Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 302-313 doi:10.4236/jep.2010.13036 Published Online September 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP Sol-Gel Materials with Pesticide Delivery Properties Malina Raileanu1*, Ligia Todan1, Maria Crisan1, Ana Braileanu1, Adriana Rusu1, Corina Bradu2, Adrian Carpov3, Maria Zaharescu1 1Ilie Murgulescu Institute of Physical Chemistry, Romanian Academy, Bucharest, Romania; 2Faculty of Chemistry, Organic Chemis- try Department, University of Bucharest, Bucharest, Romania; 3Petru Poni Institute of Macromolecular Chemistry, Iasi, Romania. Email: mraileanu@icf.ro, malina_raileanu@yahoo.com Received March 26th, 2010; revised April 20th, 2010; accepted April 25th, 2010. ABSTRACT Pesticides are widely used in agricu lture, although they may create haza rds both to humans and to the environment. In order to reduce the harmful effects of their administration, there has been made a great effort to find solutions. The porous sol-gel silica materials which are able to entrap different organic molecules represent new studied controlled release carriers. The aim of the present work was to prepare and characterize sol-gel composites based on trichlorfon as organophosp horous pesticide embedded in silica matrices generated from three differen t SiO2 sources : tetraethylor- thosilicate (TEO S ), colloida l silica (CS), and sodium silicate (SS). Similar samples to those containing only trichlorfon have also been synthesised, in which α-, β-, and γ-cyclodextrin have been included in order to study the possibility of improving the release of the pesticide from the silica matrices. The porous sol-gel silica materials generated from TEOS and CS are able to entrap the trichlorfon and ensure an efficient delivery of the pesticide. In the absence of cyclodextrins, better results are obtained in the case of TEOS precursor, compared to colloidal silica. The addition of cyclodextrins in order to improve the release of the pesticide from the silica matrices was successful only in the case of CS as SiO2 precursor. The best release of the pesticide was obtained with β-CD. Keywords: Sol-Gel, Tetraethylorthosilicate, Colloidal Silica, Trichlorfon, Cyclodextrin 1. Introduction Nowadays, agrochemicals play an important role in making possible the continuous increase of food produc- tion. On the other hand, a high level of chemicals could become a da nger for humanity. Pesticides represent one of the most im por tant ty pes of c h em ical s used i n a gric ulture. They include a wide di versity of substances. Am ong them , the class of organophosphorous compounds (OPs) has gained an enormous commercial success, due to their properties of controlling moths, ants, cockroaches, ter- mites, fruit flies and similar insects, fleas, locusts, cater- pillars and ticks [1]. They are th e most frequently used in many cultures, at various stages of cultivation, as common insecticides, in order to control insect and arthropod pests on vegetable crops together with the increase of crop yields [2-8]. Among the organophosphorous pesticides, trichlorfon [O,O-dimethyl-(2, 2, 2-trichloro-1-hydroxy ethyl)-phosphonate] is one of the most popular, being ex- tensively used in agriculture since 1952 as a broad spec- trum insecticide [2,9-16]. One of the major problems of agriculture which must be solved refers to the controlled use of pesticides. The presence of the used pesticides in the surface and ground waters [4,6,17,18] emphasises this environmental issue. As a result of the increased aware- ness and worry regarding the potential of pesticides to have detrimental impacts on both human and ecosystem health, there is a great interest in developing less persis- tent but more selective pesticides. However, their use can be too expensive because they require more frequent application. Because of the high costs and the limitations in what the design of new pesticides is concerned, the industry and the researchers have decided not to fabricate new pesticides, but to improve the delivery systems of the current ones. An effective way is using the controlled release alternative. The controlled release systems have become more a nd more im portant d ue to their adva ntages: they minimize pesticide residues available to the envi-  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 303 ronment, thus reducing the environmental hazards and human toxicity, they increase the efficacy and the lon- gevity of the pesticide and decrease the application costs, allowing less frequent uses [17,19-22]. Hussain [21] di- vides the controlled release formulations of pesticides into four main types: polymer membrane reservoirs, matrices containing physically-trapped pesticides, covalently- bounded pesticides and coated pesticide granule systems. Some examples are already mentioned in the literature [8,22-25]. A s the property of t he sol-gel process to pre pare inorganic-organic hybrids by embedding organic mole- cules in the inorganic oxide matrix is a well known fact [26-35], Böttcher et al. [20] have used the sol-gel matrix as an alternative to biocide encapsulation. They prepared sol-gel composite films with controlled release properties. The aim of the present work was to prepare and char- acterize sol-gel composites powders based on trichlorfon as organophosphorous pesticide embedded in silica