Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 908-916 doi:10.4236/jbise.2010.39121 Published Online September 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online September 2010 in SciRes. http:// www.scirp.org/journal/jbise Accelerated chondrogenesis in nanofiber polymeric scaffolds embedded with BMP-2 genetically engineered chondrocytes Robert T. Gorsline1,2, Prasam Tangkawattana3, John J. Lannutti4, Mamoru Yamaguchi3, Christopher C. Kaeding2, Alicia L. Bertone1,2 1Comparative Orthopaedic Research Laboratories, The Ohio State University, Columbus, USA; 2Department of Orthopaedics, College of Medicine, The Ohio State University, Columbus, USA; 3Department of Veterinary Biosciences, College of Veterinary Medicine, The Ohio State University, Columbus, USA; 4Department of Materials Science and Engineering, College of Engineering, The Ohio State University, Columbus, USA. Email: bertone.1@osu.edu Received 28 June 2010; revised 19 July 2010; accepted 20 July 2010. ABSTRACT This study evaluated chondrogenesis within a nanofi- ber polymeric scaffold seeded with isolated untreated chondrocytes, isolated chondrocytes genetically engi- neered with adenoviral (Ad) bone morphogenetic protein (BMP)-2, or isolated chondrocytes genetically engineered with green fluorescent protein (Ad-GFP). Electrospun polycaprolactone scaffolds (150-200 m thickness, 700 m fiber diameter, 30 m pore size) were optimally seeded with 1 x 107 isolated chondro- cytes by using a 20% serum gradient culture system. Chondrocyte- scaffold constructs (untreated, Ad-B- MP-2 and Ad-GFP) were generated from 5 adult ho- rses, cultured in triplicate for 7 or 14 days, and quan- titatively analyzed for cell proliferation (DNA content; Hoechst assay), viability, morphology (confocal mi- croscopy), matrix production (proteoglycan content; DMMB assay), and mRNA expression of collagen I, collagen II, and aggrecan. Chondrocytes transduced with Ad-BMP-2 demonstrated greater cell numbers and significantly greater expression of chondrogenic markers including aggrecan, collagen II, and proteo- glycan through 14 days of culture as compared to un- transduced or Ad-GFP controls. This study demons- trated that chondrocytes can be driven to seed a pol- ycaprolactone nanofiber scaffold by serum gradient and a polycaprolactone nanofiber scaffold containing Ad-BMP2 transduced chondrocytes resulted in grea- ter and accelerated chondrogenesis than controls. Th- is cell engineered construct has potential use in one- step cartilage repair in vivo. Keywords: Nanofiber; Scaffold; Chondrogenesis; BMP- 2; Adenovirus 1. INTRODUCTION Articular cartilage defects heal poorly and combinations of progenito r chondrocytes, bioactive factors, and matri- ces are being applied as focal synthetic devices [1-3]. Synthetic biodegradable polymers have served as scaf- folds for articular cartilage tissue engineering. Studies have demonstrated that properties such as pore size, ma- terial composition, and diameter of the fibers are impor- tant for cell proliferation, adhesion, migration and main- tenance of a chondrogenic phenotype of seeded cells [4-12]. Successful biodegradable polymeric scaffolds permissive to cell and tissue in-growth have been studied both in vitro and in vivo, including for chondrocytes [4,6-13]. Properties of successful scaffolds include in- terconnected pores permissive to cell adhesion, prolif- eration and migration, and fluid transport as well as bio- compatibility. Scaffolds have been created from bioac- tive materials such as plasma, starch, collagen, hydrogel, hydroxyapatite, alginate, and periosteum [9,11,13-15] as well as the synthetic materials such as porous poly (l-lactic acid) [5,8,16], poly (glycerol sebacate) (PGS)[6], poly (1,8 octanediol- co-citrate) (POC) [6], poly (ethyl- ene glycol)-terephtha- late/poly (butylene terephthalate (PEGT/PBT) [17] poly- caprolactone (PCL) [4 - 7 , 1 0 ,12 , 17- 2 3] and poly (gamma- glutamic acid)-graft-chondroitin sul- fate/PCL composites [24], polydioxone [25] and other PCL composites[8,13]. Recent publications support the use of a nanoscale fib- er size and PCL material for tissue engineering of chon- drocytes [4-6,9-10,12,21,23]. Use of a fiber material in a mesh pattern has been reported for chondrocytes an d the optimum fiber material will consider fiber size and topo- graphy. Nanofiber technology and its influence on cell behavior is advancing the science of tissue engineering by providing a fiber on the scale of biologic fibers [18- 20,23]. It is proposed that fibers which closely mimic  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 909 normal extra-cellular matrix or