Paper Menu >>
Journal Menu >>
 Modeling and Numerical Simulation of Material Science, 2013, 3, 23-27 Published Online January 2013 (http://www.SciRP.org/journal/mnsms) Copyright © 2013 SciRes. MNSMS Synthesis of Barium Nickel Titanium Oxide Stabilized by Citric Acid K. Y. Chew, M. Abu Bakar*, N.H.H. Abu Bakar Nanoscience Research Laboratory, School of Chemical Sciences, Universiti Sains Malaysia, 11800 USM Pulau Pinang, Malaysia. Email: bmohamad@usm.my Received 2012 ABSTRACT Barium nickel titanium oxide particles (Ba2NiTi5O13) were synthesized in the presence of citric acid by using a two step sol-gel method followed by calcination. The addition of citric acid as a stabilizer (mole ratio of 0.5, 1.0, 2.0, 4.0) re- sulted in the formation of Ba2NiTi5O13 particles with various morphology (i.e. sphere, cube, rod). These various mor- phology changes were deduced to be caused by citric acid that tends to absorbed on certain dimension of the Ba2NiTi5O13 particles when different concentration of citric acid was added. Besides that, the growth of Ba2NiTi5O13 particles from incorporation of bulky micelles which act as a protective 'shell' that control particle sizes by attaching on the surfaces of particles. Keywords: Barium Nickel Titanium Oxide; Citric Acid; Morphology 1. Introduction Ceramic perovskite materials have gained a lot of interest because they are exploited as industrial electrodes [1], catalysts [2, 3], electronic components [4] and so on. The high demand for these materials is ascribed to their unique characteristics such as high mechanical strength, high temperature stability and lowered sintering temperature of ceramic materials as well as high surface area. Recently, perovskite structured metal oxides (AB O 3) has become a promising heterogenous catalyst as it provides features suitable for green sustainable techno lo g y [2,3] and also has high porosity which re- sulted in a better catalyst life [3]. The structure of perovskite ceramics (ABO3) is where A is the larger cations from alkaline metal groups whereas B is the smaller cations from transition metal groups. Either site of A/B or both cations are responsible for the respective catalytic reaction. Therefore, this material can reduce the usage amount of the commonly expensive noble metals such as Pd, Pt and Rh [3]. Certain binary, ternary and quaternary metal oxides that contain 2, 3 and 4 different metal elements also belong to the perovskite-type mixed oxides materials. Barium nickel titanium oxide (Ba2NiTi5O13) is an example of quaternary perovskite structured material [5]. Conventional solid state milling methods require high calcination temperatures and pressure, which can cause agglomeration of particles which in turn can cause the coarsening the surface of particles. This is undesirable as it can affect the chemical and physical properties of the particles [6,7]. Therefore, many wet chemical synthesis methods have been introduced to compensate these problems such as hydrothermal [8], coprecipitation [9] molten salt route [10] and sol gel [11]. The sol gel method is a common wet chemical method because it exhibits several advan- tages such as low processing cost, excellent chemical homogeneity, low processing temperature as well as good stoichiometrically control of the elements of the synthesized compound. However, refined shaped and non agglomerated particles are commonly achieved by addition of supports which are then difficult to be re- moved. These supports such as polymers and surfactants have often been used in these supports assisted