ma- trices generated from three different SiO2 sources: tetra- ethylorthosilicate-TEOS, colloidal silica-CS, and sodium silicate-SS. Both routes of the sol-gel method (the al koxide and the aqueous one) have been used. The aqueous route selection was based both on economical and ecological reasons: the aqueous SiO2 precursors are less expensive than TEOS and the soil attack is almost inexistent in a system in which the pesticide proceeds from an aqueous medium. Because the ability of cyclodextrins to form inclusion complexes with a wide variety of agricultural chemicals is well known [36-42], the possibility of using them in order to improve the release of the pesticide from the silica matrices has been studied. A comparison be- tween the performances of the prepared materials has been made, in order to establish the most propitious re- agents (e.g. silica precursor and type of cyclodextrin) and synthesis conditions which could lead to the best results from the point of view of the applicability. 2. Materials and Methods 2.1. Preparation of Samples Composite samples, consisting of pesticide embedded in silica gels (pest icide/SiO2 = 1/3 in weight), have been pre- pared using both routes of the sol-gel method: the alkoxide and the colloidal one. The pesticide was an organophos- phorous one, trichlorfon respectively: O,O-dimethyl-(2, 2, 2-trichloro-1-hydroxy ethyl)-phosphonate (T), from Ji- angsu Hongze Chemical and Industry Co., Ltd. The SiO2 precursors were: tetraethylorthosilicate (TEOS) from Merck, colloidal silica Ludox SM-30 type (CS) from Aldrich, and a sod ium silicate solution 26 wt.% SiO2 (SS) from Merck, respectively. In the case of TEOS, the ethanol (from Riedel de Haën) has been used as solvent. The gelation of all prepared sol-gel materials was ac- complished at neutral pH, which is also totally harmless for the soil in case of application. The pH adjustment was realized with a 25 wt.% HNO3 solution. The experiments were made at the room temperature. All samples have been prepared under continuous stirring, in a few steps. First, the alkoxide was solv ed in the correspo nding quan- tity of alcohol (the molar ratio of SiO2/ROH = 0.075/ 0.515), respectively the colloidal silica and the sodium silicate solutions have been diluted with water, in order to ensure the same SiO2 concentration in all samples (solutions “a”). Then, the aqueous solution of trichlorfon (solution “b”) was added drop by drop to solution “a”, resulting solution “c”. Its pH was checked and adjusted to the value of 6. For each of the obtained composite samples the corresponding SiO2 matrices have been pre- pared, in the same experimental conditions but in the absence of trichlorfon. In order to study the influence of the presence of cyclodextrins over the release of th e pes- ticide from the prepared materials, similar samples to those containing only trichlorfon have been synthesised at the same time, in which α-, β-, and γ-cyclodextrin (CD) from Wacker-Chemie GMBH have been included. All cyclodextrin types have been introduced in the reaction mixture as aqueous solutions, simultaneously with the trichlorfon, in the quantity imposed by the solubility limit. The composition of the corresponding samples was: 19 wt.% T, 24 wt.% CD and 57 wt.% SiO2, respectively. In the present work, a number of 15 gels have been prepared. Their names were established depending on the used SiO2 precursor, as follows: TEOS, CS, and SS (for the silica matrices); TEOS + T, CS + T, and SS + T (for the silica/trichlorfon composites); TEOS + T + CD (α-, β-, or γ-), CS + T + CD (α-, β-, or γ-) and SS + T + CD (α-, β-, or γ-), respectively (for those composites in which both trichlorfon and cyclodextrin have been in- cluded in the silica matrices). 2.2. Methods of Characterization The structural characterization of the prepared samples was accomplished by IR spectroscopy, using a FT-IR NICOLET 6700 (400-4000 cm-1) spectrophotometer. Their thermal behaviour was studied up to 800℃, usin g a Mettler Toledo Star System TGA/SDTA851/LF 1600℃, with a heating rate of 10 K min-1, dynamic air atmos- phere, and a flow rate of 50 mL min-1. The release of trichlorfon from the silica matrix was investigated during a series of batch experiments. In these tests, a quantity of sample corresponding to 100.0 mg trichlorfon (between 0.3863 g and 0.8803 g sample) was contacted with 100 mL ultrapure water and continu- ously mixed (150 rpm.) in a closed glass vessel at the temperature of 21 1℃ using a thermostated shaker (GFL 3033). Trichlorfon concentrations from the aque- 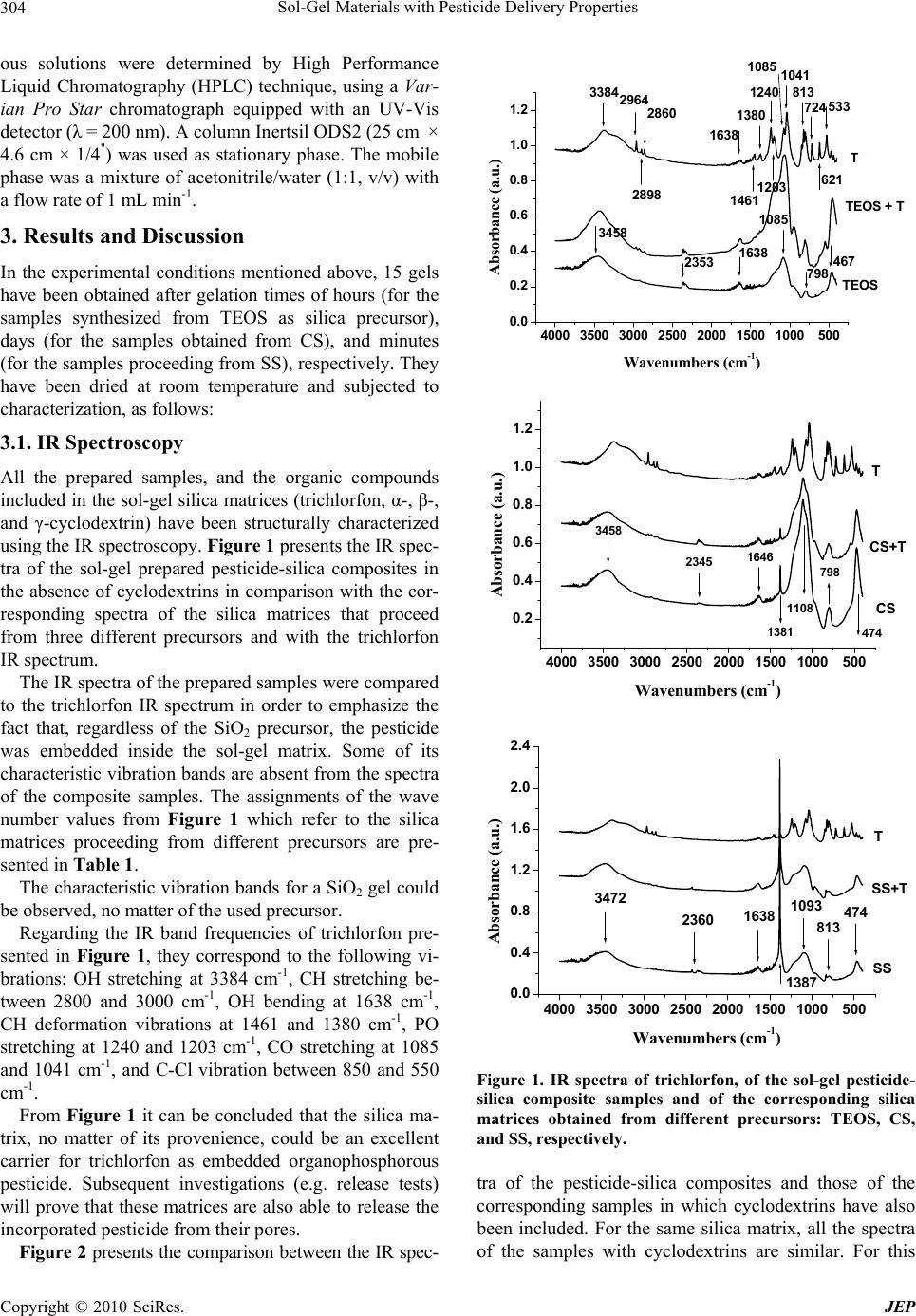 Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 304 ous solutions were determined by High Performance Liquid Chromatography (HPLC) technique, using a Var- ian Pro Star chromatograph equipped with an UV-Vis detector (λ = 200 nm). A column Inertsil ODS2 (25 cm × 4.6 cm × 1/4") was used as stationary phase. The mobile phase was a mixture of acetonitrile/water (1:1, v/v) with a flow rate of 1 mL min-1. 