, which can change the heme group and start the oxidation of myoglobin, which causes the color loss of the meat [13]. With regards to odor, it was observed that on the first day all MDCM treatments showed a characteristic odor. On the third day the SE1 and SE2 treatments showed no change in odor in comparison to the first day, while the SC, AA1 and AA2 and Control treatments presented acidic odors. On the fifth day the SE1 and SE2 treatments also had a characteristic odor, whereas the AA1, AA2 and Control treatments demonstrated a sulfide odor. Lipids contribute to desirable traits of juiciness, flavor and aroma, although easily oxidized, resulting in the formation of toxic and undesirable products [14].
The strong antioxidant effect of Erythorbate prevents the development of oxidative rancidity when applied in concentrations above 100 ppm, and in lower concentrations it can accelerate the development of oxidative rancidity [15]. Of the natural antioxidants, Ascorbic acid stands out as one of the most used agents in food products [16].
Table 1 shows the results for pH, peroxide and acidity values on the 1st shelf-life day of MDCM. The results for acidity were lower (p ≤ 0.05) in the SE1, SE2, AA1 and AA2 treatments when compared with the Control, whereas when compared with Control, the SC treatment did not differ. The SE1, SE2, AA1 and AA2 treatments showed no differences between treatments, whereas SC differed from treatment AA2. Therefore both chemical agents used showed similar effects on the acidity of MDCM after one day of storage. The peroxide value did not differ (p ≤ 0.05) between treatments on the 1st day of MDCM as no peroxides were detected in the studied samples in detectable amounts by the methodology.
Regarding pH determination, it was observed that the pH in the SE1 and AA2 treatments was higher (p ≤ 0.05) than in the Control.
Table 2 shows the mean values of pH, peroxide and acidity on the 3rd day of MDCM with and without the addition of agents. After the 3rd shelf-life day SE1 was the only treatment with the lowest acid value, therefore more effective in inhibiting acidity (p ≤ 0.05) than the other treatments. However, SE1 did not differ from the AA1 and AA2 treatments (p ≤ 0.05).
In an experiment using chicken breast fillets Mantilla and others [17] reported that considerable pH variations occurred only after the 12th day.
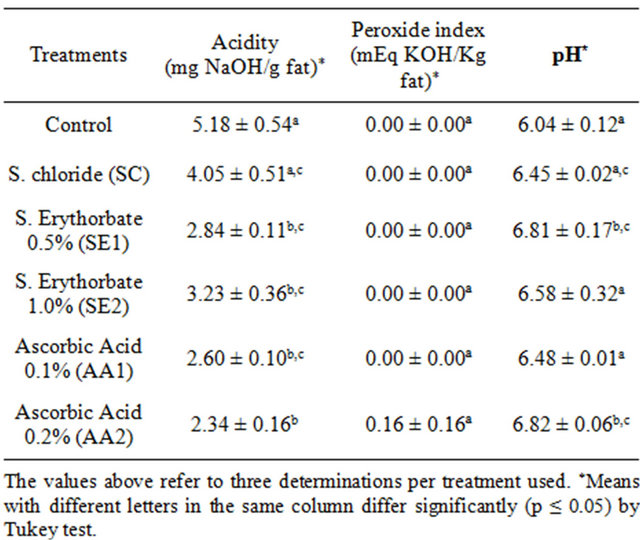
Table 1. Mean values of pH, peroxide and acidity value on the 1st day of MDCM, with and without addition of agents.