sol gel synthesis are able to control particles sizes and modify particles morphology [12]. However, total removal of carbonaceous substances by calcinations after the incor- poration of these supports is difficult [12]. Citric acid stabilized sol gel method, which is commonly known as the Pechini method, are practiced by using citrate salts or mixtures of co mmo n salts with citric acids as precursor. This citrate will form complexations via its carboxylate groups with empty orbitals of metals [13]. Because of this, citric acid is well known as a stabilizer in nanoparticles synthesis by functioning as ligand, or act ing as a particle surface modifier [4,7,14]. Therefore, the objective of this study is *Corresponding author. 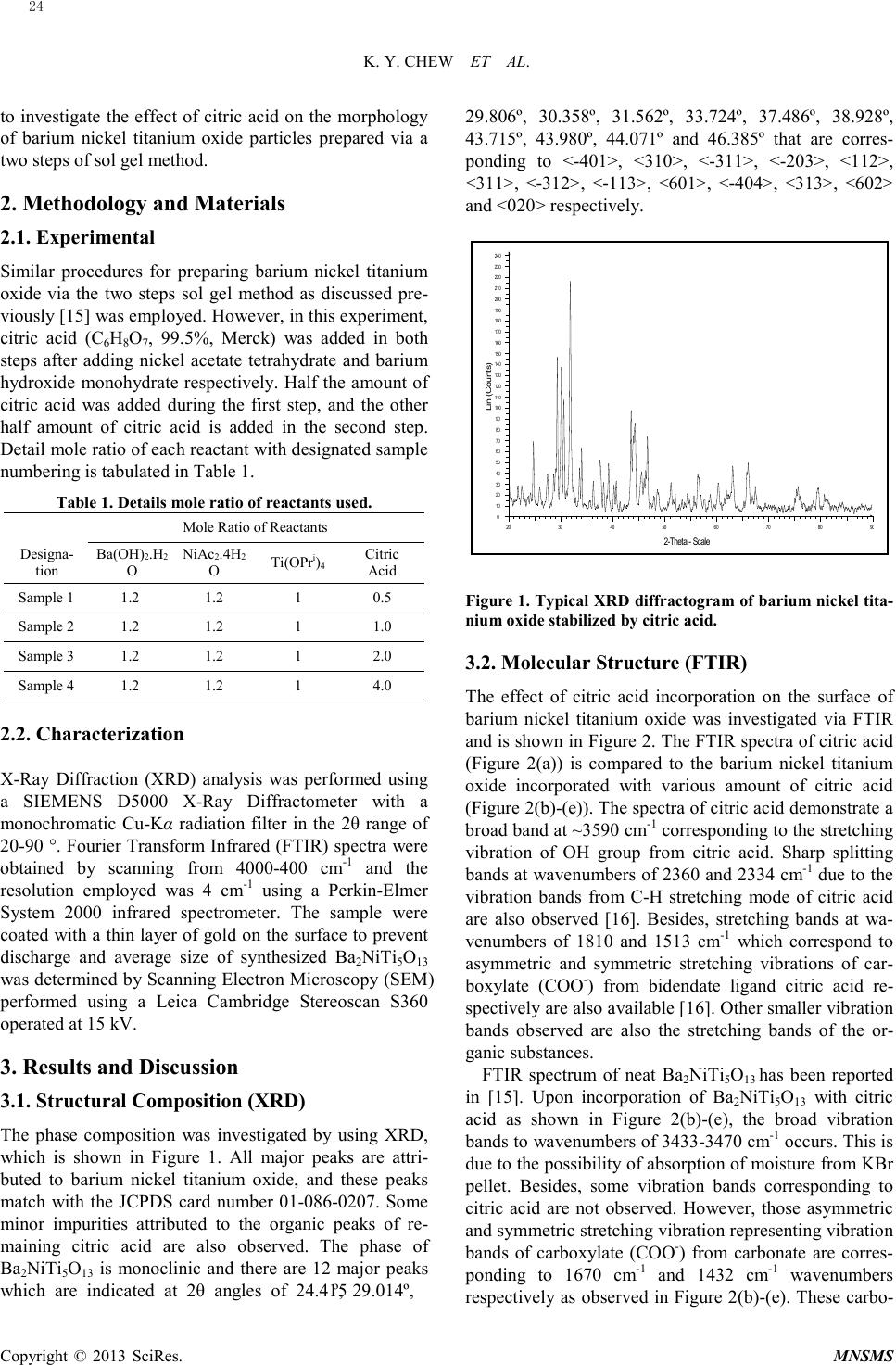 K. Y. CHEW ET AL. Copyright © 2013 SciRes. MNSMS to investigate the effect of citric acid on the morphology of barium nickel titanium oxide particles prepared via a two steps of sol gel method. 2. Methodology and Materials 2.1. Experimental Similar procedures for preparing barium nickel titanium oxid e via the two steps sol gel method as discussed pre- viously [15] was employed. However, in this experiment, citric acid (C6H8O7, 99.5%, Merck) was added in both steps after adding nickel acetate tetrahydrate and barium hydroxide monohydrate respectively. Half the amount of citric acid was added during the first step, and the other half amount of citric acid is added in the second step. Detail mole ratio of each reactant with designated sample numbering is tabulated in Table 1. Table 1. Details mole ratio of reactants used. Mole Ratio of Reactan ts Desi gn a- tion Ba(OH)2.H2 O Ni Ac2.4H2 O Ti(O Pr i)4 Citric Acid Sample 1 1 .2 1 .2 1 0.5 Sample 2 1 .2 1 .2 1 1.0 Sample 3 1 .2 1 .2 1 2.0 Sample 4 1 .2 1 .2 1 4.0 2.2. Characterization X-Ray Diffraction (XRD) analysis was performed using a SIEMENS D5000 X-Ray Diffractometer with a monochromatic Cu-Kα radiation filter in the 2θ range of 20-90 °. Fourier Transform Infrared (FTIR) spectra were obtained by scanning from 4000-400 cm-1 and the resolution employed was 4 cm-1 using a Perkin-Elmer System 2000 infrared spectrometer. The sample were coated with a thin layer of gold on the surface to prevent discharge and average size of synthesized Ba2NiTi5O13 was determined by Scanning Electron Microscopy (SEM) performed using a Leica Cambridge Stereoscan S360 operated at 15 kV. 3. Results and Discussion 3.1. Structural Composition (XRD) The phase composition was investigated by using XRD, which is shown in Figure 1. All major peaks are attri- buted to barium nickel titanium oxide, and these peaks matc h with the JCPDS card number 01-086-0207. Some minor impurities attributed to the organic peaks of re- maining citric acid are also observed. The phase of Ba2NiTi5O13 is monoclinic and there are 12 major peaks which are indicated at 2θ angles of 24.415º, 29.014º, 29.806º, 30.358º, 31.562º, 33.724º, 37.486º, 38.928º, 43.715º, 43.980º, 44.071º and 46.385º that are corres- ponding to <-401>, <310>, <-311>, <-203>, <112>, <311>, <-312>, <-113>, <601>, <-404>, <313>, <602> and <020> respectively. Figure 1. Typical XRD diffractogram of barium nickel tita- nium oxide stabilized by citric acid. 3.2. Molecular Structure (FTIR) The effect of citric acid incorporation on the surface of barium nickel titanium oxide wa s investigated via FTIR and is shown in Figure 2. The FTIR spectra of citric acid (Figure 2(a)) is compared to the barium nickel titanium oxide incorporated with various amount of citric acid (Figure 2(b)-(e)). The