3. Results and Discussion In the experimental conditions mentioned above, 15 gels have been obtained after gelation times of hours (for the samples synthesized from TEOS as silica precursor), days (for the samples obtained from CS), and minutes (for the samples proceeding from SS), respectively. They have been dried at room temperature and subjected to characterization, as follows: 3.1. IR Spectroscopy All the prepared samples, and the organic compounds included in the sol-gel silica matrices (trichlorfon , α-, β-, and γ-cyclodextrin) have been structurally characterized using the IR spectroscopy. Figure 1 presents the IR spec- tra of the sol-gel prepared pesticide-silica composites in the absence of cyclodextrins in comparison with the cor- responding spectra of the silica matrices that proceed from three different precursors and with the trichlorfon IR spectrum. The IR spectra of the prepared samples were compared to the trichlorfon IR spectrum in order to emphasize the fact that, regardless of the SiO2 precursor, the pesticide was embedded inside the sol-gel matrix. Some of its characteristic vibration bands are absent from the spectra of the composite samples. The assignments of the wave number values from Figure 1 which refer to the silica matrices proceeding from different precursors are pre- sented in Table 1. The characteristic vibration bands for a SiO2 gel could be observed, no m at t e r of the used precursor. Regarding the IR band frequencies of trichlorfon pre- sented in Figure 1, they correspond to the following vi- brations: OH stretching at 3384 cm-1, CH stretching be- tween 2800 and 3000 cm-1, OH bending at 1638 cm-1, CH deformation vibrations at 1461 and 1380 cm-1, PO stretching at 1240 and 1203 cm-1, CO stretching at 1085 and 1041 cm-1, and C-Cl vibration between 850 and 550 cm-1. From Figure 1 it can be concluded that the silica ma- trix, no matter of its provenience, could be an excellent carrier for trichlorfon as embedded organophosphorous pesticide. Subsequent investigations (e.g. release tests) will prove that these matrices are also able to release the incorporated pesticide from their pores. Figure 2 presents the comparison between the IR spec - 4000 3500 3000 2500 2000 1500 1000500 0.0 0.2 0.4 0.6 0.8 1.0 1.2 TEOS + T 2898 2860 2964 1380 1461 1638 12401041 1203 1085 3384 621 724 813 533 1638 1085 2353 3458 798 467 T TEOS Absorbance (a.u.) Wavenumbers (cm-1) 4000 3500 3000 2500 2000 1500 1000500 0.2 0.4 0.6 0.8 1.0 1.2 2345 3458 1646 1381 1108 798 474 T CS+T CS Absorbance (a.u.) Wavenumbers (cm-1) 4000 35003000 25002000 15001000500 0.0 0.4 0.8 1.2 1.6 2.0 2.4 2360 1638 813 1093 1387 3472 474 T SS+T SS Absorbance (a.u.) Wavenumbers (cm-1) Figure 1. IR spectra of trichlorfon, of the sol-gel pesticide- silica composite samples and of the corresponding silica matrices obtained from different precursors: TEOS, CS, and SS, respectively. tra of the pesticide-silica composites and those of the corresponding samples in which cyclodextrins have also been included. For the same silica matrix, all the spectra of the samples with cyclodextrins are similar. For this 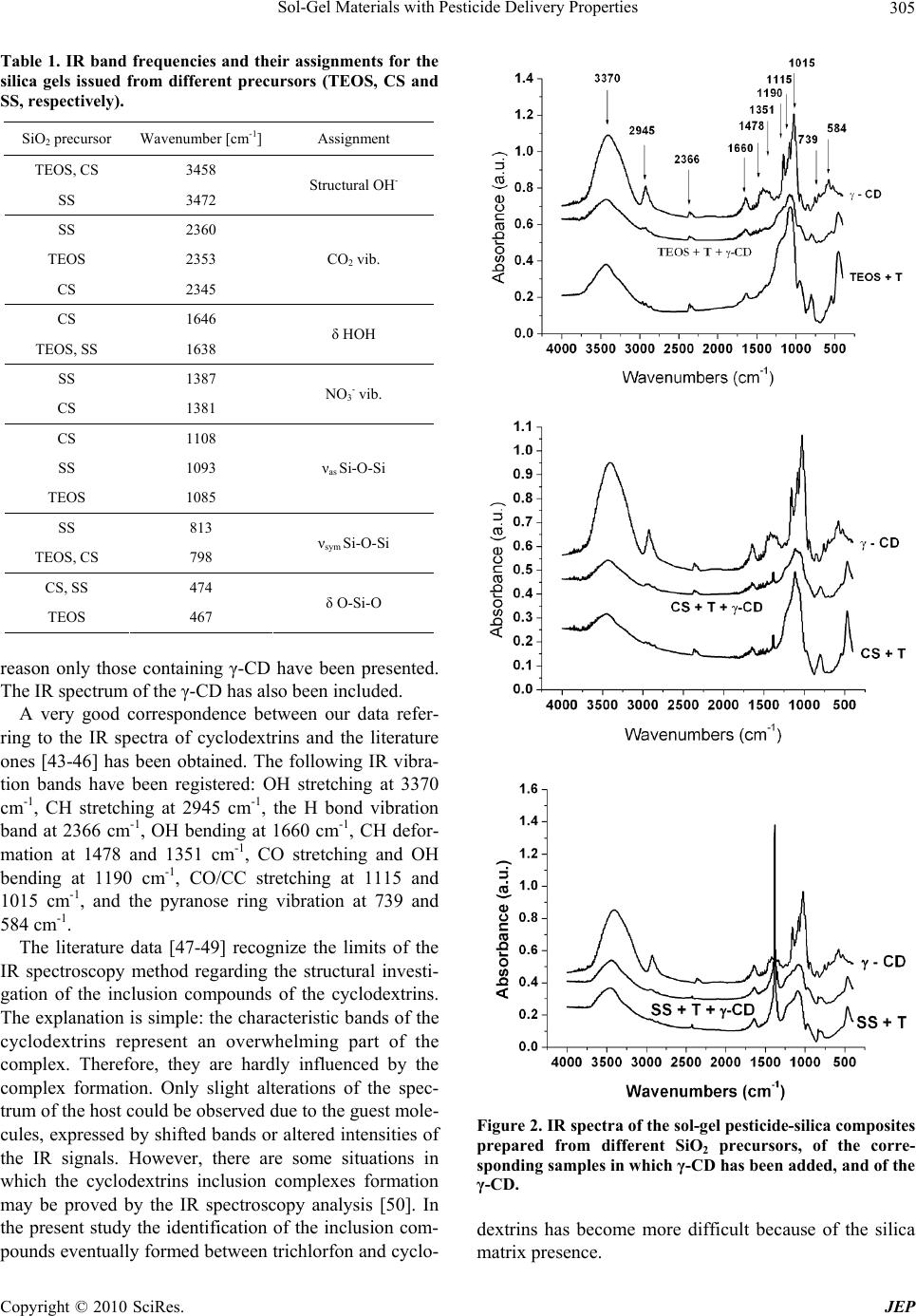 Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 305 Table 1. IR band frequencies and their assignments for the silica gels issued from different precursors (TEOS, CS and SS, respectively). SiO2 precursor Wavenumber [cm-1] Assignment TEOS, CS 3458 SS 3472 Structural OH- SS 2360 TEOS 2353 CS 2345 CO2 vib. CS 1646 TEOS, SS 1638 δ HOH SS 1387 CS 1381 NO3- vib. CS 1108 SS 1093 TEOS 1085 νas Si-O-Si SS 813 TEOS, CS 798 νsym Si-O-Si CS, SS 474 TEOS 467 δ O-Si-O reason only those containing γ-CD have been presented. The IR spectrum of the γ-CD has also been included. A very good correspondence between our data refer- ring to the IR spectra of cyclodextrins and the literature ones [43-46] has been obtained. The following IR vibra- tion bands have been registered: OH stretching at 3370 cm-1, CH stretching at 2945 cm-1, the H bond vibration band at 2366 cm-1, OH bending at 1660 cm-1, CH defor- mation at 1478 and 1351 cm-1, CO stretching and OH bending at 1190 cm-1, CO/CC stretching at 1115 and 1015 cm-1, and the pyranose ring vibration at 739 and 584 cm-1. The literature data [47-49] recognize the limits of the IR spectroscopy method regarding the structural investi- gation of the inclusion compounds of the cyclodextrins. The explanation is simple: the characteristic b ands of the cyclodextrins represent an overwhelming part of the complex. Therefore, they are hardly influenced by the complex formation. Only slight alterations of the spec- trum of the host could be observed due to the guest mole- cules, expressed by shifted bands or altered intensities of the IR signals. However, there are some situations in which the cyclodextrins inclusion complexes formation may be proved by the IR spectroscopy analysis [50]. In the present study the identification of the inclusion com- pounds eventually formed between trichlorfon and cyclo- Figure 2. IR spectra of the sol-gel pesticide-silica composites prepared from different SiO2 precursors, of the corre- sponding samples in which γ-CD has be en add ed, and of the γ-CD. dextrins has become more difficult because of the silica matrix presence.  