basement membrane str- ucture will show improved cytocompatability. Typically, naturally occurring extra-cellular matrices and basement membranes are composed of proteins including fibrone- ctin, collagen, hyaluronic acid, chondroitin sulfate, der- matan sulfate and proteoglycans. These proteins are fib- rous and are on a nanofiber scale in size. PCL fibers in the 30-1500 nm diameter range have been demonstrated to have optimal structural integrity and, particularly und- er dynamic loading, supported a desirable cellular respo- nse in culture, including chondrocyte proliferation and matrix production [23]. Electrospinning is a well-estab- lished process that can produce a random meshwork of nanofibers, including PCL, with appropriate pore size to support cellular infiltration of the scaffold, includ ing ch- ondrocytes [4-5,9-10,12,23-24], stem cells [21,22,26], glio- ma cells [27], and endothelial cells [28]. Cell-based therapy in conjunction with scaffolds for tissue engineering of cartilage is supported in vitro [3,6, 8,10-12,23,24,26] and in vivo [7,14,17,21-22,25] and is rapidly advancing toward clinical application [1-4]. [1-4] Typically the cell source for cartilage engineering is chondrocytes [3,6-12,14,21,23-25] or mesenchymal stem cells. [17,21-22,26,29-30 ] Specifically for articular carti- lage repair, animal studies support the use of biodegrad- able scaffolds seed ed with morcelized cartilage [25], ch- ondrocytes[7,14,21] or direct injection of mesenchymal stem cells.[31] Supplementation w ith growth f actors, su- ch as transforming growth factor-beta 3, a known regu- lator of cell growth and differentiation, could enhance chondrocyte density and integration [11]. Growth factor members of the TGF beta superfamily, such as the BM- Ps, are particularly beneficial in promoting chondrogen- esis of mesenchymal stem cells [29-34] and can support cartilage matrix production [35,36]. Specifically, bone morphogenetic protein (BMP)-2 can regulate chondroc- yte differentiation in progenitor cells [34], enhance bone formation through the endochondral ossification pathw- ay[31,37], and can increase chondrocyte extracellular ma- trix production in vitro [35-36]. Mesenchymal stem cells genetically engineered to produce BMP-2 can enhance articular cartilage repair during articular fracture repair in vivo [31]. Our study focuses on the ab ility of the BMP-2 gene to support phenotype, proliferation, and matrix pro- duction of chondrocytes suspended in a biodegradable nanofiber PCL scaffold. We investigated whether BMP- 2 could successfully promote chondrogenesis in this 3- dimensional scaffold for potential use in articular carti- lage tissue engineering. 2. METHODS 2.1. Study Design Chondrocytes were isolated and expanded in primary mo- nolayer culture. Chondrocytes were seeded onto the sur- face of three dimensional polycaprolactone nanofiber sca- ffolds and evaluated for scaffold penetration by histology cryosection and morphology by confocal microscopy un- der conditions of fetal bovine serum gradient (10 or 30%), cell seeding density (5.0 × 105/ml, 1.0 × 106 ml, or 5.0 × 106 ml) and duration of cell growth (day 2,7, or 14). The best condition was selected and chondrocytes from 5 hor- ses were seeded onto similar scaffolds, in duplicate, and were evaluated at two time points (day 7 or 14) for three cell preparations; isolated cells (untreated control), iso- lated cells transduced with Ad-GFP (vector control), and isolated cells transduced with Ad human (h) BMP-2 (ex- perimental gene). Chondrocyte transduction and BMP-2 production were confirmed. Outcome assessments were cell proliferation, cell morphology, cell viability, BMP-2 production, extracellular proteoglycan matrix production, and chondrocyte gene expression of Type I and Type II collagen as well as aggrecan. 2.2. Generation of Adenoviral Vector Constructs Recombinant adenoviral vector containing these 1547 base-pairs of human BMP-2 under the cytomegalovirus promoter were propagated [31]. Expression of transgene was verified in cell culture. 