spectra of citric acid demonstrate a broad band at ~3590 cm-1 corresponding to the stretching vibration of OH group from citric acid. Sharp splitting bands at wavenumbers of 2360 and 2334 cm-1 due to the vibration bands from C-H stretching mode of citric acid are also observed [16]. Besides, stretching bands at wa- venumbers of 1810 and 1513 cm-1 which correspond to asymmetric and symmetric stretching vibrations of car- boxylate (COO-) from bidendate ligand citric acid re- spectively are also available [16]. Other smaller vibration bands observed are also the stretching bands of the or- ganic substances. FTIR spectrum of neat Ba2NiTi5O13 has been reported in [15]. Upon incorporation of Ba2NiTi5O13 with citric acid as shown in Figure 2(b)-(e), the broad vibration bands to wavenumbers of 3433-3470 cm-1 occurs. This is due to the possibility of absorption of moisture from KBr pellet. Besides, some vibration bands corresponding to citric acid are not observed. However, those asymmetric and symmetric stretching vibration representing vibration bands of carboxylate (COO-) from carbonate are corres- ponding to 1670 cm-1 and 1432 cm-1 wavenumbers respectively as observed in Figure 2(b)-(e). These carbo- Lin (Counts) 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 2-Theta - Scale 20 30 40 50 60 70 80 90 24  K. Y. CHEW ET AL. Copyright © 2013 SciRes. MNSMS nate bands formed from calcination of citric acid with addition of Ba2NiTi5O13. This may indicate that incorporation of citric acid on Ba2NiTi5O13 particles by forming complexation has occurred previously before calcination [16]. In addition, 857 cm-1 wavenumber are corresponded to asymme tric stretching of carbonate [15]. Formation of carbonate in Ba2NiTi5O13 even after calci- nations may be due to incomplete calcinations of organic substances in those samples. On the other hand, a broad absorption at wavenumber ~540 cm-1 represents charac- teristic stretching vibration of Ti-O from perovskite phase structure formation as in Ba2NiTi5O13 [17] can also be observed. Therefore, incorporation of citric acid on Ba2NiTi5O13 has occurred. Figure 2. FTIR spectra of (a) citric acid; (b) Sample 1; (c) Sample 2 (d) Sample 3 and (e) Sample 4 after calcination at 900 °C for 8 hours. 3.3. Morphology of Ba2NiTi 5O13 (SE M) The morphology of citric acid stabilized Ba2NiT i 5O13 is not homogeneously spherical as observed for the neat Ba2NiTi5O13 [15]. In Sample 1 which is shown in Figure 3, when the mole ratio of citric acid used is 0.5, cubic shape d of Ba2NiTi5O13 particles were formed. An inhomogeneous size of particles existed (average size = 0.78 ± 0.56 μm). By continuously increasing the mole ratio of citric acid to 1.0 (Sample 2) as shown in Figure 4, the particles fo rmed is still cubic shaped ho wever with better size distribution (0.35 ± 0 .13 μ m). When the mole ratio of citric acid is 2.0 (Sample 3) as shown in Figure 5, some smal l cubic shaped particles still exist (average size = 0.31 ± 0.18 μm) however most of the particles are in the form of rods with an average length 7.01 ± 3.19 μm. By adding citric acid up to a mole ratio of 4.0 (Sample 4) which is shown in Figure 6, neither cube shape nor rod shape particles can be seen. Instead spherical shaped particles with an average size of 0.60 ± 0.39 μm particles are observed. The particles size transformation of barium nickel tita- nium oxide with increasing amount of citric acid as sta- bilizer can be explained according to the ‘protective shell ’ concept. When the amount of citric acid increases, the precursor nuclei