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 306 3.2. Thermal Analysis The use of the thermoanalytical techniques in order to investigate the formation of the cyclodextrins inclusion compounds is well recognized for three decades [41,42, 50,51]. All the prepared samples and the organic compounds included in the sol-gel silica matrices: trichlorfon, α-, β-, and γ-cyclodextrin, respectively, have been characterized by thermal analysis. Table 2 and Figures 3-5 present the obtained re sults. In what the three types of cyclodextrins are concerned, the obtained results presented in Table 1 are in agreement with the literature data [41,47,50,52-54]. Because the registered derivatograms of the sol-gel samples obtained with different cyclodextrins (α-, β-, and γ-CD, respectively) are similar, Figures 3-5(c) present only the experimental results obtained using γ-CD. As seen in Figures 3-5, the nature of the silica matrix has a significant influence over the thermal behavior of the material. The trichlorfon elimination (which starts in the pure co mpound at 196℃) is modified in the presence of the matrix: in the case of the organic precursor TEOS, it takes place at 409℃, in the case of the inorganic pre- cursors, the corresponding temperatures are lower: 182℃ for the colloidal silica and 245℃, respectively, for the sodium silicate. Regarding the elimination of trichlorfon in the presence of cyclodextrins (α-, β-, and γ-CD), only in the case of the matrix proceeded from TEOS, the process takes place at temperatures (195-202℃) compa- rable with those of the pure trichlorfon (196-258℃). In the case of the inorganic SiO2 precursors (CS and SS, respectively), the trichlorfon elimination starts at modi- fied temperatures (156-164℃ for CS and 155-196℃ for SS). The influence of the silica matrix on the thermal behavior of the sol-gel prepared materials can also be seen in the major modification of the temperatures cor- responding to the combustion of cyclodextrins. No mat- ter the type (α-, β-, or γ-CD), they are eliminated by combustion from the samples at much lower tempera- tures (α-CD: 305-330℃, β-CD: 295-311℃, and γ-CD: 310-335℃), compared to the temperatures of combustion of the pure cyclodextrins which are around 500℃ (α-CD: 499℃, β-CD: 48 0℃, and γ-CD: 498℃). It can be assumed that the shift of the decomposition temperatures is due not only to the silica matrices but also to the inclusion compounds formation. The supposi- tion could be correct for all the sol-gel prepared samples containing both trichlorfon and cyclodextrin, because the melting step corresponding to the trichlorfon could not be observed. In fact, the lack of the melting step of the guest molecule from the derivatograms is considered to be the proof of the inclusion compound formation [41,42, Temperature/℃ Mass/% (a) Temperature/℃ Mass/% (b) Temperature/℃ Mass/% (c) Figure 3. Thermal behavior of the silica matrix obtained from TEOS (a) and of the sol-gel samples prepared from TEOS in the presence of T (b) and of both T and γ-CD (c). 50]. Therefore, it can be concluded that the thermal analysis indicates that the pesticide molecules have in-  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 307 Temperature/℃ Mass/% (a) Temperature/℃ Mass/% (b) Temperature/℃ Mass/% (c) Figure 4. Thermal behavior of the silica matrix obtained from CS (a) and of the sol-gel samples prepared from CS in the presence of T (b) and of both T and γ-CD (c). teracted with the cavity of the cyclodextrins host mole- cules, forming the inclusion complexes. Some doubts still Temperature/℃ Mass/% (a) Temperature/℃ Mass/% (b) Temperature/℃ Mass/% (c) Figure 5. Thermal behavior of the silica matrix obtained from SS (a) and of the sol-gel samples prepared from SS in the presence of T (b) and of both T and γ-CD (c). exist, taking into account the fact that in the samples prepared without cyclodextrins, the silica matrix (no 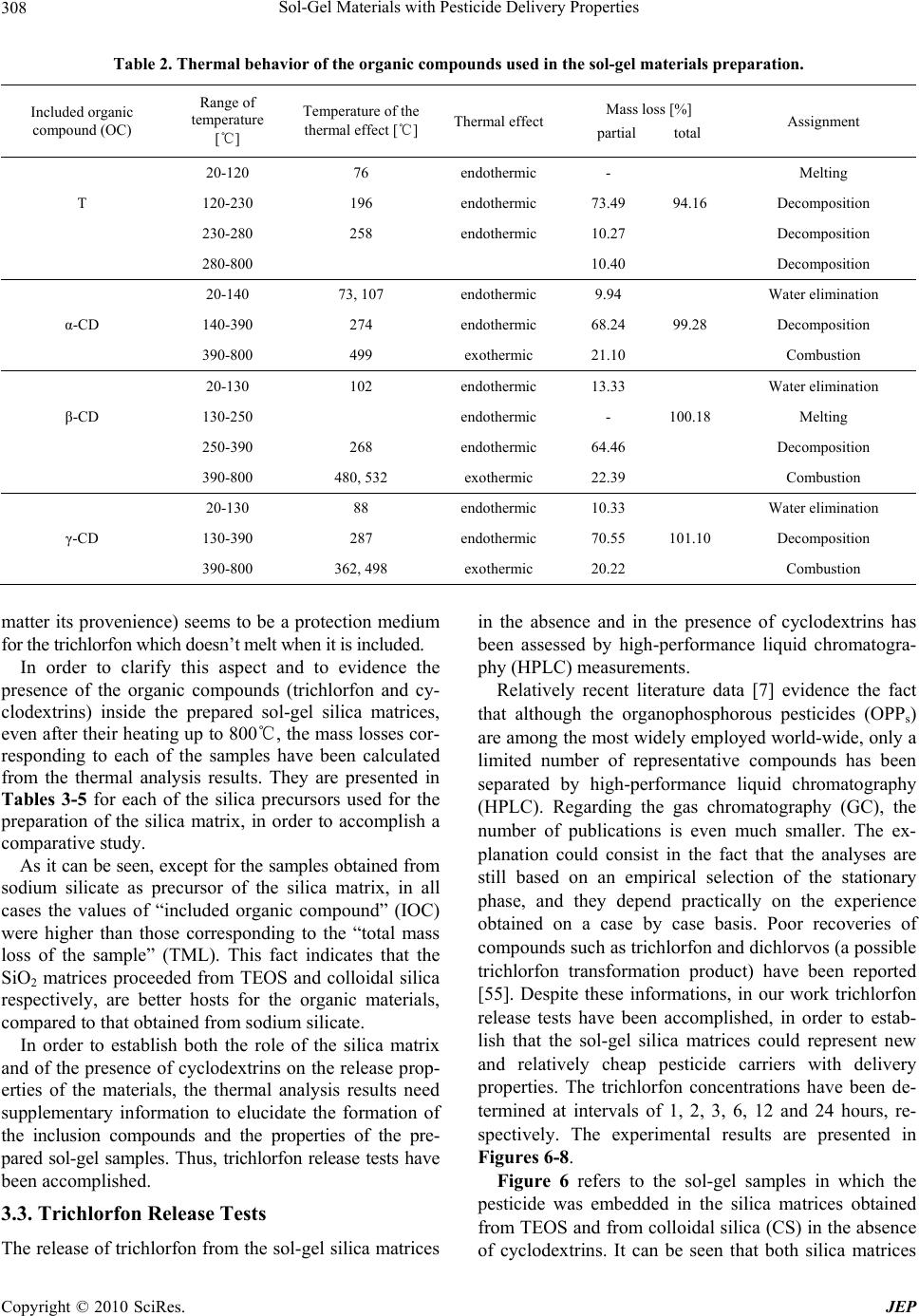 Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 308 Table 2. Thermal behavior of the organic compounds used in the sol-gel materials preparation. Included organic compound (O C ) Range of temperature [℃] Temperature of the thermal effect [℃]Thermal effect Mass loss [%] partial total Assignment 20-120 76 endothermic - Melting T 120-230 196 endothermic 73.49 94.16 Decomposition 230-280 258 endothermic 10.27 Decomposition 280-800 10.40 Decomposition 20-140 73, 107 endothermic 9.94 Water elimination α-CD 140-390 274 endothermic 68.24 99.28 Decomposition 390-800 499 exothermic 21.10 Combustion 20-130 102 endothermic 13.33 Water elimination β-CD 130-250 endothermic - 100.18 Melting 250-390 268 endothermic 64.46 Decomposition 390-800 480, 532 exothermic 22.39 Combustion 20-130 88 endothermic 10.33 Water elimination γ-CD 130-390 287 endothermic 70.55 101.10 Decomposition 390-800 362, 498 exothermic 20.22 Combustion matter its provenience) seems to be a protection medium for the trichlorfon which doesn’t melt when it is included. In order to clarify this aspect and to evidence the presence of the organic compounds (trichlorfon and cy- clodextrins) inside the prepared sol-gel silica matrices, even after their heating up to 800℃, the mass losses cor- responding to each of the samples have been calculated from the thermal analysis results. They are presented in Tables 3-5 for each of the silica precursors used for the preparation of the silica matrix, in order to accomplish a comparative study. As it can be seen, except for the samples obtained from sodium silicate as precursor of the silica matrix, in all cases the values of “included organic compound” (IOC) were higher than those corresponding to the “total mass loss of the sample” (TML). This fact indicates that the SiO2 matrices proceeded from TEOS and colloidal silica respectively, are better hosts for the organic materials, compared to that obtained from sodium silicate. In order to establish both the role of the silica matrix and of the presence of cyclodextrins on the release prop- erties of the materials, the thermal analysis results need supplementary information to elucidate the formation of the inclusion compounds and the properties of the pre- pared sol-gel samples. Thus, trichlorfon release tests have been accomplished. 