2.3. Chondrocyte Preparation Chondrocytes from 5 adult horses [5-9 years] were harv- ested aseptically from articular cartilage of the femorop- atellar joint and isolated by collagenase digestion. Cho- ndrocytes were expanded in monolayer in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Sigma-Aldri- ch, St. Louis, MO) supp lemented with sodium penicillin at a concentration of 50 units/ml, streptomycin at a con- centration of 100 units/ml, and L-glutamine at a concen- tration of 29.2 mg/ml (supplemented DMEM) with 10% fetal bovine serum (FBS). At 75% confluence, cells were lifted, counted and pooled in equal concentrations and cultured in monolayer in flasks. Chondrocyte monolayer flasks had Ad vector transduction with Ad-GFP or Ad- BMP-2 performed at a multiplicity of infection (moi) of 17:1 (Adeno-XTM Rapid Titer Kit, BD Biosciences Clontech, Palo Alto, CA) at 37˚C for a transduction time of 2 h, washed and allowed to incubate overnight to ach- ieve expression of transgene product. Transduction effi- ciency was determined by calculating the percent of cells fluorescing [525 nm wavelength] per microscopic field (200X) in the monolayer chondrocytes treated with Ad- GFP. Chondrocytes were harvested and allocated to PCL culture systems. 2.4. Polycaprolactone Scaffolds Nanofibrous [~700 nm diameter] PCL [MW 40,000,  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 910 Sigma Aldrich, St. Louis, MO] matrices were created through an electrospinning process [18-22] to form 150- 200 m thick 3 dimensional sheets containing ~88% porosity and an average pore size of 30m.[18] Sheets were cut into 25 mm diameter discs using a 25 mm cir- cular leather punch and sterilized by ethanol treatment. 2.5. Chondrocyte Culture on PCL Matrices The 25 mm diameter sterile 3D nano -fibrous PCL matri- ces were placed into Corning Costar Snapwell 12 mm in- sertsTM [Corning Inc., Corning, NY] designed to fit into standard 6-well culture plates. Chondrocytes were seed- ed onto the PCL scaffolds by placing cells in suppleme- nted DMEM into the Snapwell® inserts. Inserts were pla- ced into standard 6-well culture plates containing suppl- emented DMEM with the assigned concentration of FBS to create a gradient of FBS across the scaffold construct. 2.6. Preliminary Study Isolated chondrocytes were cultured on PCL matrices in triplicate for 18 different conditions representing 3 cell seeding densities of 5.0 × 105/ml, 1.0 × 106/ml, or 5.0 × 106/ml, 2 serum gradients of 10% or 30%, and duration of culture of 2, 7 and 14 days. PCL/cell matrices were embedded in OCT medium, snap frozen in liquid nitro- gen, cryosectioned (10 microns), and stained with tolu- idine blue for microscopy or fixed for surface scanning electron microscopy (SEM) to assess fiber pattern and cell distribution. The cell seeding density and serum gradient that supported the greatest number and depth of penetration of chondrocytes in the PCL matrix was se- lected for use in subsequent experiments. 2.7. Chondrogenesis of Genetically Engineered PCL/ Cell Matrices Using the best seeding conditions, isolated chondrocytes from 5 horses were cultured on PCL matrices in 12 repli- cates each of untreated, transduced with Ad-GFP, or tra- nsduced with Ad-hBMP-2 for 14 days. Media were changed on days 2, 7, and 14 and stored at –80°C. Con- st- ructs from at least 2 replicates from each horse from each treatment (untreated, Ad-GFP-treated and Ad-BM- P-2-treated) cultured for 7 or 14 days were quantitatively evaluated for each parameter of cell proliferation (DNA [g/ml]; Hoechst assay), % viability (live/dead stain) and morphology (confocal microscopy), matrix proteo- glycan expression (ng/ml; DMMB assay) and gene ex- pression (mRNA quantitative RT-PCR) of aggrecan (ddCT), collagen 1 (ddCT) and collagen II (ddCT) using equine specific primers and probes. Aggrecan and col- lagen gene expression intensity was expressed as a ration to 18sRNA and between Ad-BMP-2 treated chondro- cytes to untreated and Ad-GFP controls (ddCT). BMP-2 protein concentration (ng/ml; ELISA) in the media and chondrocyte GFP expression intensity were compared among untreated, Ad-GFP-treated and Ad-BMP-2-tre- ated PCL/ cell matrices. 2.8. Transgene and Protein Expression GFP fluorescence was quantified using an in vivo imag- ing system (IVIS®, Xenogen Corporation, Alameda, CA) at day 2 post-transduction. GFP production was quanti- fied as flux (photons of light produced per second per square centimeter per steradian, photons/s/cm2/sr) [31]. Aliquots of media from PCL/cell matrix culture sys- tems were frozen at –80°C on days 2, 7, and 14. Produc- tion of hBMP-2 was quantified using enzyme-linked immunosobent assays (ELISAs) for recombinant human (rh) BMP-2 (Quantikine®, R & D Systems, Minneapolis, MN) and expressed as picograms/milliliter/day. 2.9. Cytomorphology of PCL/ Cell Matrices Cell morphology and viability were quantified using special stains [LIVE/DEAD® Viability Kit, Molecular Probes Inc. OR] and confocal microscopy [Leica DM- 1RE2, Leica Microsystems Inc., Bannockburn, IL]. Cell morphology was scored after staining the cytoskeleton (actin) with phallotoxin (Alexa Fluoro 647 Phalloidin, Molecular Cell Probes Inc. Oreg on) and the nu cleu s with DAPI (Molecular