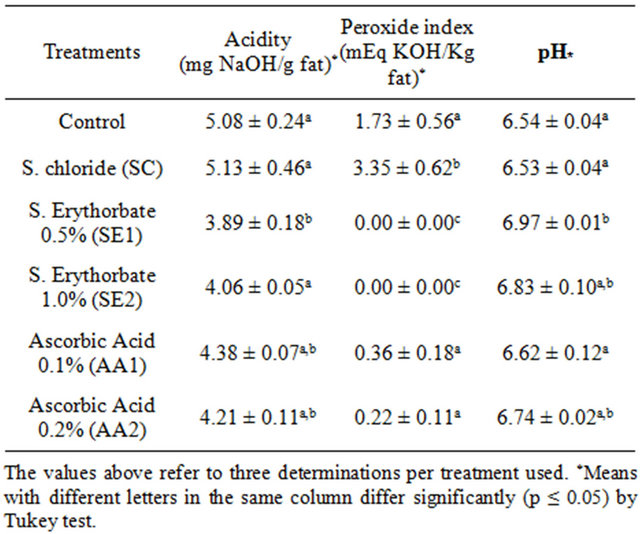
Table 2. Mean values of pH, peroxide and acidity value on the 3rd day of MDCM, with and without the addition of agents.
In contrast, Pollonio [18], in a study with mechanically separated chicken meat observed that the pH results ranged between 6.20 and 6.37 in frozen and stored samples for six months.
The peroxide values on the 3rd day showed that the SE1 and SE2 treatments were more efficient in inhibiting peroxide formation in MDCM (p ≤ 0.05). The AA1 and AA2 treatments were less effective than other agents used to inhibit the formation of peroxides, with similar results for the Control treatment (p ≤ 0.05). Regarding the pH determination, it was found that the treatments with the lowest acidity levels had higher pH levels.
Table 3 shows the values obtained on the 5th analysis day of MDCM. There were no significant differences among the SE1, SE2, AA1 and AA2 treatments regarding the acidity analysis, and they were lower than the SC treatment and Control (p ≤ 0.05). It was found that treatments with sodium erythorbate and ascorbic acid were effective in inhibiting acidity of MDCM, displaying on the 5th day a similar acidity to the Control on the 3rd day. The peroxide determination showed that SC treatment had higher peroxide values than the other treatments, but this treatment showed no difference when compared with Control (p ≤ 0.05). According to Olivo [19], peroxides are products of the first lipid oxidation step and are not toxic, but the secondary products of oxidation can be toxic. According to Bellaver and Zanotto [20], the peroxide index (PI) is commonly used to detect the rancidity of fat. A rancid odor probably indicates that the oxidation process is in its final phase. A low PI in its final phase coincides with high concentrations of secondary products (aldehydes, ketones, alcohols and esters).
MDCM is highly susceptible to rancidity due to the

Table 3. Mean values of pH, peroxide and acidity value on the 5th day of MDCM, with and without the addition of agents.
large area in contact with oxygen and obtention manner, displaying high levels of fat, lipids and calcium in its composition. Antioxidants are widely used to retard or inhibit lipid oxidation in foods. The antioxidants’ mechanisms of action happen when competitively binding to oxygen, slowing the initiation step, interrupting the propagation step by destroying or binding the free radicals, inhibiting the catalyzers or stabilizing the hydroperoxides. Antioxidants should not be toxic, display high activity at low concentrations, should concentrate on the surface of the food grease phase, should withstand food processing, and also contribute to the stability of the final product [21].
The pH values obtained showed no difference among the treatments under study—however a reduction was found when compared with the values obtained after the 3rd day, possibly due to the product’s increased acidity after 5th day since acidity corresponds to the base quantity (in mg) (KOH or NaOH) required to neutralize the free fatty acids in 1 g of fat present in the MDCM. The use of sodium erythorbate and ascorbic acid antioxidants, in the levels studied, was effective in inhibiting the formation of peroxides in MDCM until the 5th day under refrigeration. In a study of turkey MDM, it was found that the antioxidants studied, for 7 months under freezing conditions, were less effective in this order; ascorbic acid, Vitamin C in aqueous solution, synthetic vitamin E, and the antioxidant with the highest efficiency over time was rosemary extract [22]. The use of casein-derived bioactive peptides in MDCM was also a natural antioxidant alternative [23]. Hassan and Fan [24] compared the synthetic antioxidants BHA and BHT with cocoa leaf-derived polyphenols, which were less effective, though very similar to the oxidation of MDCM.