3.3. Trichlorfon Release Tests The release of trichlorfon from the sol-gel silica matrices in the absence and in the presence of cyclodextrins has been assessed by high-performance liquid chromatogra- phy (HPLC) measurements. Relatively recent literature data [7] evidence the fact that although the organophosphorous pesticides (OPPs) are among the most widely employed world-wide, only a limited number of representative compounds has been separated by high-performance liquid chromatography (HPLC). Regarding the gas chromatography (GC), the number of publications is even much smaller. The ex- planation could consist in the fact that the analyses are still based on an empirical selection of the stationary phase, and they depend practically on the experience obtained on a case by case basis. Poor recoveries of compounds such as trichlorfon and dichlorvos (a possible trichlorfon transformation product) have been reported [55]. Despite these informations, in our work trichlorfon release tests have been accomplished, in order to estab- lish that the sol-gel silica matrices could represent new and relatively cheap pesticide carriers with delivery properties. The trichlorfon concentrations have been de- termined at intervals of 1, 2, 3, 6, 12 and 24 hours, re- spectively. The experimental results are presented in Figures 6-8. Figure 6 refers to the sol-gel samples in which the pesticide was embedded in the silica matrices obtained from TEOS and from colloidal silica (CS) in the absence of cyclodextrins. It can be seen that both silica matrices 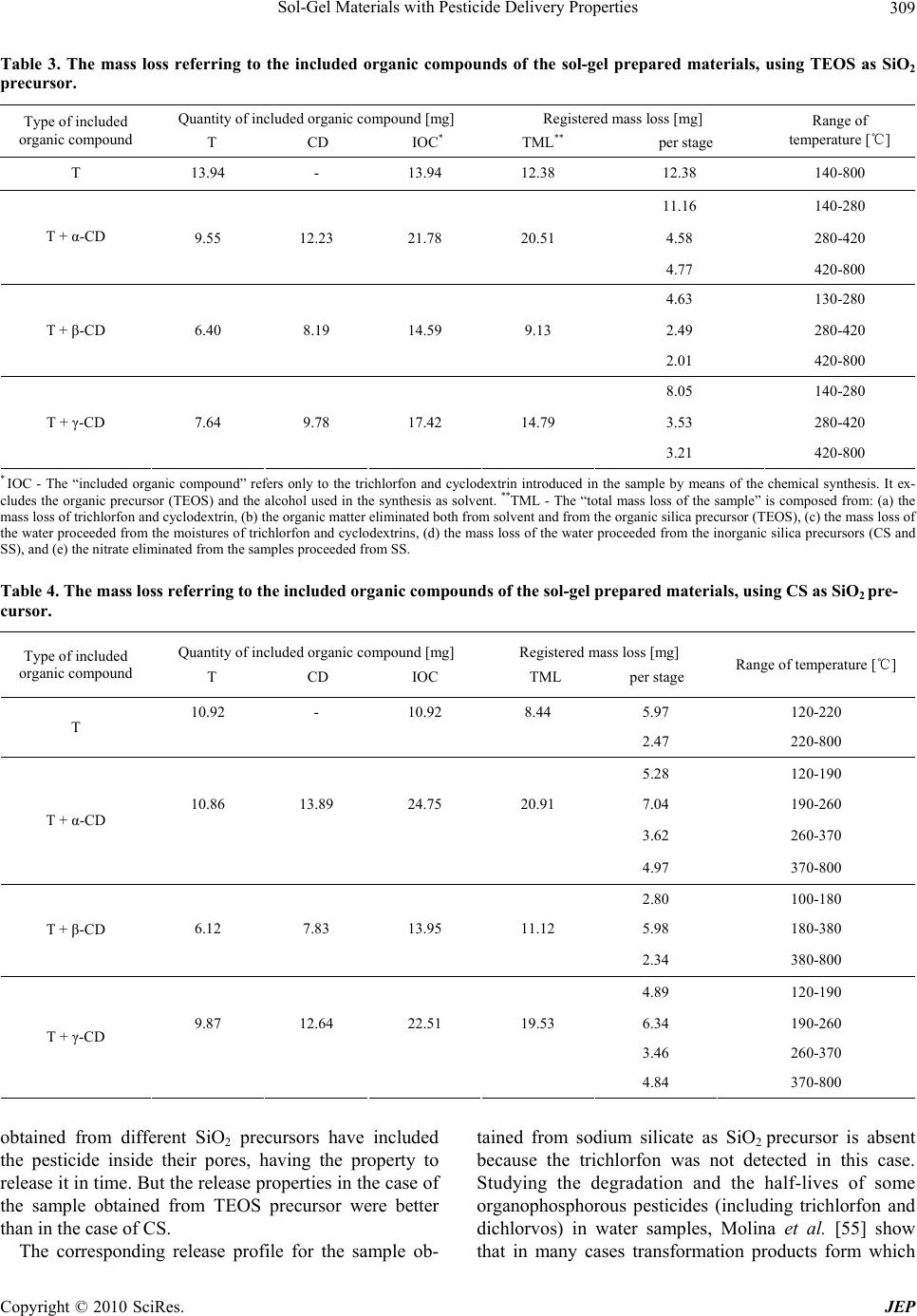 Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 309 Table 3. The mass loss referring to the included organic compounds of the sol-gel prepared materials, using TEOS as SiO2 precursor. Type of included organic compound Quantity of included organic compound [m g ] T CD IOC * Registered mass loss [mg] TML** per stage Range of temperature [℃] T 13.94 - 13.94 12.38 12.38 140-800 11.16 140-280 9.55 12.23 21.78 20.51 4.58 280-420 T + α-CD 4.77 420-800 4.63 130-280 6.40 8.19 14.59 9.13 2.49 280-420 T + β-CD 2.01 420-800 8.05 140-280 7.64 9.78 17.42 14.79 3.53 280-420 T + γ-CD 3.21 420-800 * IOC - The “included organic compound” refers only to the trichlorfon and cyclodextrin introduced in the sample by means of the chemical synthesis. It ex- cludes the organic precursor (TEOS) and the alcohol used in the synthesis as solvent. **TML - The “total mass lo ss of the sample” is composed from: ( a) the mass loss of trichlorfon and cyclodextrin, (b) the organic matter eliminated both from solvent and from the organic silica precursor (TEOS), (c) the mass loss of the water p roceeded f rom the mois tures of t richlorfo n and cyclod extrins, (d ) the mass l oss of th e water proceed ed from th e inorganic s ilica pr ecursors (CS and SS), and (e) the nitrate eliminated from the samples proceeded from SS. Table 4. The mass loss referring to the included organic compounds of the sol-gel prepared materials, using CS as SiO2 pre- cursor. Type of included organic compound Quantity of included organic compound [m g ] T CD IOC Registered mass loss [mg] TML per stage Range of temperature [℃] 10.92 - 10.92 8.44 5.97 120-220 T 2.47 220-800 5.28 120-190 10.86 13.89 24.75 20.91 7.04 190-260 3.62 260-370 T + α-CD 4.97 370-800 2.80 100-180 6.12 7.83 13.95 11.12 5.98 180-380 T + β-CD 2.34 380-800 4.89 120-190 9.87 12.64 22.51 19.53 6.34 190-260 3.46 260-370 T + γ-CD 4.84 370-800 obtained from different SiO2 precursors have included the pesticide inside their pores, having the property to release it in time. But the release properties in the case of the sample obtained from TEOS precursor were better than in the case of CS. The corresponding release profile for the sample ob- tained from sodium silicate as SiO2 precursor is absent because the trichlorfon was not detected in this case. Studying the degradation and the half-lives of some organophosphorous pesticides (including trichlorfon and dichlorvos) in water samples, Molina et al. [55] show that in many cases transformation products form which 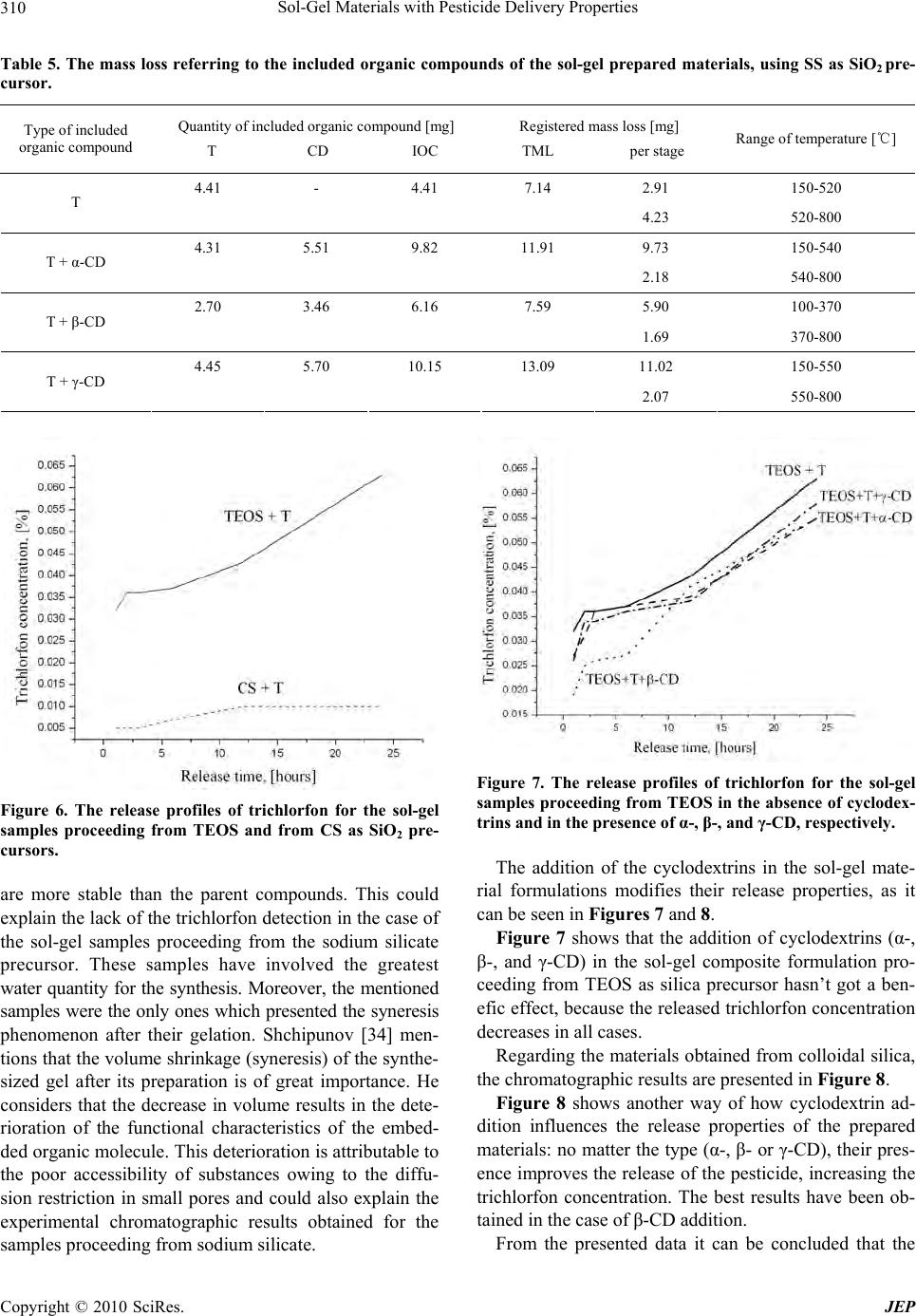 Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 310 Table 5. The mass loss referring to the included organic compounds of the sol-gel prepared materials, using SS as SiO2 pre- cursor. Type of included organic compound Quantity of included organic compound [m g ] T CD IOC Registered mass loss [mg] TML per stage Range of temperature [℃] 4.41 - 4.41 7.14 2.91 150-520 T 4.23 520-800 4.31 5.51 9.82 11.91 9.73 150-540 T + α-CD 2.18 540-800 2.70 3.46 6.16 7.59 5.90 100-370 T + β-CD 1.69 370-800 4.45 5.70 10.15 13.09 11.02 150-550 T + γ-CD 2.07 550-800 Figure 6. The release profiles of trichlorfon for the sol-gel samples proceeding from TEOS and from CS as SiO2 pre- cursors. are more stable than the parent compounds. This could explain the lack of the