Probes Inc. OR). Three representative fields were scored 0-4 with 0 representing the most healthy adherent cell and 4 representing the most pykno- tic and crenated cell. 2.10. Cellular Content Cellular content was determined by analyzing DNA con- tent of PCL/cell matrices using a modification of the previously described Hoechst 33258 Fluorometeric as- say. Discs of PCL/matrices containing chondrocytes cultured for 7 or 14 days were placed into a 2.0 ml Ep- pendorf tube with 1 ml of papain (Acros Organics) dis- solved at 125 g/ml in sterile 1X PBS pH 6.0, with 5mM cysteine HCl and 5mM Na2EDTA and incubated for 24h at 60˚C. Papain digested samples were centrifuged at 1500 rpm (0.2 rcf) for 30 seconds to pellet debris and th e supernatant isolated. DNA standards of double stranded calf thymus DNA (Sigma) dissolved in TN buffer (50 mM Tris pH 7.5, 150 mM NaCl) and serially diluted ranged from 10 g/ml to 500 g/ml. in a Costar® 96 well, black, clear bottom assay plate (Corning, Inc.) 50l of papain digested sample or standard was placed in 200l of Hoechst 33258 dye (AnaSpec, Inc) diluted to 0.2 g/ml in TN buffer. The plates were read on a UV/Vis spec- trometer (Lambda 45, Perkin Elmer) at an excitation of 360 nm, and emission of 460 nm, with a 430 nm cutoff filter. DNA concentrations of samples were determined 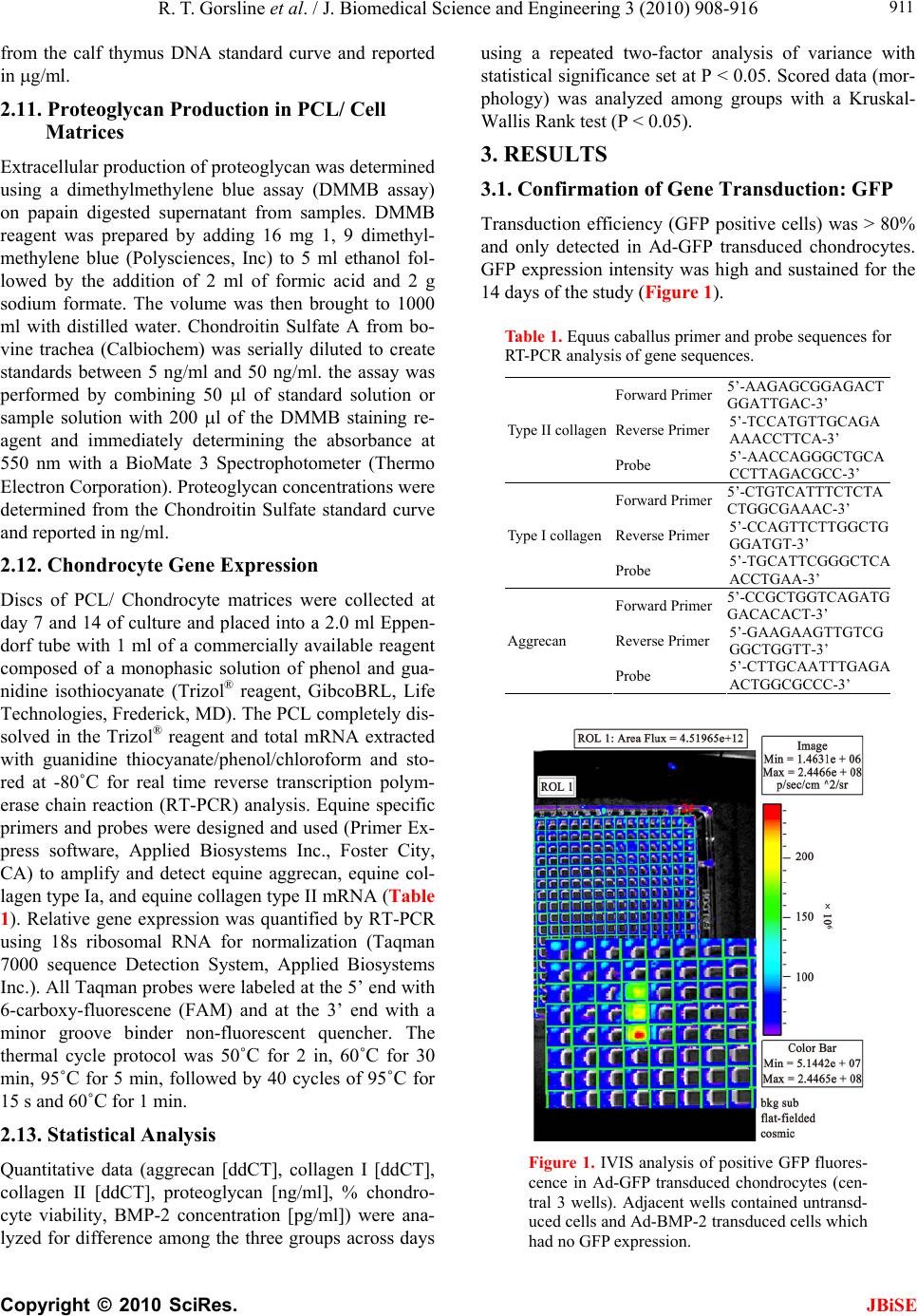 R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 911 from the calf thymus DNA standard curve and reported in g/ml. 2.11. Proteoglycan Production in PCL/ Cell Matrices Extracellular production of proteoglycan was determined using a dimethylmethylene blue assay (DMMB assay) on papain digested supernatant from samples. DMMB reagent was prepared by adding 16 mg 1, 9 dimethyl- methylene blue (Polysciences, Inc) to 5 ml ethanol fol- lowed by the addition of 2 ml of formic acid and 2 g sodium formate. The volume was then brought to 1000 ml with distilled water. Chondroitin Sulfate A from bo- vine trachea (Calbiochem) was serially diluted to create standards between 5 ng/ml and 50 ng/ml. the assay was performed by combining 50 l of standard solution or sample solution with 200 l of the DMMB staining re- agent and immediately determining the absorbance at 550 nm with a BioMate 3 Spectrophotometer (Thermo Electron Corporation). Proteoglycan concentrations were determined from the Chondroitin Sulfate standard curve and reported in ng/ml. 2.12. Chondrocyte Gene Expression Discs of PCL/ Chondrocyte matrices were collected at day 7 and 14 of culture and placed into a 2.0 ml Eppen- dorf tube with 1 ml of a commercially available reagent composed of a monophasic solution of phenol and gua- nidine isothiocyanate (Trizol® reagent, GibcoBRL, Life Technologies, Frederick, MD). The PCL completely dis- solved in the Trizol® reagent and total mRNA extracted with guanidine thiocyanate/phenol/chloroform and sto- red at -80˚C for real time reverse transcription polym- erase chain reaction (RT-PCR) analysis. Equine specific primers and probes were designed and used (Primer Ex- press software, Applied Biosystems Inc., Foster City, CA) to amplify and detect equine aggrecan, equine col- lagen type Ia, and equine collagen type II mRNA (Table 1). Relative gene expression was quantified by RT-PCR using 18s ribosomal RNA for normalization (Taqman 7000 sequence Detection System, Applied Biosystems Inc.). All Taqman prob es were labeled at the 5’ end with 6-carboxy-fluorescene (FAM) and at the 3’ end with a minor groove binder non-fluorescent quencher. The thermal cycle protocol was 50˚C for 2 in, 60˚C for 30 min, 95˚C for 5 min, followed by 40 cycles of 95˚C for 15 s and 60˚C for 1 m i n. 2.13. Statistical Analysis Quantitative data (aggrecan [ddCT], collagen I [ddCT], collagen II [ddCT], proteoglycan [ng/ml], % chondro- cyte viability, BMP-2 concentration [pg/ml]) were ana- lyzed for difference among the three groups across days using a repeated two-factor analysis of variance with statistical significance set at P < 0.05. Scored data (mor- phology) was analyzed among groups with a Kruskal- Wallis Rank test (P < 0.05). 3. RESULTS 3.1. Confirmation of Gene Transduction: GFP Transduction efficiency (GFP positive cells) was > 80% and only detected in Ad-GFP transduced chondrocytes. GFP expression intensity was high and sustained for the 14 days of the study (Figure 1). Table 1. Equus caballus primer and probe sequences for RT-PCR analysis of gene sequences. Forward Primer 5’-AAGAGCGGAGACT GGATTGAC-3’ Reverse Primer 5’-TCCATGTTGCAGA AAACCTTCA-3’ Type II collag en Probe 5’-AACCAGGGCTGCA CCTTAGACGCC-3’ Forward Primer 5’-CTGTCATTTCTCTA CTGGCGAAAC-3’ Reverse Primer 5’-CCAGTTCTTGGCTG GGATGT-3’ Type I collagen Probe 5’-TGCATTCGGGCTCA ACCTGAA-3’ Forward Primer 5’-CCGCTGGTCAGATG GACACACT-3’ Reverse Primer 5’-GAAGAAGTTGTCG GGCTGGTT-3’ Aggrecan Probe 5’-CTTGCAATTTGAGA ACTGGCGCCC-3’ Figure 1. IVIS analysis of positive GFP fluores- cence in Ad-GFP transduced chondrocytes (cen- tral 3 wells). Adjacent wells contained untransd- uced cells and Ad-BMP-2 transduced cells which had no GFP expression.  