According to the data obtained in this work, it was observed that the addition of sodium chloride had no effect on retarding lipid oxidation. This corroborates other authors who claim that sodium chloride should be avoided in fresh meat to be frozen, as it acts as a pro-oxidant, promoting oxidative rancidity and an undesirable brown color of metmyoglobin [25]. According to Torres and others [26], the addition of salt in meat products is problematic for their quality because it has been associated to lipid oxidation and meat discoloration due to the presence of metals acting as catalysts.
4. Conclusions
The treatments with 0.5% (SE1) and 1.0% (SE2) sodium erythorbate were effective in terms of MDCM oxidative rancidity, because they reduced pigment formation and characteristic odors resulting from lipid oxidation. However the treatments with 0.1% (AA1) and 0.2% (AA2) ascorbic acid were effective on the color and odor only on first evaluation day. The treatment with 1.5% sodium chloride (SC) showed no lipid oxidation inhibition.
The results showed that sodium erythorbate and ascorbic acid are effective in reducing oxidative rancidity in mechanically deboned chicken meat, and require further studies to optimize the concentrations added.
5. Acknowledgements
The researchers would like to thank the Research Support Program of the Federal Technological University of Paraná (UTFPR)—Campus Francisco Beltrão.
REFERENCES
- S. Magazine, “Poultry in Paraná,” 2011. http://www.youblisher.com/p/90930-Revista-Sindiavipar-n-20/
- R. M. Gonçalves, J. R. Gonçalves, R. R. Oliveira, R. A. Oliveira and M. E. Lage, “Physical-Chemical Evaluation of and Heavy Metals Contents in Broiler and Beef Mechanically Deboned Meat (MDM) Produced in the State of Goiás, Brazil,” Brazilian Animal Science, Vol. 10, No. 2, 2009, pp. 553-559.
- C. Móri, E. A. Garcia, C. Andrighetto and K. Pelicia, “Mechanical Separated Poultry Meat,” 2006. http://www.veterinaria.org/revistas/redvet/n040406/040602.pdf
- M. G. Pereira, “Application of Natural Antioxidants in Mechanically Separated Meat (MDCM) Poultry,” Ph.D. Dissertation, Federal University of Santa Maria, Santa Maria, 2009.
- Brazil Ministry of Agriculture, Livestock and Food Supply, “Normative Instruction. 04, March 31, 2000. Approves the Technical Regulation for Establishment of Identity and Quality of mechanically separated meat (MDCM) Poultry, Beef and Pork,” Federal Official Gazette, Section 1, 2000, pp. 6-10.
- M. J. Thomas, “The Role of Free Radicals and Antioxidants,” Nutrition, Vol. 16, No. 7/8, 2000, pp. 16-18.
- L. R. B. Mariutti and N. Bragagnolo, “Lipid Oxidation in Chicken Meat and the Impact of the Addition of Sage (Salvia officinalis L.) and Garlic (Allium sativum L.) as Natural Antioxidants,” Journal of the Adolfo Lutz Institute, Vol. 68, No. 1, 2009, pp. 1-11.
- M. Rafecas, F. Guardiola, M. Illera, R. Codony and J. Boatella, “Liquid Chromatographic Determination of Phenolic Antioxidants in Bakery Products,” Chromatographia, Vol. 822, No. 2, 1998, pp. 305-309. doi:10.1016/S0021-9673(98)00601-3
- Brazil Ministry of Agriculture, Livestock and Food Supply, “Normative Instruction. 20, July 21, 1999. Analytical Methods for Physical and Chemical Control of Meat Products and Their Ingredients—Salt and Brine—DAS,” Federal Official Gazette, Section 1, 1999.
- IAL, “Analytical Standards Institute Adolfo Lutz: Physico-Chemical Methods for Analysis of Foods,” Melhoramentos, Brasilia, 2005.
- AOAC, “Official Methods of the Association of Official Agricultural Chemist’s International,” Gaitheersburg, Washington DC, 2000.