trichlorfon detection in the case of the sol-gel samples proceeding from the sodium silicate precursor. These samples have involved the greatest water quantity for the synthesis. Moreover, the mentioned samples were the only ones which presented the syneresis phenomenon after their gelation. Shchipunov [34] men- tions that the volume shrinkage (syneresis) of th e synthe- sized gel after its preparation is of great importance. He considers that the decrease in volume results in the dete- rioration of the functional characteristics of the embed- ded organic molecule. Th is deterioration is attributable to the poor accessibility of substances owing to the diffu- sion restriction in small pores and could also explain the experimental chromatographic results obtained for the samples proceeding from sodium silicate. Figure 7. The release profiles of trichlorfon for the sol-gel samples proceeding from TEOS in the absence of cyclodex- trins and in the presence of α-, β-, and γ-CD, re spectively. The addition of the cyclodextrins in the sol-gel mate- rial formulations modifies their release properties, as it can be seen in Figures 7 and 8. Figure 7 shows that the addition of cyclodextrins (α-, β-, and γ-CD) in the sol-gel composite formulation pro- ceeding from TEOS as silica precursor hasn’t got a ben- efic effect, because the released trichlorfon concentration decreases in all cases. Regarding the materials ob tained from colloidal silica, the chromatographic results are presented in Figure 8. Figure 8 shows another way of how cyclodextrin ad- dition influences the release properties of the prepared materials: no matter the type (α-, β- or γ-CD), their pres- ence improves the release of the pesticide, increasing the trichlorfon concentration. The best results have been ob- tained in the case of β-CD addition. From the presented data it can be concluded that the  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 311 Figure 8. The release profiles of trichlorfon for the sol-gel samples proceeding from CS in the absence of cyclodextrins and in the presence of α-, β-, and γ-CD, respectively. porous sol-gel silica materials generated from different SiO2 sources (except the sodium silicate) are able to en- trap the organophosphorous pesticide (e.g. trichlorfon) in order to create new controlled release carriers. In all cases (except sample CS + T) the release of trichlorfo n in time is slow and continuous during the studied interval of time (1-24 hours). In the absence of cyclod extrins, better results are obtained in the case of the materials obtained from TEOS, compared to those proceeding from colloid- dal silica as SiO2 precursor. The use of cyclodextrins in order to improve the controlled release of the pesticide from the silica matrices showed that only in the case of CS precursor, the addition was successful. 4. Conclusions Two series of sol-gel composite samples have been pre- pared, using proper recipes. The first consists in trichlor- fon as pesticide, embedded in silica gels generated from different silica sources (TEOS, CS, and SS). The second series is likewise synthesized with the first one. The only difference between them is the presence of cyclodextrins in the reaction mixtures. The release properties of the prepared materials de- pend both on the nature of the silica matrix and on the presence or absence of cyclodextrin in the reaction mix- ture. Thus, best results have been obtained for the sam- ples proceeding from TEOS in the absence of cyclodex- trins and for those obtained from CS, in the presence of cyclodextrins. The delivery properties of the sol-gel silica matrices being confirmed, it can be concluded that they can be considered as a new, ecological type of pesticides carri- ers. REFERENCES [1] S. Paliwal, M. Wales, T. Good, J. Grimsley, J. Wild and A. Simonian, “Fluorescence-Based Sensing of P-Nitroph- enol and P-Nitrophenyl Substituent Organophosphates,” Analytica Chimica Acta, Vol. 596, No. 1, 2007, pp. 9-15. [2] M. G. Dantas Silva, A. Aquino, H. S. Dórea and S. Navickiene, “Simultaneous Determination of Eight Pesti- cide Residues in Coconut Using MSPD and GC/MS,” Talanta, Vol. 76, No. 3, 2008, pp. 680-684. [3] B. Kuswandi, C. I. Fikriyah and A. A. Gani, “An Optical Fiber Biosensor for Chlorpyrifos Using a Single Sol-Gel Film Containing Acetylcholinesterase and Bromothymol Blue,” Talanta, Vol. 74, No. 4, 2008, pp. 613-618. [4] M. Waibel, H. Schulze, N. Huber and T. T. Bachmann, “Screen-Printed Bienzymatic Sensor Based on Sol-Gel Imm obilized Nippostrongylus Brasiliensis Acetylcolineste- rase and a Cytochrome P450 BM-3 (CYP102-A1) Mu- tant,” Biosensors and Bioelectronics, Vol. 21, 2006, pp. 1132-1140. [5] K. S. Yao, D. Y. Wang, C. Y. Chang, et al., “Photocata- lytic Disinfection of Phytopathogenic Bacteria by Dye- Sensitized TiO2 Thin Film Activated by Visible Light,” Surface & Coatings Technology, Vol. 202, No. 4-7, 2007, pp. 1329-1332. [6] A. N. Ivanov, G. A. Evtugyn, R. E. Gyurcsányi, K. Tóth and H. C. Budnikov, “Comparative Investigation of Elec- tro-Chemical Cholinesterase Biosensors for Pesticide De- termination,” Analytica Chimica Acta, Vol. 404, No. 1, 2000, pp. 55-65. [7] N. Fidalgo-Used, E. Blanco-González and A. Sanz-Medel, “Evaluation of Two Commercial Capillary Columns for the Enantioselective Gas Chromatographic Separation of Organophosphorous