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 912 3.2. Transgene Protein Expression Gene transduction and protein expression were confir- med for the Ad-BMP-2-transduced chondrocytes in the PCL matrices on days 7 and 14 of culture. BMP concen- tration was > 150,000 pg/ml in Ad-BMP-2-transduced PCL/ cell matrices and <100 pg/ml in untreated and Ad- GFP PCL/ cell matrices. 3.3. Scanning Electron Microscopy Scanning electron microscopy of PCL scaffolds demon- strated a random woven pa ttern of fiber s in the 300-1000 nm range with an average pore size of ~30 nm (Figure 2). Toluidine blue stained frozen cross sections of the PCL/ cell scaffolds demonstrated penetration to ¾ depth by 5 × 105and 1 × 106 cells/ scaffold in a 30% serum gr- adient by day 7 (Figures 3A-B). Cell seeding density of 1 × 105 cells, 10% serum gradient and 2 days were insu- fficient to produce chondrocyte penetration of PCL ma- trices. Confocal microscopy of stained untreated chon- drocytes and GFP expressing chondrocytes seeded on PCL matrices demonstrated an even distribution of cells with normal morphology, sustained gene expression, and cell viability in situ for 14 days (Figures 4A-B). 3.4. Cytomorphology of PCL/ Cell Matrices Cell morphology score (median 4; range 3-4) and nucl- ear morphology score (median 3; rang e 3-4) w as not d if- ferent (P > 0.05) among untreated, Ad-GFP, and Ad- BMP-2 PCL/ cell matrices at both days 7 and 14. Figure 2. Scanning electron microscopy of the scaffold surface (1000X). (a) (b) Figure 3. Toluidine blue positive chondrocytes (a) on the sur- face of the PCL at day 2. Toluidine blue positive chondrocytes (b) penetrating most of the scaffold with a 20% FBS gradient and 1 × 106 cell seeding density. (a) (b) Figure 4. Cells transduced with Ad-GFP and suspended in scaffold demonstrated fluorescence under confocal microscopy (a) and had similar cell numbers and morphology to other transduced and untransduced cells at day 7 (b). 3.5. Cell Content DNA content (g/ml) was not different among untreated, Ad-GFP, and Ad-BMP-2 PCL/cell matrices at day 7, but was significantly decreased in Ad-GFP and increa- sed in Ad-BMP-2 by day 14 (P < 0.01) (Figure 5A). 3.6. Proteoglycan Production in PCL/ Cell Matrices Proteoglycan content (ng/ml) within the PCL/cell matri- ces was significantly greater in the Ad-BMP-2 treated matrices by day 7 and continued to significantly in crease only in the Ad-BMP-2 matrices (P < 0.05) (Fi gure 5B ). 3.7. Chondrocyte Gene Expression Quantitative gene expression was expressed as the inve- rse of Delta CT values so that positive values correlated to increased gene expression. Ad-BMP-2 matrices has significantly greater Type II collagen (P < 0.002) and ag- grecan (P < 0.001) gene expression than untreated and Ad-GFP matrices by day 7 and sustained until at least day 14. There was no difference in type I collagen gene expression among groups (Figures 6A-C). 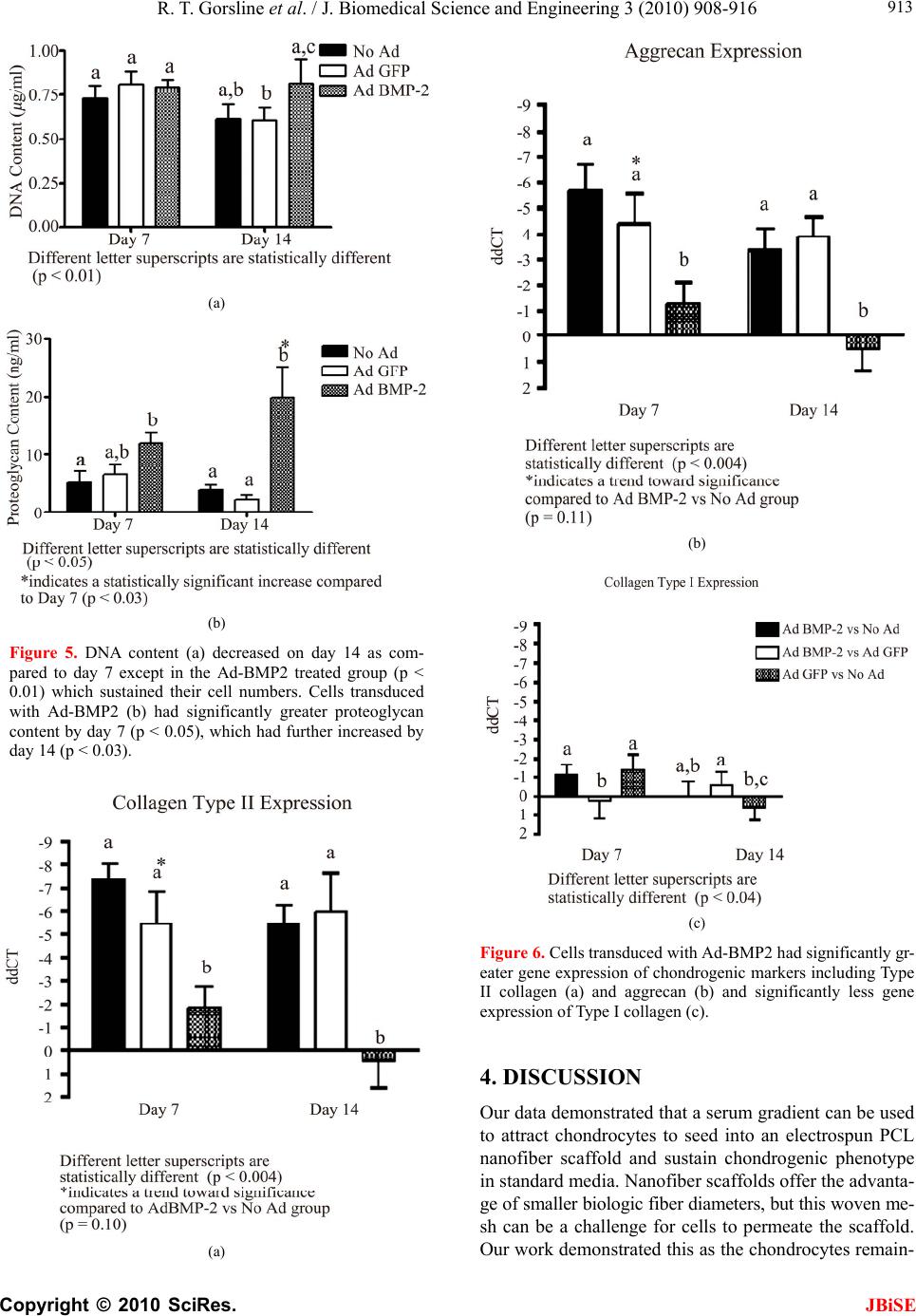 R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 913 (a) (b) Figure 5. DNA content (a) decreased on day 14 as com- pared to day 7 except in the Ad-BMP2 treated group (p < 0.01) which sustained their cell numbers. Cells transduced with Ad-BMP2 (b) had significantly greater proteoglycan content by day 7 (p < 0.05), which had further increased by day 14 (p < 0.03). (a) (b) (c) Figure 6. Cells transduced with Ad-BMP2 had significantly gr- eater gene expression of chondrogenic markers including Type II collagen (a) and aggrecan (b) and significantly less gene expression of Type I collagen (c). 