are repulsed by the increasing amount of steric hindrance from the long carbon chain of citric acid. Thus, the precursor nuclei are forced apart before they have the chance to collide with each other and form bigger precursor nuclei. Therefore, the larger the amount of citric acid, the smaller the particles formed. Nevertheless, the spherical particle sizes are larger than the non-citric acid incorporated on Ba2NiTi5O13 particles reported in [15]. In this case, citric acid may be facilitat- ing formation of bigger micelles and thus bigger particles as compared to non-citric acid incorporated Ba2NiT i 5O13 particles as the formation of them do not need to be re- stricted by the micelles. Citric acid is believed to act as a surfactant. When dissolved in water, surfactant attracts with each other to form micelles. These micelles are the core center of particles formation. As observed, the morphology transformation of barium nickel titanium oxide occurred with different amounts of citric acid. Lower amounts of citric acid addition helped the surfactant to form rod shaped particles. Nevertheless, when the amount of citric acid increases high enough as in Sample 4, citric acid tends to form spherical micelles that facilitate the formation of spherical particles. Citric acid may be tends to absorbed on certain dimension of Ba2NiTi5O13 par- ticles when different amount of citric acid is added. When the mole ratio of citric acid is 0.5%, citric acid is absorbed on both horizontal and vertical axial forming cubic shaped Ba2NiTi5O13 particles. As increasing the citric acid concentration, the increased amount of citric acid tends to absorbed more on horizontal dimension and thus forming rod shaped Ba2NiTi5O13 particles. However, when the mole ratio of citric acid increases to 4.0%, the excess of citric acid are absorbed on every dimension of Ba2NiTi5O13 particles and thus forming many smaller and spherical shaped particles. Figure 7 shows the tentative schematic diagram of these morphology changes of Ba2NiTi5O13 particles. Therefore, citric acid is not only able to control the size of Ba2NiTi5O13 particles, but also able to control the morphology of particles. 4. Conclusion Complexation of citric acid incorporation on Ba2NiTi5O13 was found to occur. Formation of a protecting shell of citric acid was deemed to be able to stabilize as well as modify the morphology of core barium nickel titanium oxide particles. Rod shaped or cubic shaped particles formed when small amount of citric acid was used. However, when the citric acid con- tent wa s increased to 4.0 mole %, more homogenous and nearly spherical particles with an average size of 0.60 ± 0.39 μm wer e observed. 25  K. Y. CHEW ET AL. Copyright © 2013 SciRes. MNSMS 5. Acknow l edge ments This work is financially supported by the USM Research University Postgraduate Research Grant Scheme (RUPGRS). Great appreciation is given to USM Fellowship and to lab technicians for helping in operating instruments and equipments. Figure 3. SEM micrograph of Sample 1 magnified 20000 times. Figure 4. SEM micrograph of Sample 2 magnified 20000 times. Figure 5. SEM micrograph of Sample 3 magnified 20000 times. Figure 6. SEM micrograph of Sample 4 magnified 20000 times. Figure 7. Tentative schematic diagram for morphology changes of Ba2NiTi5O13 particle s . REFERENCES [1] Y. Shimizu and N. Yamashita, “Solid Electrolyte CO2 sensor using NASICON and Perovskite-Type Oxide Electrode”, Sensors and Actuators, Vol. B62, 2000, pp. 102-106. [2] B. Seyfi, M. Baghalha and H. Kazemian, “Modified La- CoO3 Nano-Perovskite Catalysts for the Enviromental Application of Automotive CO Oxidation”, Chemical Engineering Journal, Vol. 148, 2009, pp. 306-311. [3] M. Misono, “ Recent Progress in the Practical Applica- tions of Heteropolyacid and Perovskite Catalysts: Cata- lytic Technology for the Sustainable Society”, Catalysis Today, Vol. 144, 2009, pp. 285-291. [4] Z.