Pesticides,” Talanta, Vol. 70, No. 5, 2006, pp. 1057-1063. [8] F. Sopeña, C. Maqueda and E. Morillo, “Controlled Release Formulations of Herbicides Based on Micro- Encapsulation,” Ciencia e Investigación Agrarian, Vol. 35, No. 1, 2009, pp. 27-42. [9] A. T. Doherty, S. Ellard, E. M. Parry and J. M. Parry, “A Study of the Aneugenic Activity of Trichlorfon Detected by Centromere-Specific Probes in Human Lymphoblas- toid Cell Lines,” Mutation Research, Vol. 372, No. 2, 1996, pp. 221-231. [10] X. Hong, J. Qu, J. Chen, et al., “Effects of Trichlorfon on Progesterone Production in Cultured Human Granulosa- Lutein Cells,” Toxicology in vitro, Vol. 21, No. 5, 2007, pp. 912-918. [11] X. Hong, J. Qu, Y. Wang, et al., “Study on the Mecha- nism of Trichlorfon-Induced Inhibition of Progesterone Synthesis in Mouse Leydig Tumor Cells (MLTC-1),” Toxicology, Vol. 234, No. 1-2, 2007, pp. 51-58. [12] S. Cukurcam, F. Sun, I. Betzendahl, I. D. Adler and U. Eichenlaub-Ritter, “Trichlorfon Predisposes to Aneuploi- dy and Interferes with Spindle Formation in in vitro Maturing Mouse Oocytes,” Mutation Research, Vol. 564, No. 2, 2004, pp. 165-178.  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 312 [13] R. Ranaldi, G. Gambuti, U. Eichenlaub-Ritter and F. Pacchierotti, “Trichlorfon Effects on Mouse Oocytes Following in vivo Exposure,” Mutation Research, Vol. 651, No. 1-2, 2008, pp. 125-130. [14] B. K. Catalgol, S. Ozden and B. Alpertunga, “Effects of Trichlorfon on Malondialdehyde and Antioxidant System in Human Erythrocytes,” Toxicology in vitro, Vol. 21, No. 8, 2007, pp. 1538-1544. [15] A. Mehl, T. M. Schanke, A. Torvik and F. Fonnum, “The Effect of Trichlorfon and Methylazoxymethanol on the Development of Guinea Pig Cerebellum,” Toxicology and Applied Pharmacology, Vol. 219, No. 2-3, 2007, pp. 128- 135. [16] N. M. Brito, S. Navickiene, L. Polese, E. F. G. Jardim, R. B. Abakerli and M. L. Ribeiro, “Determination of Pesti- cide Residues in Coconut Water by Liquid-Liquid Extrac- tion and Gas Chromatography with Electron-Capture Plus Thermionic Specific Detection and Solid-Phase Extrac- tion and High-Performance Liquid Chromatography with Ultraviolet Detection,” Journal of Chromatography A, Vol. 957, No. 2, 2002, pp. 201-209. [17] A. G. S. Prado and C. Airoldi, “The Toxic Effect on Soil Microbial Activity Caused by the Free or Immobilized Pesticide Diuron,” Thermochimica Acta, Vol. 394, No. 1-2, 2002, pp. 155-162. [18] C. Blasco, G. Font and Y. Picó, “Comparison of Micro- Extraction Procedures to Determine Pesticides in Oranges by Liquid Chromatography-Mass Spectrometry,” Journal of Chromatography A, Vol. 970, No. 1-2, 2002, pp. 201- 212. [19] D. K. Rodham, “Colloid and Interface Science in For- mulation Research for Crop Protection Products,” Cur- rent Opinion in Colloid and Interface Science, Vol. 5, No. 5-6, 2000, pp. 280-287. [20] H. Böttcher, C. Jagota, J. Trepte, K. H. Kallies and H. Haufe, “Sol-Gel Composite Films with Controlled Re- lease of Biocides,” Journal of Controlled Release, Vol. 60, No. 1, 1999, pp. 57-65. [21] M. Hussain, “Atoms in Agriculture: Nuclear Techniques in ‘Controlled Release’ Pesticide Research,” IAEA Bulle- tin, Vol. 31, No. 2, 1989, pp. 36-40. [22] A. Khazaei, D. Soudbar, M. Sadri and H. Hosseini, “Synthesis and Characterization of Poly(biphenyl-2-yl p-styrenesulphonate) as Profungicide in Controlled Re- lease Technique,” Journal of the Chinese Chemical Soci- ety, Vol. 54, No. 3, 2007, pp. 763-766. [23] M. Y. Arica, M. Yiğitoğlu, M. Lale, F. N. Kök and V. Hasirci, “Control led Release of Aldicarb from Carboxy me- thylcellulase Microcapsules,” Turkish Journal of Chemis- try, Vol. 21, No. 2, 1997, pp. 100-104. [24] K. Y. Choi, K. S. Min, I. H. Park, K. S. Kim and T. Chang, “Microcapsulation of Pesticides by Interfacial Polymerization 1. Polyurethane Microcapsules Contain- ing Oilsoluble Drug,” Polymer (Korea), Vol. 14, No. 4, 1990, pp. 392-400. [25] M. Y. El-Shoura, S. T. Badr, S. A. El-Khishen and M. M. Abu Elamayem, “Effect of Controlled Release Formula- tions of Carbofuran Soil Fertilizers and their Mixtures on Root-Knot Nematode on Tomato Plants,” Journal of King Saud University. Agricultural Sciences, Vol. 4, No. 1, 1992, pp. 69-77. [26] H. Schmidt, “Chemistry of Material Preparation by the Sol-Gel Process,” Journal of Non-Crystalline Solids, Vol. 100, No. 1-3, 1988, pp. 51-64. [27] J. D. Mackenzie, “Hybrid Organic-Inorganic Materials,” In: J. E. Mark, C. Y.-C. Lee and P. A. Bianconi, Eds., Hybrid Organic-Inorganic Composites, ACS Symposium Series 585, Washington, D.C., 1998, pp. 226-236. [28] J. Livage, F. Beteille, C. Roux, M. Chatry and P. David- son, “Sol-Gel Synthesis of Oxide Materials,” Acta Mate- rialia, Vol. 46, No. 3, 1998, pp. 743-750. [29] J. D. Wright and N. A. J. M. Sommerdijk, “The Chemis- try of Sol-Gel Silicates,” In: D. Phillips, P. O’Brien and S. Roberts, Eds., Advanced Chemistry Texts, OPA N.V., Gordon and Breach Science Publishers, New York, 2001, pp. 33-52. [30] Y. A. Shchipunov and T. Y. Karpenko, “Hybrid Polysac- charide-Silica Nanocomposites Prepared by the Sol-Gel Technique,” Langmuir, Vol. 20, No. 10, 2004, pp. 3882- 3887. [31] Y. A. Shchipunov, T. Y. Karpenko, I. Y. Bakunina, Y. V. Burtseva and T. N. Zvyagintseva, “A New Precursor for the Immobilization of Enzymes inside Sol-Gel Derived Hybrid Silica Nanocomposites Containing Polysaccha- rides,” Journal