4. DISCUSSION Our data demonstrated that a serum gradient can be used to attract chondrocytes to seed into an electrospun PCL nanofiber scaffold and sustain chondrogenic phenotype in standard media. Nanofiber scaffolds offer the adva nta- ge of smaller biologic fiber diameters, but this woven me- sh can be a challenge for cells to permeate the scaffold. Our work demonstrated this as the chon drocytes remain-  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 914 ed on the surface in the control specimens. This is partic- ularly challenging for relatively nonmobile ch ondrocytes that must dedifferentiate to a fibroblastic phenotype to migrate along fibrils and into pores. In our study, BMP2 sustained chondrogenic phenotype and extracellular ma- trix production while permitting these cells to enter the scaffold. For cartilage engineering, migration is particu- larly relevant to accommodate the thickness of a scaffold desired for cartilage regeneration (100-300 uM). Althou- gh it is possible to incorpor ate cells into a scaffold using an in situ cell seeding protocol [38], or by modifying the electrospinning protocol to produce larger fibers (in the micrometer range with larger pore sizes of >100 uM [23,29]), these methods are potentially handicapped by injury or contamination of cells or loss of the bioactivity advantage of the nanofibrous scale. In vivo generation of a serum gradient in th e base of a cartilage defect is pote- ntially achievable w ith the use of autologous serum pro- ducts placed in the bed of debrided cartilage defects.[40] Commercially available platelet rich plasma or plasma concentrate products have been shown to have increased growth factor concentration of TGFbeta1 and 2 as well as other growth factors that may serve as a chemoattrac- tant to chondrocytes layered on the surface of a scaffold in vivo. [41] Studies to further investigate this are war- ranted. These biologic plasma/fibrin products offer the additional advantage of serving as a biologic glue that may be able to help secure the scaffold into the defect. Fibrin glue is reported to secure cells within full-thick- ness cartilage defects in animal models [42]. In our study, the chondrocytes engineered to express BMP-2 had accelerated and amplified chondrogenesis within the scaffold as compared to control cells. In a pr e- vious in vitro study, chondrocytes incorporated in a hyd- rogel had enhanced matrix generation when the media was supplemented with TGFbeta3, a known chondrog- enic growth factor. [11] Supplementing cells within a scaffold with a growth factor solution is technically dif- ficult in vivo, other than by using a serum/plasma prod- uct as described above. Saturation of the scaffold with a TGFbeta solution is likely to have the TGFbeta rapidly leave the site or be diluted by join t fluid. Engineering of chondrocytes with chon drogenic genes offers an alterna- tive for sustained trophic influences on the cells within the scaffold as demonstrated with BMP2 in our study. Additionally the release of soluable BMP2 will have a paracrine effect on other cells migrating into the site to heal the defect such as bone marrow-derived cells or sy- novial fibroblasts. Other genes such as insulin-like gro- wth factor or TGFbeta may function similarly and could be used in chondrocyte engineering. The process of cho- ndrocyte transduction with adenovirus did not nullify this trophic effect in our study. Methods of cell transdu- ction other than use of adenovirus may offer advantages, most notably a more sustained gene expression [43]. Our data provided evidence that engineering cells can pro- vide enhanced chondrogenesis in Electrospun PCL nan- ofiber scaffolds for use in cartilage tissue eng ineering. Current techniques under investigation for autologous chondrocyte transplantation include direct intraoperative processing of autologous articular cartilage to expose or isolate chondrocytes for immediate reimplantation [25] or chondrocyte expansion in culture prior to reimplanta- tion at a second surgery. G ene transduction with BMP-2 can occur in under 2 hours and could be a practical ge- netic engineering technique to augment autologous cells with genes promoting chondrogenesis in the operating room. Other techniques that focus on autologous chondr- ocyte expansion prior to reimplantation at a second surg- ery would also be readily amenable to genetic engineer- ing as described in this report. This study provides evi- dence that this process holds merit for potential clinical application. Our study was limited to in vitro processing and further studies to confirm this potential in an in vivo animal model are warranted. REFERENCES [1] Simon, T.M. and Jackson, D.W. (2006) Articular carti- lage: Injury pathways and treatment options. Sports Medicine and Arthroscopy Review, 14(3), 146-154. [2] Chung, C. and Burdick, J.A. (2008) Engineering carti- lage tissue. Advanced Drug Delivery Reviews, 60(2), 243-262. [3] Kino-Oka, M., Maeda, Y., Yamamoto, T., Sugawara, K. and Taya, M. (2005) A kinetic modeling of chondrocyte culture for manufacture of tissue-engineered cartilage. Journal of Bioscience and Bioengineering, 99(3), 197-207. [4] Thorvaldsson, A., Stenhamre, H., Gatenholm, P. and Walkenstrom, P. (2008) Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules, 9(3), 