- Q. Liu, C. Lanari and D. M. Schaefer, “A Review of Dietary Vitamin E Supplementation for Improvement of Beef Quality,” Journal of Animal Science, Vol. 73, No. 10, 1995, pp. 3131-3140.
- M. P. Lynch, J. P. Kerry, D. J. Buckley, C. Faustman and P. A. Morrissey, “Effect of Dietary Vitamin E Supplementation on the Color and Lipid Stability of Fresh, Frozen and Vacuum-Packaged Beef,” Meat Science, Vol. 52, No. 1, 1999, pp. 95-99. doi:10.1016/S0309-1740(98)00153-3
- M. Shimokomaki, R. Olivo, N. N. Terra and B. D. G. M. Franco, “Current Issues in Science and Technology of meat,” Varela, São Paulo, 2006.
- J. I. Gray and A. M. Pearson, “Rancidity and WarmedOver Flavor,” In: A. M. Pearson and T. R. Dutson, Eds., Advances in Meat Research, Van Nostrand Reinhold Co., New York, 1987, pp. 221-269.
- E. N. Frankel, “Antioxidants in Lipid Foods and Their Impact on Food Quality,” Food Chemistry, Vol. 57, No. 1, 1996, pp. 51-55. doi:10.1016/0308-8146(96)00067-2
- P. S. S. Mantilla, E. B. Santos, C. A. Conte Júnior, S. B. Mano, H. C. Vital and R. M. Franco, “Spoilage Bacteria on Chicken Fillets Packed in Air, Vacuum and Irradiated: Bacteriological Parameters of Development and Commercial Term,” Tropical Agricultural Research, Vol. 39, No. 4, 2009, pp. 271-277.
- M. A. R. Pollonio, “Study of the Functional Properties of Myofibrillar Proteins and Lipid Oxidation of Mechanically Deboned Chicken Meat,” Ph.D. Dissertation, University of Campinas, Campinas, 1994.
- R. Olivo, “The World of Chicken: Meat Production Chain of Chicken,” Do Autor, Criciúma, 2006.
- M. Oetterer, M. A. B. Regitano-d’Arce and M. H. F. Spoto, “Essentials of Food Science and Technology,” Manole, Barueri, 2006.
- M. B. Mielnik, K. Aaby and G. Skrede, “Commercial Antioxidants Control Lipid Oxidation in Mechanically Debones Turkey Meat,” Meat Science, Vol. 65, No. 3, 2003, pp. 1147-1155. doi:10.1016/S0309-1740(02)00345-5
- C. Bellaver and D. L. Zanotto, “Quality Parameters in Fats and Protein Byproducts of Animal Origin,” 2010. http://www.cnpsa.embrapa.br/sgc/sgc_arquivos/palestras_k9r8d4m.pdf
- K. Rossini, C. P. Z. Noreña, F. C. Olivera and A. Brandelli, “Casein Peptides with Inhibitory Activity on Lipid Oxidation in Beef Homogenates and Mechanically Deboned Poultry Meat,” LWT—Food Science and Technology, Vol. 42, No. 4, 2009, pp. 862-867.
- O. Hassan and L. S. Fan, “The Anti-Oxidation Potential of Poliphenol Extract from Cocoa Leaves on Mechanically Deboned Chicken Meat (MDCM),” LWT—Food Science and Technology, Vol. 38, LWT—Food Science and Technology 2005, pp. 315-321.
- R. O. Roça, “Cura de Carnes,” 2010. http://pucrs.campus2.br/~thompson/Roca111.pdf.
- E. A. F. S. Torres, C. D. Rimoli, R. Olivo, M. K. Hatano and M. Shimokomaki, “Role of Iodized Salt on Lipid Oxidation in Beef and Pork Burgers (Mixed) or Chicken,” Food Science and Technology, Vol. 18, No. 1, 1998, pp. 49-52.
NOTES
*Corresponding author.