Ž. Lazarević, M. Vijatović, Z. Dohčević-Mitrović, N.Ž. Romčević, M.J. Romčević, N. Paunović and B.D. Stojanović, “The Characterization of the Barium Titanate Ceramic Powders Prepared by the Pechini Type Reaction Route and Mechanically Assisted Synthesis”, Journal of European Ceramic Society, Vol. 30, 2010, pp. 623-628. [5] R.D. Adams, R. Layland and C. Payen, “A New Mixed-Metal Titanate. The Synthesis and Characteriza- tion of Ba2Ni Ti5O13”, Chemistry of Materials, Vol. 7, 1995, pp. 2168-2170. [6] A.C. Caballero, J.F. Fernández, C. Moure, P. Durán and Y.-M. Chiang, “Grain Growth Control and Dopant Dis- tribution in ZnO-Doped BaTiO3”, Journal of American Ceramic Society, Vol. 81, 1998, pp. 939-944. [7] V. Vinothini, P. Singh and M. Balasubramanian, “Syn- thesis of Barium Titanate Nanopowder Using Polymeric 26  K. Y. CHEW ET AL. Copyright © 2013 SciRes. MNSMS Precursor Method”, Ceramic International, Vol. 32, 2006, pp. 99-103. [8] M.Z.-C. Hu, V. Kurian, E.A. Payzant, C.J. Rawn, and R.D. Hunt, “Wet-Chemical Synthesis of Monodispersed Barium Titanate Particles - Hydrothermal Conversion of TiO2 Microspheres to Nanocrystalline BaTiO3”, Powder Technology, Vol. 110, 2000, pp. 2-14. [9] S.K. Lee, T.J. Park, G.J. Choi, K.K. Koo and S.W. Kim, “Effects of KOH/BaTi and Ba/Ti ratios on Synthesis of BaTi O 3 Powder by Coprecipitation/Hydrothermal Reac- tion”, Materials Chemistry and Physics, Vol. 82, 2003, pp. 742-749. [10] Y. Zhang, L. Wang and D. Xue, “Molten Salt Route of Well Dispersive Barium Titanate Nanoparticles”, Powder Technology, Vol. 217, 2012, pp. 629-633. [11] S. Sreekantan, A.F.M. Noor, Z.A. Ahmad, R. Othman and A. West, “Structural and Electrical Characteristic of Crystalline Barium Titanate Synthesized by Low Tem- perature Aqueous Method”, Journal of Materials Processing Technology, Vol. 195, 2008, pp. 171-177. [12] S. Zhang, F. Jiang, G. Qu, and C. Lin, “Synthesis of Sin- gle-Crystalline Perovskite Barium Titanate Nanorods by A Combine Route Based on Sol-Gel and Surfac- tant-Templated Methods”, Materials Letters, Vol. 62, 2008, pp. 2225-2228. [13] A. Abreu Jr, S.M. Zanetti, M.A.S. Oliveira and G.P. Thim, “Effect of Urea on Lead Zirconate Tita- nate-Pb (Zr0.52 Ti0.48)O3-Nanopowders Synthezied by the Pechini Method”, Journal of the European Ceramic So- ciety, Vol. 25, 2005, pp. 743-748. [14] K.K. Lee, Y.C. Kang, K.Y. Jung and J.H. Kim, “Prepara- tion of Nano-Sized BaTiO3 Particle by Citric Ac- id-Assisted Spray Pyrolysis”, Journal of Alloys and Compounds, Vol. 395, 2005, pp. 280-285. [15] K.Y. Chew, M. Abu Bakar, “Two Steps Synthesis of Quaternary Metal Titanate of Ba2Ni Ti5O13 and Characte- rization”, Advanced Materials Research, Vol. 364, 2012, pp. 353-358. [16] A. Hardy, J.D’ Haen, M.K. Van Bael and J. Mullens, “An Aqueous Solution-Gel Citratoperoxo-Ti(IV) Precursor: Synthesis, Gelation, Thermo-Oxidative Decomposition and Oxide Crystallization”, Journal of Sol-Gel Science and Technology, Vol. 44, 2007, pp. 65-74. [17] W.-D. Yang, K.-M. Hung and C.-F. Kao, “Preparation and Dielectric Properties of Semiconducting Lantha- num-Doped Strotium Ttitante Ceramics from Titanyl Ci- trate Precursors”, Ceramics International, Vol. 26, 2000, pp. 475-484. 27 |