1044-1049. [5] Li, W.J. and Tuan, R.S. (2009) Fabrication and applica- tion of nanofibrous scaffolds in tissue engineering. Cur- rent Protocols in Cell Biology, Chapter 25(25), Unit 25.2. [6] Jeong, C.G. and Hollister, S.J. (2010) A comparison of the influence of material on in vitro cartilage tissue en- gineering with PCL, PGS and POC 3D scaffold archi- tecture seeded with chondrocytes. Biomaterials, 31(15), 4304-4312. [7] Martinez-Diaz, S., Garcia-Giralt, N., Lebourg, M., et al. (2010) In vivo evaluation of 3-dimensional polycaprolac- tone scaffolds for cartilage repair in rabbits. American Journal of Sports Medicine, 38(3), 509-519. [8] He, L., Liu, B., Xipeng, G., et al. (2009) Microstructure and properties of nano-fibrous PCL-b-PLLA scaffolds for cartilage tissue engineering. European Cells and Ma- terials, 18, 63-74. [9] Martins, A., Pinho, E.D., Faria, S., et al. (2009) Surface  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. JBiSE 915 modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological per- formance. Small, 5(10), 1195-1206. [10] da Silva, M.A., Crawford, A., Mundy, J., et al. (2009) Evaluation of extracellular matrix formation in poly- caprolactone and starch-compounded polycaprolactone nanofiber meshes when seeded with bovine articular cho- ndrocytes. Tissue Engineering Part A, 15(2), 377-385. [11] Maher, S.A., Mauck, R.L., Rackwitz, L. and Tuan, R.S. (2010) A nanofibrous cell-seeded hydrogel promotes in- tegration in a cartilage gap model. Journal of Tissue En- gineering and Regenerative Medicine, 4(1), 25-29. [12] Li, W.J., Danielson, K.G., Alexander, P.G. and Tuan, R.S. (2003) Biological response of chondrocytes cultured in three-di- mensional nano fibrous p oly (epsilon-cap rolactone) scaffolds. Journal of Medical Materials Research, 67(4), 1105-1114. [13] Lee, J.J., Yu, H.S., Hong, S.J., Jeong, I., Jang, J.H. and K im, H.W. ( 2009 ) Nanofibrou s membrane of collagen- poly- caprola- ctone for cell growth and tissue regeneration. Journal of M aterials Sc ience: Materials in Medicine, 20(9), 1927-1935. [14] Mierisch, C. M., Wilson, H.A., Turner, M. A., et al. (2003) Chondrocyte transplantation into articular cartilage de- fects with use of calcium alginate: the fate of the cells. Journal of Bone and Joint Surgery, 85A(9), 1757-1767. [15] I to, Y., Fitzsimmons, J.S., Sanyal, A., Mello, M.A., Mukher- jee, N. and O'Driscoll, S.W. (2001) Localization of chon- drocyte precursors in periosteum. Osteoarthritis and Cartilage, 9(3), 215-223. [16] Li, J., Chen, Y., Mak, A.F., Tuan, R.S., Li, L. and Li, Y. (2009) A one-step method to fabricate PLLA scaffolds with deposition of bioactive hydroxyapatite and collagen using ice- based microporogens. Acta Biomaterialia, 6(6), 2013-2019. [17] Mendes, S.C., Bezemer, J., Claase, M.B., et al. (2003) Ev aluation of two biodegradable polymeric syste ms as sub- strates for bone tissue engineering. Tissue Engineering, 9 (Suppl 1), 91-101. [18] Duling, R.R., Dupaix, R.B., Katsube, N. and Lannutti, J. (2008) Mechanical characterization of electrospun poly- caprolac- tone (PCL): A potential scaffold for tissue en- gineering. Journal of Biomechanical Engineering, 130(1), 011006. [19] Gaumer, J ., Pr asa d, A ., Le e, D. and Lannu tti, J. (2009) Struc- ture-function relationships and source-to-ground distance in electrospun polycaprolactone. Acta Biomaterialia, 5(5), 1552-1561. [20] Johnson, J., Niehaus, A., Nichols, S., et al. (2009) Electr- ospun PCL in vitro: a microstructural basis for mechani- cal property changes. Journal of Biomaterials Sicence, Polymer Edition, 20(4), 467-481. [21] Li, W.J., Chiang, H., Kuo, T.F., Lee, H.S., Jiang, C.C. and Tuan, R.S. (2009) Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: A pilot study. Journal of Tissue Engineer- ing and Regenerative Medicine, 3(1), 1-10. [22] Fecek, C., Yao, D., Kacorri, A., et al. (2008) Chondro- genic derivatives of embryonic stem cells seeded into 3D poly- caprolactone scaffolds generated cartilage tissue in vivo. Tissue Engineering Part A, 14(8), 1403-1413. [23] Nam, J., Rath, B., Knobloch, T.J., Lannutti, J.J. and Agarwal, S. (2009) Novel electrospun scaffolds for the molecular analysis of chondrocytes under dynamic com- pression. Tissue Engineering Part A, 15(3), 513-523. [24] Chang, K.Y., Cheng, L.W., Ho, G.H., Huang, Y.P. and Lee, Y.D. (2009) Fabrication and characterization of poly (gamma- glutamic acid)-graft-chondroitin sulfate/ polycaprolacto- ne porous scaffolds for cartilage tissue engineering. Acta Biomaterialia, 5(6), 1937-1947. [25] Frisbie, D.D., Lu, Y., Kawcak, C.E., DiCarlo, E.F., Bi- nette, F. and McIlwraith, C.W. (2009) In vivo evaluation of