of Biochemical and Biophysical Methods, Vol. 58, No. 1, 2004, pp. 25-38. [32] Y. A. Shchipunov, T. Y. Karpenko and A. V. Krekoten, “Hybrid Organic-Inorganic Nanocomposites Fabricated with a Novel Biocompatible Precursor Using Sol-Gel Processing,” Composite Interfaces, Vol. 11, No. 8-9, 2005, pp. 587-607. [33] Y. A. Shchipunov, A. V. Krekoten, V. G. Kuryavyi and I. N. Topchieva, “Microporous Nanocomposite Material Synthesized by Sol-Gel Processing in the Presence of Cyclodextrins,” Colloid Journal, Vol. 67, No. 3, 2005, pp. 380-384. [34] Y. A. Schipunov, “Entrapment of Biopolymers into Sol-Gel Derived Silica Nanocomposites,” In: E. Ruiz- Hitzky, K. Ariga and Y. M. Lvov, Eds., Bio-Inorganic Hybrid Nanomaterials, Copyright WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008, pp. 75-112. [35] M. Răileanu, “The Use of Sol-Gel Method for Bio- materials Preparation,” Revue Roumaine de Chimie, Vol. 51, No. 10, 2006, pp. 941-962. [36] C. Van Hooidonk and J. C. A. E. Breebaart-Hansen, “Model Studies for Enzyme Inhibition. Part IV. The As- sociation of Some Alkyl Methylphosphonates with α- cyclodextrin in an Aqueous Medium,” Recueil, Vol. 91, 1972, pp. 958-964. [37] E. M. Del Valle, “Cyclodextrins and their Uses: A Re- view,” Process Biochemistry, Vol. 39, No. 9, 2004, pp. 1033-1046. [38] G. Petrović, B. S. Radovanović and O. Jovanović, “Cha- racterization of Pesticide-β-cyclodextrin Inclusion Com-  Sol-Gel Materials with Pesticide Delivery Properties Copyright © 2010 SciRes. JEP 313 plexes in Aqueous Solution,” Physics, Chemistry and Technology, V o l . 3, N o. 2 , 2005, pp. 151-155. [39] A. R. Hedges, “Industrial Applications of Cyclodextrins,” Chemical Reviews, Vo l. 9 8, No. 5 , 1998, pp. 2035-2044. [40] J. Szejtli, “Past, Present, and Future of Cyclodextrin Re- search,” Pure and Applied Chemistry, Vol. 76, No. 10, 2004, pp. 1825-1845. [41] J. Orgoványi, L. Pöppl, K. H. Otta and G. A. Lovas, “Thermoanalytical Method for Studying the Guest Con- tent in Cyclodextrin Inclusion Complexes,” Journal of Thermal Analysis and Calorimetry, Vol. 81, No. 2, 2005, pp. 261-266. [42] Cs. Novák, Z. Éhen, M. Fodor, L. Jicsinszky and J. Or- goványi, “Application of Combined Thermoanalytical Techniques in the Investigation of Cyclodextrin Inclusion Complexes,” Journal of Thermal Analysis and Calori- metry, Vol. 84, No. 3, 2006, pp. 693-701. [43] G. Ioniţă, R. Socoteanu and F. Savonea, “ATR/FTIR Study on Silica Prepared Using β-Cyclodextrine and Urea as Template,” Revue Roumaine de Chimie, Vol. 50, No. 1, 2005, pp. 71-77. [44] J. M. Gavira, A. Hernanz and I. Bratu, “Dehydration of β-cyclodextrin. An IR ν(OH) Band Profile Analysis,” Vi- brational Spectroscopy, Vol. 32, No. 2, 2003, pp. 137- 146. [45] I. Bratu, S. Astilean, C. Ionesc, E. Indrea, J. P. Huvenne and P. Legrand, “FT-IR and X-ray Spectroscopic Investi- gations of Na-diclofenac-cyclodextrins Interactions,” Spe- ctrochim Acta A, Vol. 54, No. 1, 1998, pp. 191-196. [46] A. Farcaş, “Semiconducting Polymers with Rotaxane Architecture,” In: Scientific Anales of the Al.I. Cuza Uni- versity, Volume XLV.XI.VI, Physics of the Condensed State, 1999-2000, pp. 217-223. [47] J. Szejtli, “Types, Formation and Structures of Inclusion Complexes,” In: J. Szejtli, Cyclodextrins and their Inclu- sion Complexes, Akadémiai Kiadó, Budapest, 1982, pp. 94-143. [48] A. Bertoluzza, M. Rossi, P. Taddei, E. Redenti, M. Zanol and P. Ventura, “FT-Raman and FT-IR Studies of 1:2.5 Piroxicam: β-cyclodextrin Inclusion Compound,” Journal of Molecular Structure, Vol. 480-481, 1999, pp. 535-539. [49] E. Bilensoy, M. A. Rouf, I. Vural, M. Sen and A. A. Hincal, “Mucoadhesive, Thermosensitive, Prolonged- Release Vaginal Gel for Clotrimazole: β-cyclodextrin Complex,” AAPS PharmSciTech, Vol. 7, No. 2, 2006, pp. E54-E60. [50] F. Taneri, T. Güneri, Z. Aigner, O. Berkesi and M. Kata, “Thermoanalytical Studies on Complexes of Clotrimazole with Cyclodextrins,” Journal of Thermal Analysis and Calorimetry, Vol. 76, No. 2, 2004, pp. 471-479. [51] L. P. Fernandes, Zs. Éhen, T. F. Moura, Cs. Novák and J. Sztatisz, “Characterization of Lippia sidoides Oil Ex- tract-β-cyclodextrin Complexes Using Combined Ther- moanalytical Techniques,” Journal of Thermal Analysis and Calorimetry, Vol. 78, No. 2, 2004, pp. 557-573. [52] J.-H. Li, N. Zhang, X.-T. Li, J.-Y. Wang and S.-J. Tian, “Kinetic Studies on the Thermal Dissociation of the In- clusion Complex of β-cyclodextrin with Cinnamic Alde- hide,” Journal of Thermal Analysis and Calorimetry, Vol. 49, No. 3, 1997, pp. 1527-1533. [53] G. Bettinetti, Cs. Novák and M. Sorrenti, “Thermal and Structural Characterization of Commercial α-, β-, and γ-cyclodextrins,” Journal of Thermal Analysis and Calorimetry, Vol. 68, No. 2, 2002, pp. 517-529. [54] J. M. Ginés, M. J. Arias, C. Novák, P. J. Sánchez-Soto, A. Ruiz-Conde and E. Morillo, “Thermal Study of Complex Formation of Triamterene with β-cyclodextrin by Spray- Drying and Co-Grinding,” Journal of Thermal Analysis and Calorimetry, Vol. 45, No. 4, 1995, pp. 659-666. [55] C. Molina, P. Grasso, E. Benfenati and D. Barceló, “Auto- mated Sample Preparation with Extraction Columns Fol- lowed by Liquid Chromatography-Ionspray Mass Spec- trometry. Interferences, Determination and Degradation of Polar Organophosphorous Pesticides in Water Sam- ples,” Journal of Chromatography A, Vol. 737, No. 1, 1996, pp. 47-58. |