autologous cartilage fragment-loaded scaffolds im- planted into equine articular defects and compared with autologous chondrocyte implantation. American Journal of Sports Medicine, 37 (Suppl 1), 71-80. [26] Kang, X., Xie, Y., Powell, H.M., et al. (2007) Adipogenesis of murine embryonic stem cells in a th r ee - d i m e n s i on a l c u l - ture system using electrospun polymer scaffolds. Bio- materials, 28(3), 450-458. [27] Johnson, J., Nowicki, M.O., Lee, C.H., et al. (2009) Quantitative analysis of complex glioma cell migration on electrospun polycaprolactone using time-lapse mi- croscopy. Tissue Engineering Part C, 15(4), 531-540. [28] Drilling, S., Gaumer, J. and Lannutti, J. (2009) Fabrica- tion of burst pressure competent vascular grafts via elec- trospinning: Effects of microstructure. Journal of Bio- medical Materials Research Part A, 88(4), 923-934. [29] Xian, C.J. and Foster, B.K. (2006) Repair of injured ar- ticular and growth plate cartilage using mesenchymal stem cells and chondrogenic gene therapy. Current Stem Cell Research & Therapy, 1(2), 213-229. [30] Puetzer, J.L., Petitte, J. and Loboa, E. (2010) Compara- tive review of growth f actors for in duction of 3-Dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tis- sue Engineering Part B, 16(4), 435-444 [31] Zachos, T., Diggs, A., Weisbrode, S., Bartlett, J. and Bertone, A. (2007) Mesenchymal stem cell-mediated gene delivery of bone morphogenetic protein-2 in an ar- ticular fracture m odel. Molecular Th erapy, 15(8), 1543-1550. [32] van der Kraan, P.M., Davidson, E.N. and van den Berg, W.B. (2010) Bone morphogenetic proteins and articular cartilage to serve and protect or a wolf in sheep clothing's? Osteoarthritis and Cartilage, 18(6), 735-741. [33] Kaps, C., Bramlage, C., Smolian, H., et al. (2002) Bone morphogenetic proteins promote cartilage differentiation and protect engineered artificial cartilage from fibroblast invasion and destruction. Arthritis & Rheumatism, 46(1), 149-162. [34] Majumdar, M.K., Wang, E. and Morris, E.A. (2001) BMP-2 and BMP-9 promotes chondrogenic differentia- tion of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. Journal of Cell Physiology, 189(3), 275-284. [35] Glansbeek, H.L., van Beuningen, H.M., Vitters, E.L., Morris, E.A., van der Kraan, P.M. and van den Berg, W.B. (1997) Bone morphogenetic protein 2 stimulates articular cartilage proteoglycan synthesis in vivo but does not counteract interleukin-1alpha effects on proteoglycan synthesis and content. Arthritis & Rheumatism, 40(6), 1020-1028. [36] Smith, P., Shuler, F.D., Georgescu, H.I., et al. (2000) Genetic enhancement of matrix synthesis by articular  R. T. Gorsline et al. / J. Biomedical Science and Engineering 3 (2010) 908-916 Copyright © 2010 SciRes. 916 JBiSE chondrocytes: Comparison of different growth factor genes in the presence and absence of interleukin-1. Ar- thritis & Rheumatism, 43(5), 1156-1164. [37] Zachos, T.A., Shields, K.M. and Bertone, A.L. (2006) Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or -6. Journal of Or- thopaedic Research, 24(6), 1279-1291. [38] Stankus, J.J., Guan, J., Fujimoto, K. and Wagner, W.R. (2006) Microintegrating smooth muscle cells into a bio- degradable, elastomeric fiber matrix. Biomaterials, 27(5), 735-744. [39] Nam, J., Huang, Y., Agarwal, S. and Lannutti, J. (2008) Materials selection and residual solvent retention in bio- degradable electrospun fibers. Journal of Applied Poly- mer Science, 107(3), 1547-1554. [40] Lopez-Vidriero, E., Goulding, K.A., Simon, D.A., San- chez, M. and Johnson, D.H. (2010) The use of plate- let-rich plasma in arthroscopy and sports medicine: Op- timizing the healing environment. Arthroscopy, 26(2), 269-278. [41] Sutter, W.W., Kaneps, A.J. and Bertone, A.L. (2004) Comparison of hematologic values and transforming gro- wth factorbeta and insulin-like growth factor concentra- tions in platelet concentrates obtained by use of buffy coat and apheresis methods from equine blood. American Journal of Veterinary Research, 65(7), 924-930. [42] Wilke, M.M., Nydam, D.V. and Nixon, A.J. (2007) En- hanced early chondrogenesis in articular defects follow- ing arthroscopic mesenchymal stem cell implantation in an equine model. Journal of Orthopaedic Research, 25(7), 913-925. [43] Santangelo, K.S., Baker, S.A., Nuovo, G., Dyce, J., Bart- lett, J.S. and Bertone, A.L. (2007) Detectable reporter ge- ne expression following transduction of adenovirus and adeno-associated virus serotype 2 vectors within full-thickness osteoarthritic and unaffected canine carti- lage in vitro and unaffected guinea pig cartilage in vivo. Journal of Orthopaedic Research, 28(2), 149-155. |

